Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (16): 4204-4218.doi: 10.12307/2026.729
Previous Articles Next Articles
Different exercise modalities improve health gains in vascular function in overweight or obese children and adolescents: a Bayesian meta-analysis
Xu Yang1, Li Xiupeng1, Yang Bing1, Yang Chunbaixue1, Zhao Zhongwei2
- 1School of Physical Education and Sport, Bohai University, Jinzhou 121013, Liaoning Province, China; 2School of Physical Education and Sport, Shenyang Normal University, Shenyang 110100, Liaoning Province, China
-
Received:2025-05-20Accepted:2025-08-26Online:2026-06-08Published:2025-11-28 -
Contact:Yang Bing, Master’s supervisor, Professor, School of Physical Education and Sport, Bohai University, Jinzhou 121013, Liaoning Province, China -
About author:Xu Yang, MS candidate, School of Physical Education and Sport, Bohai University, Jinzhou 121013, Liaoning Province, China -
Supported by:Major Commissioned Project of the Liaoning Provincial Social Science Planning Fund, No. L24ZD020 (to ZZW)
CLC Number:
Cite this article
Xu Yang, Li Xiupeng, Yang Bing, Yang Chunbaixue, Zhao Zhongwei . Different exercise modalities improve health gains in vascular function in overweight or obese children and adolescents: a Bayesian meta-analysis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4204-4218.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
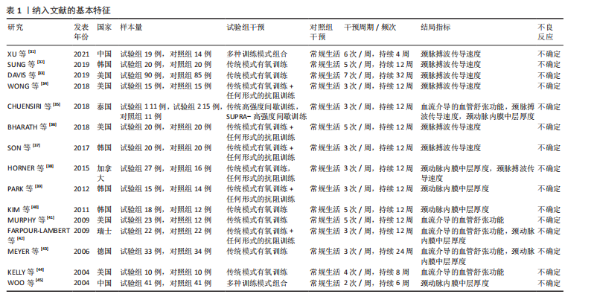
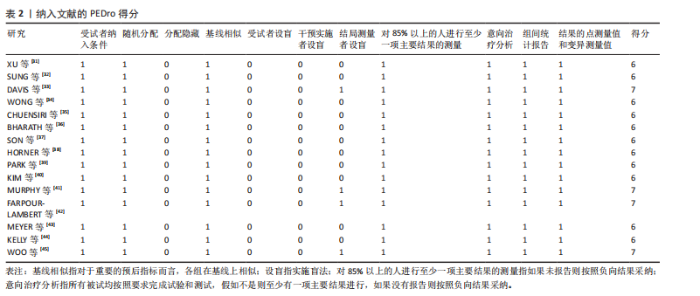
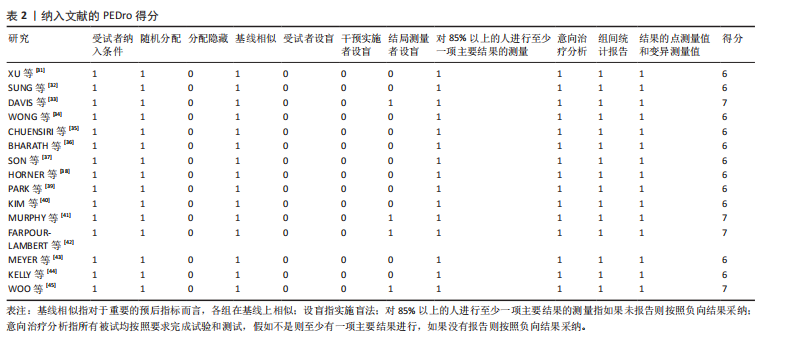
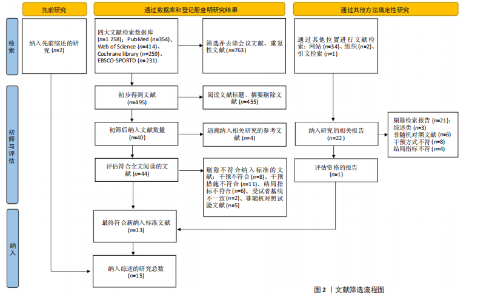
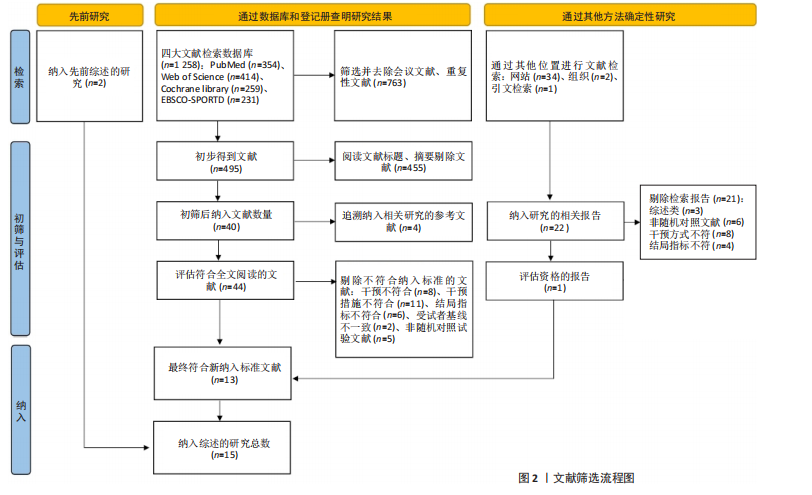
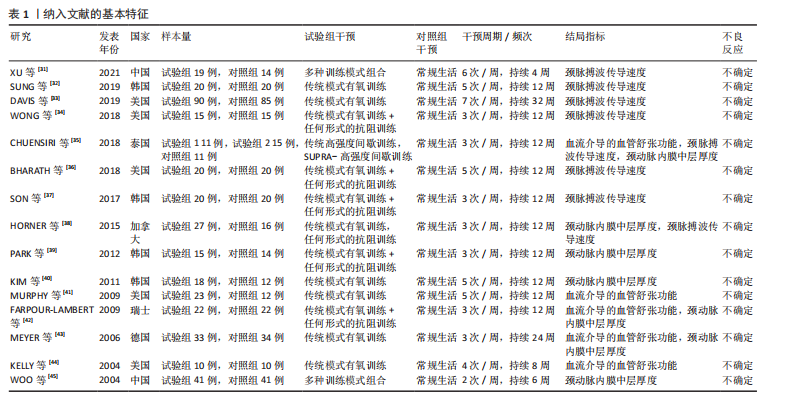
2.1 文献检索结果 通过对4个数据库进行文献检索,共检索出1 258篇文献,通过EndNote 19文献管理软件将1 258篇英文文献导入并进行重复文献筛除,初步得到495篇文献。通过逐级阅读文章标题及摘要,去除非运动干预文献后共获得44篇文献,最后通过阅读全文剔除非儿童青少年干预、非肥胖超重文献、非随机对照试验、结局指标不符合纳入标准的文献32篇,与其他方法纳入的研究合并,最终纳入符合标准的文献15篇[31-45]。文献检索流程见图2。 2.2 纳入文献的基本特征 纳入的15篇文献中,有7篇文献的试验组采用传统有氧训练方法[32-33,38,40-41,43-44],5篇文献的试验组采用有氧与抗阻结合训练的方法[34,36-37,39,42], 2篇文献的试验组采用混合训练方案[31,45],1篇文献的试验组采用间歇性训练方式[35],1篇文献的试验组采用抗阻训练的方式进行结局指标的测量[38],其中有2篇文献采用了双臂干预方式[35,38],对照组均为常规生活。纳入文献基本特征表见表1。 2.3 纳入文献质量评估结果 依据PEDro量表标准判定,得分6分以上即为较高质量文献,纳入的15篇文献得分均在6分以上,见表2。由于"
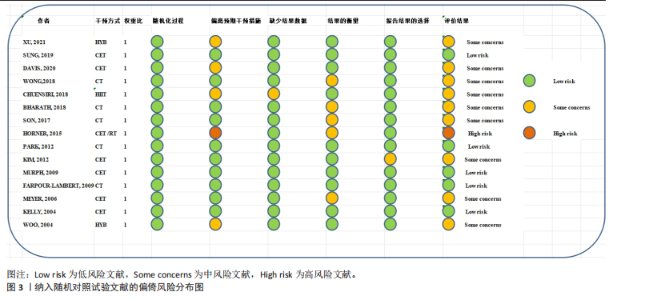
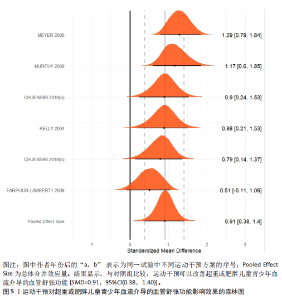
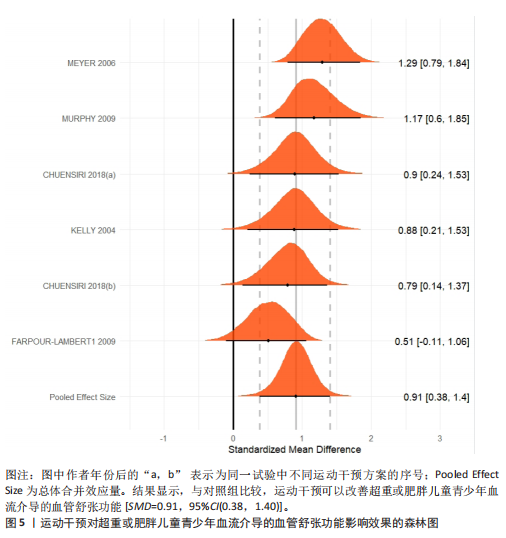
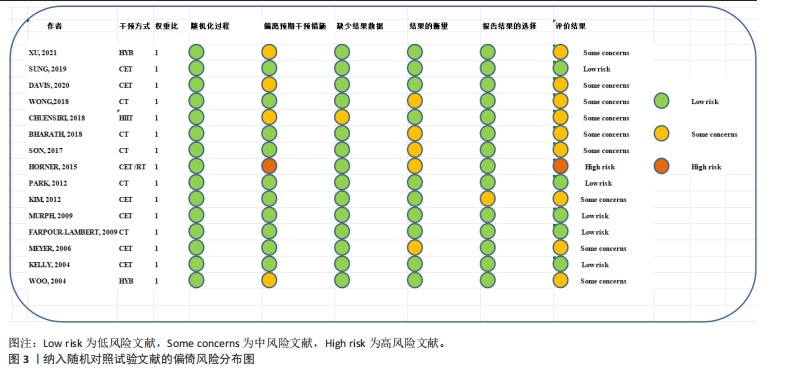
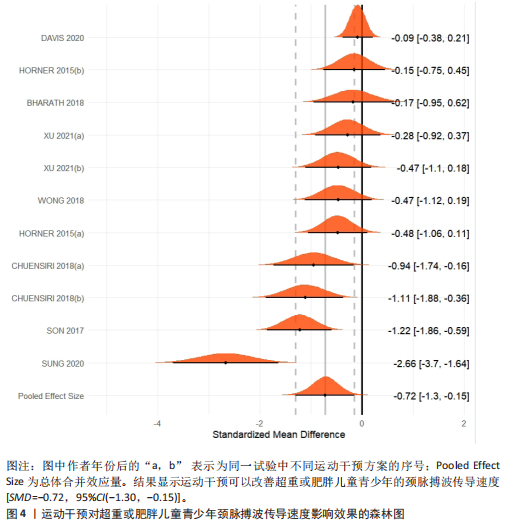
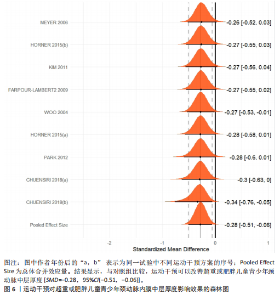
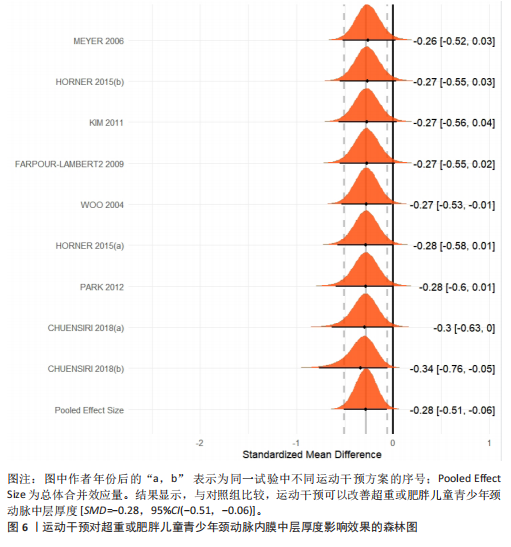
运动干预类型的可知性,导致对受试者和干预者设置盲法受阻,但仍有4篇文献对结局测量者进行设盲,提升了所取文献的可信度[33,41-42,45]。 根据Rob 2.0偏倚风险评估工具对纳入文献的随机化过程、偏离预期干预措施、结果数据的缺失、报告结果的选择进行偏倚评估,1篇文献由于设备变化和退出导致试验组与对照组样本量不均衡,被评估为高风险[38]。其余文章均为中低风险文献,其中低风险文献为5篇[32,39,41-42,44],见图3。 综上所述,文章通过 PEDro 评分量表与 RoB 2.0 偏倚风险图结合进行双重评级,最终纳入文献均在 PEDro 评分上达标,并且除1篇高偏倚风险外,其余文献均为中低风险,具备较高的可信度与方法学质量,进一步验证了该研究的可靠性。 2.4 基于贝叶斯模型下运动干预对超重或肥胖儿童青少年的Meta分析2.4.1 总体分析森林图颈脉搏波传导速度:8篇文献分析了运动干预对超重或肥胖儿童青少年颈脉搏波传导速度的影响[31-38]。异质性检验(I2=93%,P < 0.000 01)显示研究间异质性较高,采用随机效应模型进行分析。由于此次研究纳入单位不一致,故采用SMD标准化均数差进行合并效应量检验。森林图中显示的不是每个研究观察的效应量,而是基于贝叶斯模型中的每个研究估计到的效应量,结果显示运动干预可以改善超重或肥胖儿童青少年的颈脉搏波传导速度[SMD=-0.72,95%CI(-1.30,-0.15)],见图4。 血流介导的血管舒张功能:6篇文献分析了运动干预对超重或肥胖儿童青少年血流介导的血管舒张功能的影响[35,41-44]。异质性检验(I2=57%,P < 0.000 01)显示研究间异质性较高,采用随机效应模型。由于此次研究纳入单位不一致,故采用SMD标准化均数差进行合并效应量检验。森林图中显示的不是每个研究观察的效应量,而是基于贝叶斯模型中的每个研究估计到的效应量,结果显示,与对照组比较,运动干预可以改善超重或肥胖儿童青少年血流介导的血管舒张功能[SMD=0.91,95%CI(0.38,1.40)],见图5。 颈动脉内膜中层厚度:7篇文献分析了运动干预对超重或肥胖儿童青少年颈动脉内膜中层厚度的影响[35,38-40,42-43,45]。异质性检验(I2=0%,P=0.792 1)显示研究间异质性较低,采用固定效应模型。由于此次研究纳入单位不一致,故采用SMD标准化均数差进行合并效应量检验,结果显示,与对照组比较,运动干预可以改善超重或肥胖儿童青少年颈动脉中层厚度[SMD=-0.28,95%CI(-0.51,-0.06)],见图6。 2.4.2 亚组分析森林图 颈脉搏波传导速度:8篇文献分析了运动干预对超重或肥胖儿童青少年颈脉搏波传导速度的影响[31-38]。异质性检验(I2=82%,P < 0.000 01)显示研究间异质性较高,采用随机效应模型。由于此次研究纳入单位不一致,故采用SMD标准化均数差进行合并效应量检验。整体合并效应量显示,"
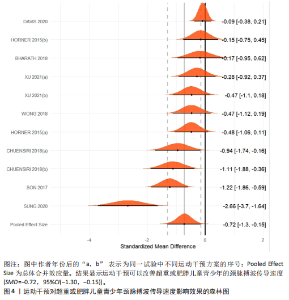
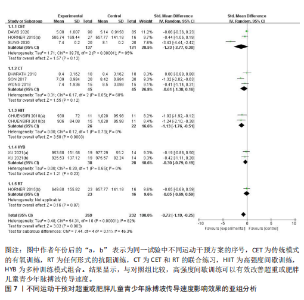
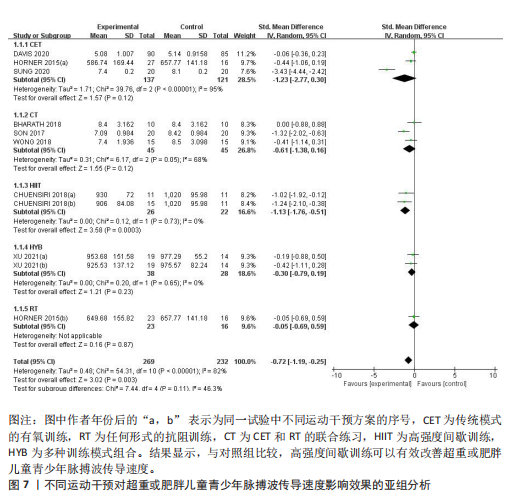
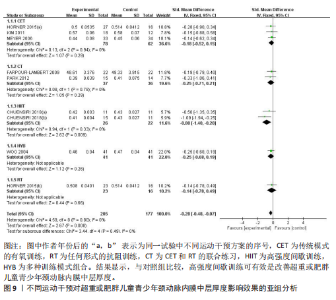
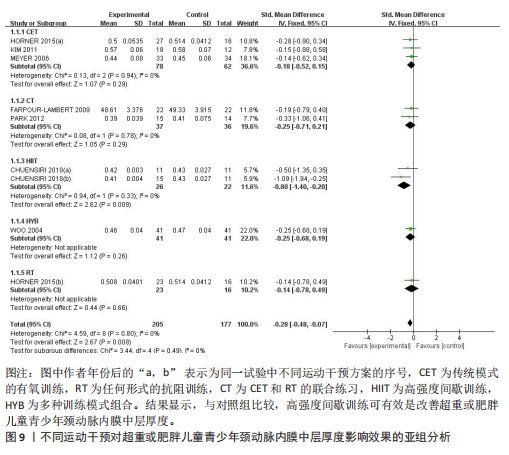
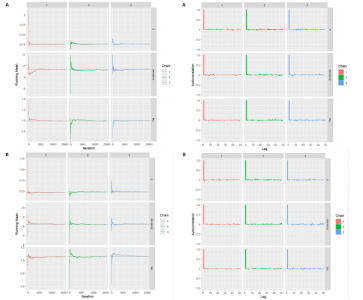
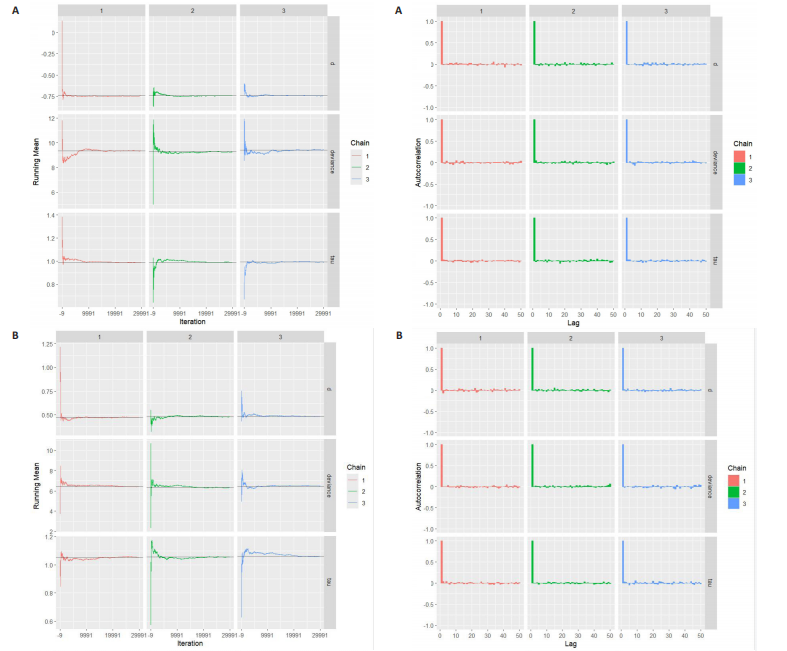
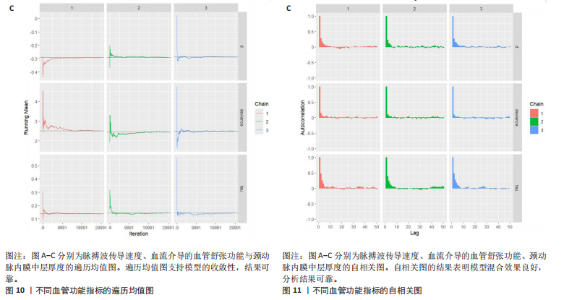
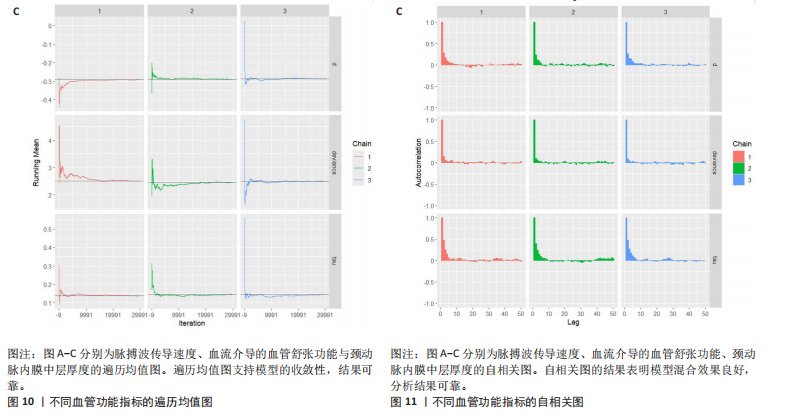
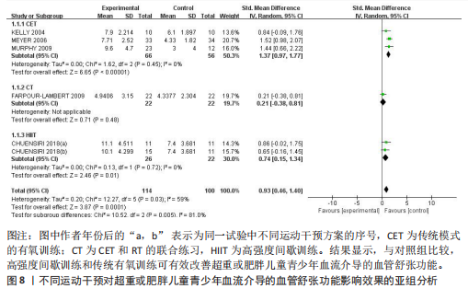
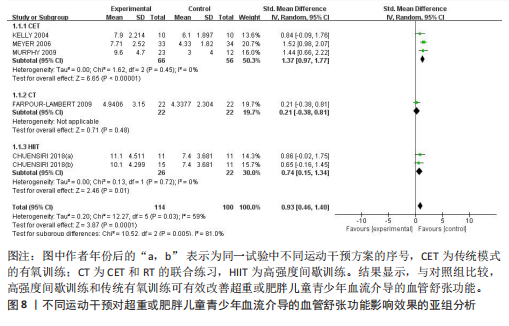
与对照组比较,运动干预可以有效改善超重或肥胖儿童青少年脉搏波传导速度[SMD=-0.72,95%CI=(-1.19,-0.25),P < 0.05];亚组分析森林图显示,与对照组比较,高强度间歇训练可以有效改善超重或肥胖儿童青少年脉搏波传导速度[SMD=-1.13,95%CI(-1.76,-0.51),P < 0.05], 联合训练[SMD=-0.61,95%CI(-1.38,0.16),P > 0.05]、传统有氧训练[SMD=-1.23,95%CI(-2.77,0.30),P > 0.05]、抗阻训练[SMD=-0.05,95%CI(-0.69,0.59),P > 0.05]和混合训练[SMD=-0.30,95%CI(-0.79,0.19),P > 0.05]未能改善超重或肥胖儿童青少年脉搏波传导速度,见图7。综上来看,高强度间歇训练对超重或肥胖儿童青少年脉搏波传导速度改善效果明显,传统有氧训练、联合训练、混合训练、阻力训练的改善效果不大,该结果可能是文章数量过少导致的。 血流介导的血管舒张功能:5篇文献分析了运动干预对超重或肥胖儿童青少年肥胖血流介导的血管舒张功能的影响[35,41-44]。异质性检验(I2=59%,P=0.03)研究间异质性较高,采用随机效应模型。由于此次研究纳入单位不一致,故采用SMD标准化均数差进行合并效应量检验。整体合并效应量显示,与对照组比较,运动干预可有效改善超重或肥胖儿童青少年血流介导的血管舒张功能[SMD=0.93,95%CI(0.46,1.40),P < 0.05];亚组分析森林图发现,与对照组比较,高强度间歇训练[SMD=0.74,95%CI(0.15,1.34),P < 0.05]和传统有氧训练[SMD=1.37,95%CI(0.97,1.77),P < 0.05]可有效改善超重或肥胖儿童青少年血流介导的血管舒张功能,联合训练未能有效改善超重或肥胖儿童青少年血流介导的血管舒张功能[SMD=0.21,95%CI(-0.38,0.81),P > 0.05],见图8。综上来看,高强度间歇训练、传统有氧训练改善超重或肥胖儿童青少年血流介导的血管舒张功能的效果显著,而联合训练改善效果不佳,该结果可能是文章数量过少导致的。 颈动脉内膜中层厚度:7篇文献分析了运动干预超重或肥胖儿童青少年颈动脉内膜中层厚度的影响[35,38-40,42-43,45]。异质性检验(I2=0%,P=0.80)显示研究间异质性较低,采用固定效应模型。由于此次研究纳入单位不一致,故采用SMD标准化均数差进行合并效应量检验。整体合并效应量显示,与对照组比较,运动干预可有效改善超重或肥胖儿童青少年颈动脉内膜中层厚度[SMD=-0.28,95%CI(-0.48,-0.07),P < 0.05];通过亚组分析森林图发现,与对照组比较,高强度间歇训练可有效改善超重或肥胖儿童青少年颈动脉内膜中层厚度[SMD=-0.80,95%CI(-1.40,-0.20), P < 0.05],传统有氧训练[SMD=-0.18,95%CI(-0.52,0.15),P > 0.05]、联合训练[SMD=-0.25,95%CI(-0.71,0.21),P > 0.05]、混合训练[SMD=-0.25,95%CI(-0.68,0.19),P > 0.05]和抗阻训练[SMD=-0.14,95%CI(-0.78,0.49),P > 0.05]均未能有效改善超重或肥胖儿童青少年颈动脉内膜中层厚度,见图9。综上来看,高强度间歇训练对超重或肥胖儿童青少年颈动脉内膜中层厚度的改善效果显著,传统有氧训练、联合训练、混合训练、抗阻训练的改善效果不大,该结果可能是文章数量过少导致的。 2.4.3 遍历均值图 图10对应脉搏波传导速度、血流介导的血管舒张功能及颈动脉内膜中层厚度3个参数的收敛性分析,展示了各参数在迭代过程中的变化情况。结果显示各链的波动平稳且重叠良好,表明模型收敛性较好;迭代次数充足,参数在早期即达到稳定状态,未观察到明显的自相关性,进一步验证了模型的有效性。总体来看,遍历均值图支持模型的收敛性,结果可靠。 2.4.4 自相关图 图11展示了颈脉搏波传导速度、血流介导的血管舒张功"
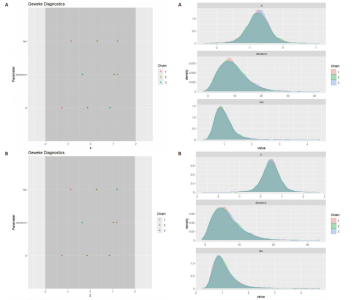
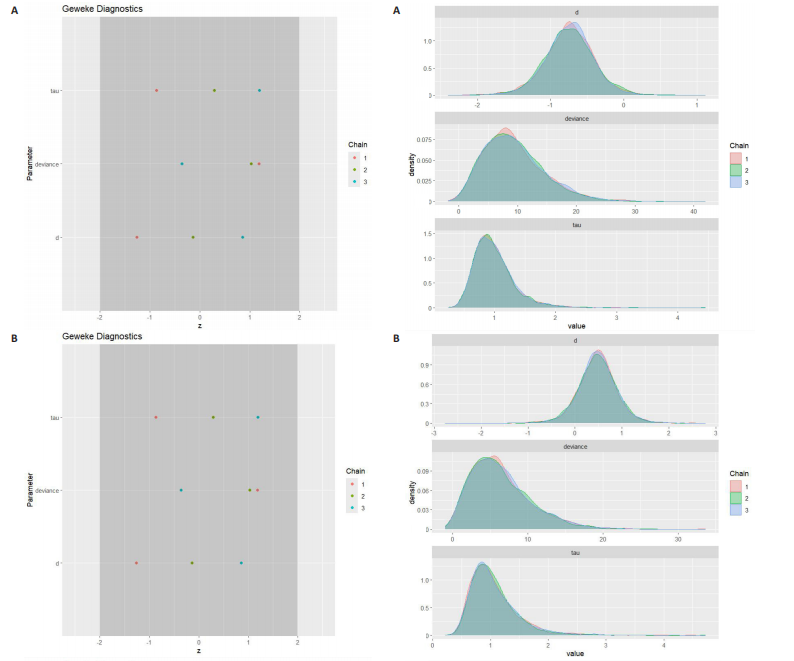
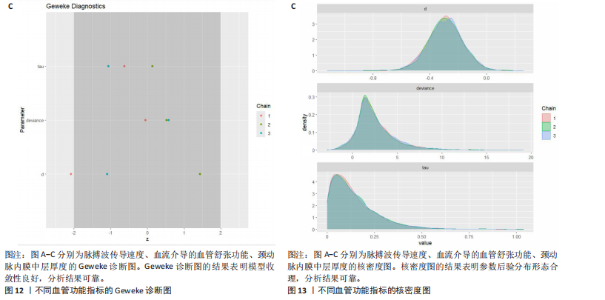
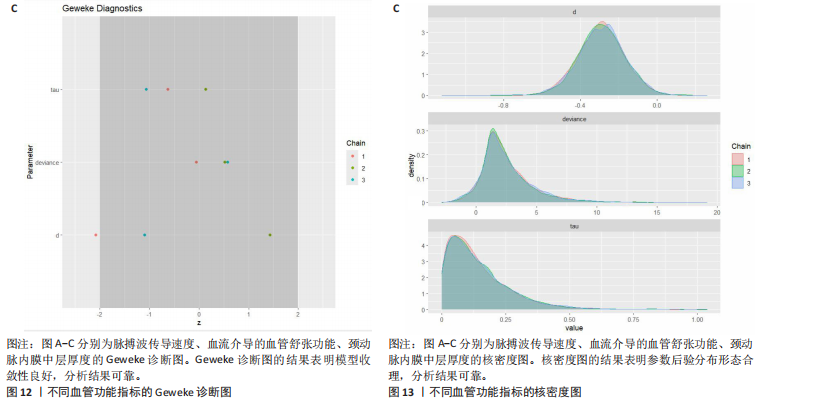
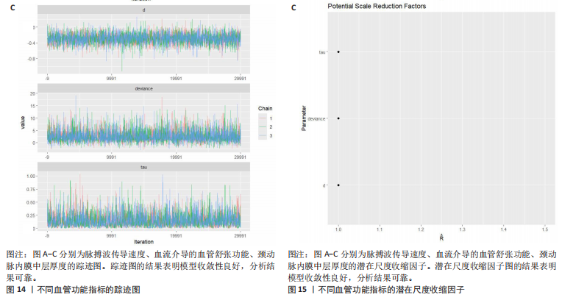
能、颈动脉内膜中层厚度参数在不同滞后阶数下的自相关性。结果显示,图中自相关系数在低滞后阶数(lag=1和lag=2)时迅速下降至接近零,表明马尔可夫链的混合效果较好,样本独立性较高;在滞后阶数达到10时,自相关系数已接近零,进一步支持模型的收敛性;未观察到自相关系数在高滞后阶数时显著高于零的情况,表明样本之间的相关性较低。总体来看,自相关图的结果表明模型混合效果良好,分析结果可靠。 2.4.5 Geweke诊断图 Geweke诊断图通过对比马尔可夫链早期与后期样本均值的差异来评估收敛性。图12中Z-score的分布集中在零附近且大部分落在[-1.96,1.96]的范围内,表明链的早期和后期均值无显著差异,支持模型收敛;未观察到明显的异常值或模式,进一步验证了模型的有效性。总体来看,Geweke诊断图的结果表明模型收敛性良好,分析结果可靠。 2.4.6 核密度图 核密度图的作用,即通过平滑曲线展示参数后验分布形态,用于直观反映参数的不确定性及其集中趋势。图13核密度图展示了参数后验分布的平滑曲线。结果显示图中曲线呈现单峰且近似对称形态,表明参数估计具有较高的确定性;曲线的尾部较短,进一步支持参数的不确定性范围较小;与先验分布相比,后验分布更为集中,表明数据对参数估计提供了较强的信息。总体来看,核密度图的结果表明参数后验分布形态合理,分析结果可靠。 2.4.7 踪迹图 踪迹图展示了马尔可夫链蒙特卡洛采样过程中参数的迭代值,用于诊断贝叶斯模型的收敛程度,当迭代次数不足时,马尔可夫链蒙特卡洛链未明显拟合,可识别出链的波动,收敛程度差,应增加迭代次数;当迭代次数达到7 000次时,各条马尔可夫链蒙特卡洛链从起始部分开始已达到稳定融合,并且在后续计算中重叠面积占链波动范围的大部分,并且不能识别单条链的波动,收敛程度满意。如图14所示,图中各链的波"
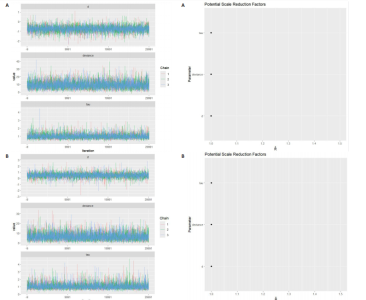
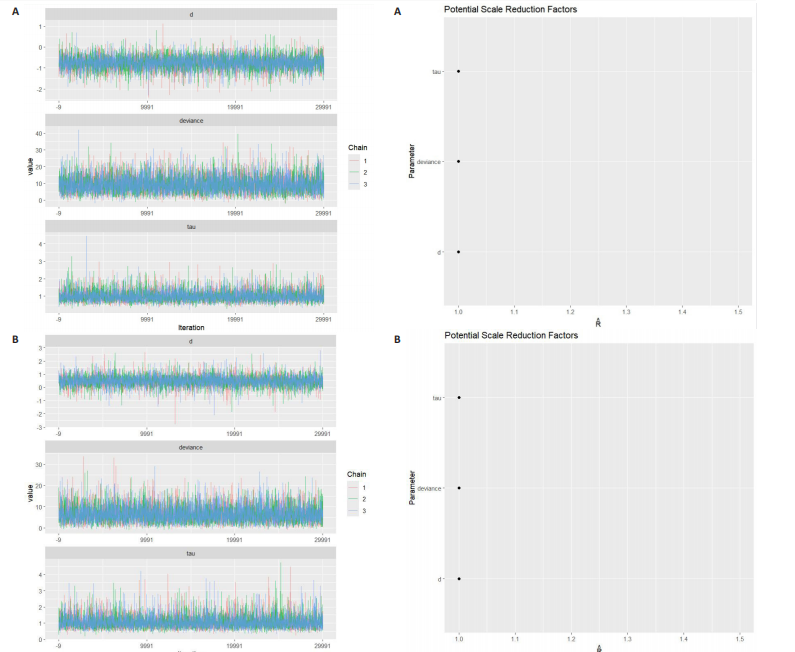
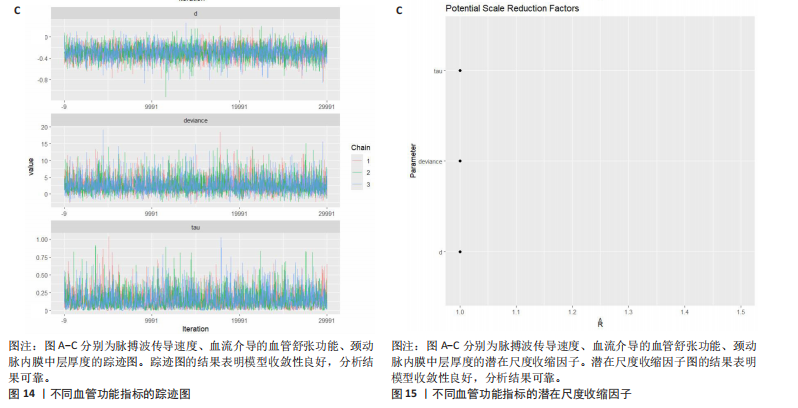
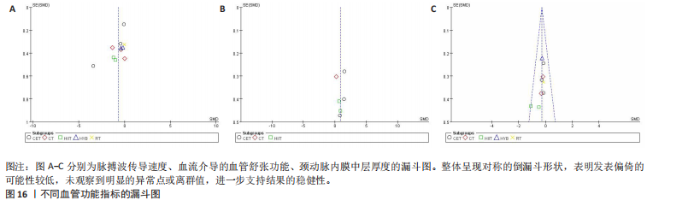
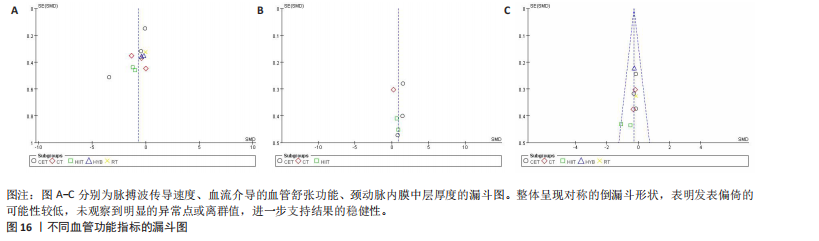
动平稳且无明显趋势,表明模型可能已经收敛;多条链在相同范围内波动且重叠良好,进一步支持模型的收敛性;迭代次数充足,参数在早期迭代后即趋于稳定;未观察到明显的异常模式,如周期性波动或突然跳跃。总体来看,踪迹图的结果表明模型收敛性良好,分析结果可靠。 2.4.8 潜在尺度收缩因子 潜在尺度收缩因子(R-hat)的作用,即通过比较链内和链间的方差来评估模型的收敛性。理想的潜在尺度收缩因子值通常小于1.1或1.05,这一范围广泛被认为是判断模型是否收敛的标准。图15中所有参数的潜在尺度收缩因子值均小于1.1,并且集中在1.0附近,表明模型收敛性良好;未观察到潜在尺度收缩因子值异常高的参数,进一步支持模型的有效性;多条链的潜在尺度收缩因子值一致性较高,表明各链之间的方差差异较小。总体来看,潜在尺度收缩因子图的结果表明模型收敛性良好,分析结果可靠。 2.4.9 漏斗图 漏斗图用于评估发表偏倚和小样本效应。图16为颈脉搏波传导速度、血流介导的血管舒张功能、颈动脉内膜中层厚度结局指标的漏斗图,图中大部分研究点集中在图形顶部,随着标准误差的减小,研究点逐渐向中间集中,整体呈现对称的倒漏斗形状,表明发表偏倚的可能性较低,未观察到明显的异常点或离群值,进一步支持结果的稳健性。"
| [1] 张瀚月,马璐,孔振兴,等.2016—2020年我国学生超重、肥胖和营养不良状况的流行趋势与防控策略[J]. 北京体育大学学报,2023,46(11):118-131. [2] LOB-CORZILIUS T. Overweight and obesity in childhood – A special challenge for public health. Int J Hyg Environ Health. 2007; 210(5):585-589. [3] DI CESARE M, SORIĆ M, BOVET P, et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17(1):212. [4] 王军利,项立敏,张松奎,等.儿童青少年超重与肥胖的成因及社会网络干预[J].上海体育学院学报,2019,43(5):30-40. [5] 蒋露芳,王莹莹,彭慧,等.学龄儿童肥胖与肠道菌群多样性及菌属丰度的关联研究[J].中华流行病学杂志,2022,43(2): 260-268. [6] KOYUNCUOĞLU GÜNGÖR N. Overweight and obesity in children and adolescents. J Clin Res Pediatr Endocrinol. 2014;6(3):129-143. [7] LEE EY, YOON KH. Epidemic obesity in children and adolescents: risk factors and prevention. Front Med. 2018;12(6):658-666. [8] MAINIERI F, LA BELLA S, CHIARELLI F. Hyperlipidemia and cardiovascular risk in children and adolescents. Biomedicines. 2023;11(3):809. [9] CAHILL PA, REDMOND EM. Vascular endothelium – gatekeeper of vessel health. Atherosclerosis. 2016;248:97-109. [10] ALEXANDER Y, OSTO E, SCHMIDT-TRUCKSÄSS A, et al. Endothelial function in cardiovascular medicine: a consensus paper of the european society of cardiology working groups on atherosclerosis and vascular biology, aorta and peripheral vascular diseases, coronary pathophysiology and microcirculation, and thrombosis. Cardiovasc Res. 2021;117(1):29-42. [11] GODO S, SHIMOKAWA H. Endothelial functions. Arterioscler Thromb Vasc Biol. 2017;37(9):e108-e114. [12] STANHEWICZ AE, WENNER MM, STACHENFELD NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol. 2018;315(6):H1569-H1588. [13] JOO TURONI C, MARAÑÓN RO, FELIPE V,et al. Arterial stiffness and endothelial function in obese children and adolescents and its relationship with cardiovascular risk factors. Horm Res Paediatr. 2013;80(4):281-286. [14] VINCZE M, DÉR H, KEREKES GY, et al. Decreased flow-mediated dilatation with increased arterial stiffness and thickness as early signs of atherosclerosis in polymyositis and dermatomyositis patients. Clin Rheumatol. 2014;33(11):1635-1641. [15] LO MH, LIN IC, LU PC, et al. Evaluation of endothelial dysfunction, endothelial plasma markers, and traditional metabolic parameters in children with adiposity. J Formos Med Assoc. 2019;118(1):83-91. [16] HILL MA, YANG Y, ZHANG L, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021; 119:154766. [17] ZHANG X, GAO F. Exercise improves vascular health: Role of mitochondria . Free Radic Biol Med, 2021;177:347-359. [18] GREEN DJ, THOMAS HJ, MARSH CE, et al.Impact of resistance and endurance exercise training on femoral artery function: sex differences in humans. J Physiol. 2025; 603(5):1045-1056. [19] OTSUKI T, NAKAMURA F, ZEMPO-MIYAKI A. Nitric oxide and decreases in resistance exercise blood pressure with aerobic exercise training in older individuals. Front Physiol. 2019;10:1204. [20] TSUKIYAMA Y, ITO T, NAGAOKA K, et al. Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J Clin Biochem Nutr. 2017;60(3):180-186. [21] EL ASSAR M, ÁLVAREZ-BUSTOS A, SOSA P, et al. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int J Mol Sci. 2022;23(15):8713. [22] PIERCE D R, DOMA K, RAIFF H, et al. Influence of exercise mode on post-exercise arterial stiffness and pressure wave measures in healthy adult males. Front Physiol. 2018;9:1468. [23] KIM HB, SEO MW, JUNG HC. Effects of aerobic vs. Resistance exercise on vascular function and vascular endothelial growth factor in older women. Healthcare. 2023; 11(18):2479. [24] DALL CH, GUSTAFSSON F, CHRISTENSEN SB, et al. Effect of moderate- versus high-intensity exercise on vascular function, biomarkers and quality of life in heart transplant recipients: a randomized, crossover trial. J Heart Lung Transplant. 2015;34(8):1033-1041. [25] BOIDIN M, ERSKINE RM, THIJSSEN DHJ, et al. Exercise modality, but not exercise training, alters the acute effect of exercise on endothelial function in healthy men. J Appl Physiol. 2021;130(6): 1716-1723. [26] CUMPSTON M, LI T, PAGE MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10(10):ED000142. [27] FARRAH K, YOUNG K, TUNIS MC, et al. Risk of bias tools in systematic reviews of health interventions: an analysis of PROSPERO-registered protocols. Syst Rev, 2019;8(1):280. [28] FATTAL R. Blue-noise point sampling using kernel density model//ACM SIGGRAPH 2011 papers. Vancouver British Columbia Canada: ACM, 2011:1-12. [29] GABRY J, SIMPSON D, VEHTARI A, et al. Visualization in bayesian workflow. J R Stat Soc Ser A Stat Soc. 2019;182(2):389-402. [30] DU H, KE Z, JIANG G, et al. The performances of gelman-rubin and geweke’s convergence diagnostics of monte carlo markov chains in bayesian analysis. J Behav Data Sci. 2022; 2(2):47-72. [31] XU L, ZOU X, GAO Z, et al. Improved fatty acid profile reduces body fat and arterial stiffness in obese adolescents upon combinatorial intervention with exercise and dietary restriction. J Exerc Sci Fitness. 2021;19(4):234-240. [32] SUNG KD, PEKAS EJ, SCOTT SD, et al. The effects of a 12-week jump rope exercise program on abdominal adiposity, vasoactive substances, inflammation, and vascular function in adolescent girls with prehypertension. Eur J Appl Physiol. 2019;119(2):577-585. [33] DAVIS CL, LITWIN SE, POLLOCK NK, et al.Exercise effects on arterial stiffness and heart health in children with excess weight: the SMART RCT. Int J Obes (Lond). 2019;44(5):1152. [34] WONG A, SANCHEZ-GONZALEZ MA, SON WM, et al. The Effects of a 12-Week Combined Exercise Training Program on Arterial Stiffness, Vasoactive Substances, Inflammatory Markers, Metabolic Profile, and Body Composition in Obese Adolescent Girls. Pediatr Exerc Sci. 2018;30(4):480-486. [35] CHUENSIRI N, SUKSOM D, TANAKA H. Effects of High-Intensity Intermittent Training on Vascular Function in Obese Preadolescent Boys. Child Obes. 2018;14(1):41-49. [36] BHARATH LP, CHOI WW, CHO JM, et al. Combined resistance and aerobic exercise training reduces insulin resistance and central adiposity in adolescent girls who are obese: randomized clinical trial. Eur J Appl Physiol. 2018;118(8):1653-1660. [37] SON WM, SUNG KD, BHARATH LP, et al. Combined exercise training reduces blood pressure, arterial stiffness, and insulin resistance in obese prehypertensive adolescent girls. Clin Exp Hypertens. 2017; 39(6):546-552. [38] HORNER K, BARINAS-MITCHELL E, DEGROFF C, et al. Effect of aerobic versus resistance exercise on pulse wave velocity, intima media thickness and left ventricular mass in obese adolescents. Pediatr Exerc Sci. 2015;27(4):494-502. [39] PARK JH, MIYASHITA M, KWON YC, et al. A 12-week after-school physical activity programme improves endothelial cell function in overweight and obese children: a randomised controlled study. BMC Pediatr. 2012;12:111. [40] KIM JY, KIM ES, JEON JY, et al. Improved Insulin Resistance, Adiponectin and Liver Enzymes without Change in Plasma Vaspin Level after 12 Weeks of Exercise Training among Obese Male Adolescents. Korean J Obes. 2011;20(3):138. [41] MURPHY ECS, CARSON L, NEAL W, et al. Effects of an exercise intervention using Dance Dance Revolution on endothelial function and other risk factors in overweight children. Int J Pediatr Obes. 2009;4(4):205-214. [42] FARPOUR-LAMBERT NJ, AGGOUN Y, MARCHAND LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;4(25):2396-2406. [43] MEYER AA, KUNDT G, LENSCHOW U, et al. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol. 2006;48(9):1865-1870. [44] KELLY AS, WETZSTEON RJ, KAISER DR, et al.Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. J Pediatr. 2004;145(6):731-736. [45] WOO KS, CHOOK P, YU CW, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109(16):1981-1986. [46] COLLINS AR, LYON CJ, XIA X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104(6):e42-e54. [47] CERCATO C, FONSECA FA. Cardiovascular risk and obesity. Diabetol Metab Syndr. 2019;11(1):74. [48] GU C, YAN J, ZHAO L, et al. Regulation of mitochondrial dynamics by aerobic exercise in cardiovascular diseases. Front Cardiovasc Med. 2022;8:788505. [49] PINCKARD K, BASKIN KK, STANFORD KI. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med. 2019;6:69. [50] XIAO H, PENG K, SUN L, et al. Effects of anaerobic exercise training on human function based on multiple linear regression. Front Phys. 2023;11:1168765. [51] SCHJERVE IE, TYLDUM GA, TJØNNA AE, et al. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci. 2008;115(9):283-293. [52] BRAGA VA, COUTO GK, LAZZARIN MC, et al. Aerobic exercise training prevents the onset of endothelial dysfunction via increased nitric oxide bioavailability and reduced reactive oxygen species in an experimental model of menopause. PLoS One. 2015;10(4):e0125388. [53] KOZAKOVA M, PALOMBO C. Vascular Ageing and Aerobic Exercise. Int J Environ Res Public Health. 2021;18(20):10666. [54] SHIMIZU R, HOTTA K, YAMAMOTO S, et al.Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol. 2016;116(4):749-757. [55] DELGADO-FLOODY P, IZQUIERDO M, RAMÍREZ-VÉLEZ R, et al. Effect of high-intensity interval training on body composition, cardiorespiratory fitness, blood pressure, and substrate utilization during exercise among prehypertensive and hypertensive patients with excessive adiposity. Front Physiol. 2020;11:558910. [56] DA SILVA MR, WACLAWOVSKY G, PERIN L,et al. Effects of high-intensity interval training on endothelial function, lipid profile, body composition and physical fitness in normal-weight and overweight-obese adolescents: a clinical trial. Physiol Behav. 2020;213:112728. [57] PEDRALLI ML, MARSCHNER RA, KOLLET DP, et al. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: a randomized clinical trial. Sci Rep. 2020; 10(1):7628. [58] OTSUKI T, NAMATAME H, YOSHIKAWA T, et al. Combined aerobic and low-intensity resistance exercise training increases basal nitric oxide production and decreases arterial stiffness in healthy older adults. J Clin Biochem Nutr. 2020;66(1):62-66. [59] ASHOR AW, LARA J, SIERVO M, et al. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(10):e110034. [60] LI Y, HANSSEN H, CORDES M, et al. Aerobic, resistance and combined exercise training on arterial stiffness in normotensive and hypertensive adults: a review. Eur J Sport Sci. 2015;15(5):443-457. [61] SARDELI AV, GÁSPARI AF, CHACON-MIKAHIL MP. Acute, short-, and long-term effects of different types of exercise in central arterial stiffness: a systematic review and meta-analysis . J Sports Med Phys Fitness. 2018;58(6):923-932. [62] BATRAKOULIS A, JAMURTAS AZ, DRAGANIDIS D, et al. Hybrid neuromuscular training improves cardiometabolic health and alters redox status in inactive overweight and obese women: a randomized controlled trial. Antioxidants. 2021;10(10):1601. |
| [1] | Wang Shijie, Hu Xiaoyu, Duan Zhuoran, Tang Yingfeng, Wang Wei . Association between grip strength to weight ratio and new-onset cardiovascular and cerebrovascular diseases: a big data analysis of the China Health and Retirement Longitudinal Study [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3190-3197. |
| [2] | Wang Zhenze, Liu Fende, Zhang Rui, Li Wujun. Mesenchymal stem cells in treatment of arteriosclerosis obliterans of lower extremities: systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1869-1876. |
| [3] | Li Guan, Wang Haopeng, Wang Jinbao, Song Xinhao, Qin Guochu, Shao Yang. Feasibility of optimizing radiation dose for three-dimensional printing of the maxillofacial bone based on low-dose CT technology [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1384-1389. |
| [4] | Wang Kairu, Fu Shizhe, Li Jiahui, Yan Ru, Ma Yuru, Shi Bo, Ye Congyan , Yan Rui, Cong Guangzhi, Jia Shaobin. Spermidine/spermine N1-acetyltransferase 1 participates in vascular smooth muscle cell calcification [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6836-6842. |
| [5] | Zhang Shaoqun, Zheng Chuanjiang, Liu Jiafu, Jiang Shunwan. Effect of positioning and non-positioning cervical rotatory manipulation on tensile mechanical properties of internal carotid artery with different degrees of atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(30): 4788-4794. |
| [6] | Zhao Guangjian, Liu Danan, Zhou Bo, Wang Yao. Action mechanism by which fibronectin type III domain-containing protein 5 inhibits macrophage pyroptosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4005-4012. |
| [7] | Pei Jiansheng, Yang Wenjuan, He Jing, Yan Ru, Huang Hui, Jia Shaobin. Bone morphogenetic protein-2 mediated homocysteine promotes vascular calcification [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4027-4033. |
| [8] | Wang Yao, Liu Danan, Zhao Guangjian, Zhou Bo, Wang Xiang. Irisin/peroxisome proliferator-activated receptor alpha signaling pathway mediates vascular smooth muscle cell proliferation in mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5320-5326. |
| [9] | Wang Shurui, Zhang Yilei, Jiao Weijie, Liu Zhihua, Zhang Kuiming, Cui Yinglin. Effects of Yiqi Tongmai Fang on serum amino acid metabolism of atherosclerotic mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(28): 4441-4447. |
| [10] | Zhang Yuwei, Liu Chuanchuan, Mao Jiaqi, Zhang Qingqing, Liu Hong, Chen Ying, Ma Lan. Umbilical cord mesenchymal stem cell-derived exosomes treated with hypoxic preconditioning inhibits proliferation of pulmonary artery smooth muscle cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(19): 2986-2992. |
| [11] | Ou Hangjun, Zhao Guangjian, Pan Yujia, Gong Caiwei, Zhao Quanwei, Liu Danan. Construction of a lentiviral vector overexpressing fibronectin type III domain containing 5 to inhibit apoptosis of endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 216-222. |
| [12] | Wang Xiang, Wei Chaojun, Wang Yao, Pan Yujia, Liu Danan. Lentiviral vector overexpressing FNDC5 inhibits proliferation and migration of smooth muscle cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(35): 5670-5675. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||