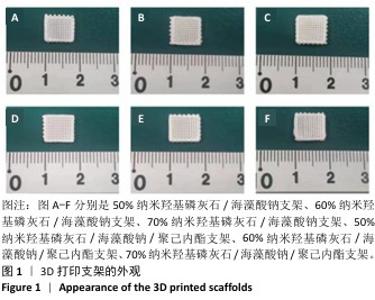
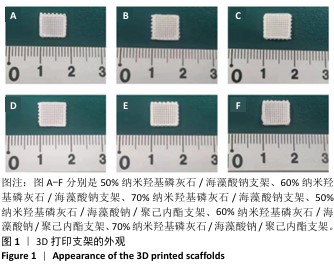
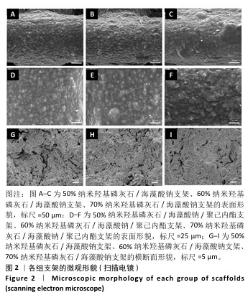
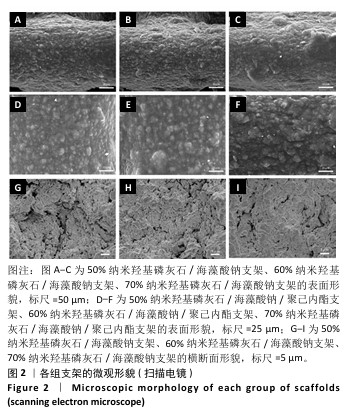
Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (8): 1962-1970.doi: 10.12307/2026.029
Previous Articles Next Articles
Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold
Zhou Hongli1, 2, Wang Xiaolong3, Guo Rui3, Yao Xuanxuan1, Guo Ru1, Zhou Xiongtao1, He Xiangyi1
- 1Institute of Stomatology, Lanzhou University, Lanzhou 730000, Gansu Province, China; 2Huizhou Health Sciences Polytechnic, Huizhou 516000, Guangdong Province, China; 3Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, Gansu Province, China
-
Received:2024-08-09Accepted:2025-01-24Online:2026-03-18Published:2025-07-16 -
Contact:He Xiangyi, Professor, Institute of Stomatology, Lanzhou University, Lanzhou 730000, Gansu Province, China -
About author:Zhou Hongli, MS, Institute of Stomatology, Lanzhou University, Lanzhou 730000, Gansu Province, China; Huizhou Health Sciences Polytechnic, Huizhou 516000, Guangdong Province, China -
Supported by:Key Research & Development Plan of Gansu Provincial Science and Technology Project, No. 21YF5GA100 (to HXY); Gansu Provincial Key Laboratory of Dentomaxillofacial Reconstruction and Bio-Intelligent Manufacturing Open Fund Project, No. 20JR10RA653-ZDKF20210103 (to WXL); Lanzhou University Research Project, No. 533000-071100191 (to HXY)
CLC Number:
Cite this article
Zhou Hongli, Wang Xiaolong, Guo Rui, Yao Xuanxuan, Guo Ru, Zhou Xiongtao, He Xiangyi. Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1962-1970.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
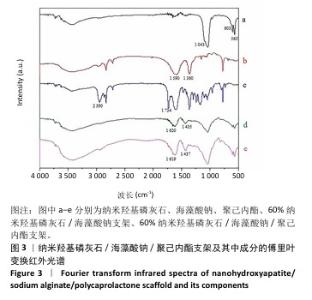
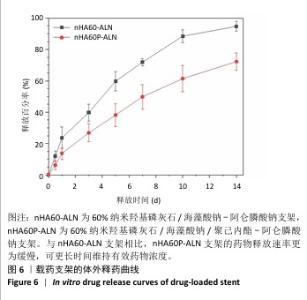
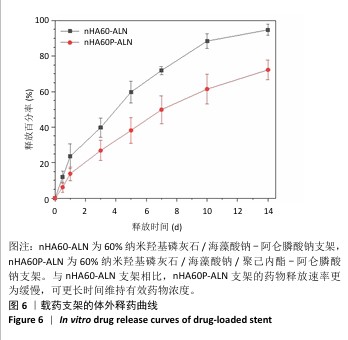
2.3 nHA60P支架傅里叶变换红外光谱检测结果 对纳米羟基磷灰石、海藻酸钠、聚己内酯及nHA60、nHA60P支架进行红外特征吸收峰的表征,见图3。纳米羟基磷灰石在565 cm-1和603 cm-1处出现对应于PO43-的变形振动峰,在1 043 cm-1附近的吸收峰对应于PO43-的伸缩振动峰;海藻酸钠在1 590 cm-1和1 360 cm-1附近出现较强的吸收峰,分别对应为-COO-的不对称伸缩振动和对称伸缩振动峰;聚己内酯线谱在2 950 cm-1处存在较强的特征吸收峰,代表的是聚己内酯的-CH2,在1 734 cm-1处的特征峰则代表C=C的特征伸缩振动峰。 在nHA60、nHA60P支架所对应的线谱中,均存在代表纳米羟基磷灰石的PO43-变形振动和伸缩振动特征峰,以及代表海藻酸钠的-COO-不对称伸缩振动和对称伸缩振动特征峰。与海藻酸钠原材料相比,在两组支架中代表-COO-的特征峰向高频方向移动到1 620 cm-1和1 435 cm-1附近,这主要是由于海藻酸钠中的-COO-和Ca2+发生反应形成蛋壳结构。与nHA60支架相比,nHA60P支架内出现代表聚己内酯的饱和-CH2和C=C的特征峰,说明聚己内酯成功负载于支架上。 "
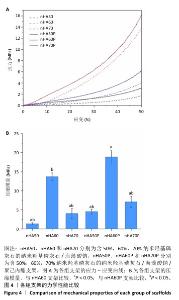
2.4 各组支架的力学性能检测结果 6组支架的应力-应变曲线与压缩模量见图4A。nHA50、nHA60、nHA70支架的压缩模量分别为(1.33±0.27),(13.70±0.99),(4.00±1.29) MPa,nHA60支架的压缩模量高于nHA50、nHA70支架(P < 0.05);nHA50P、nHA60P、nHA70P支架的压缩模量分别为(4.50±0.61),(18.89±1.65),(7.12±1.44) MPa,nHA60P支架的压缩模量高于nHA50P、nHA70P(P < 0.05);nHA60支架的压缩模量高于nHA50P、nHA70P(P < 0.05),低于nHA60P支架(P < 0.05),见图4B。"

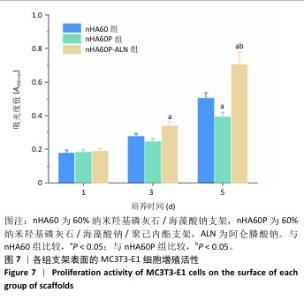
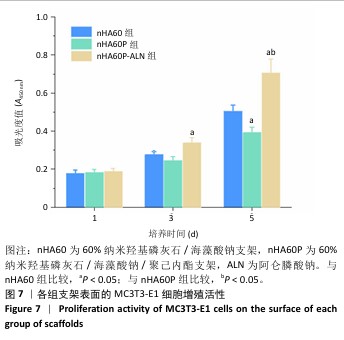
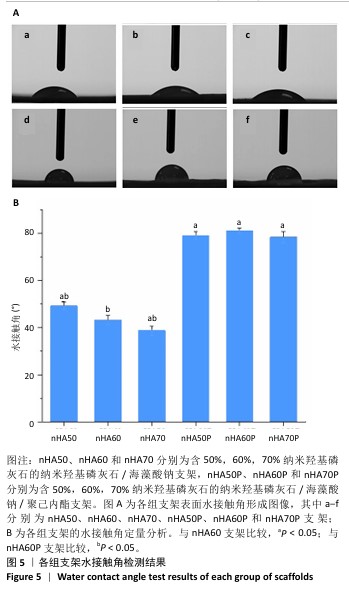
2.5 各组支架的亲水性能检测结果 6组支架的水接触角检测结果见图5。nHA50、nHA60和nHA70支架的水接触角分别为(49.20±1.52)°,(43.27±2.01)°,(38.77±1.89)°,3组间两两比较差异均有显著性意义(P < 0.05)。nHA50P、nHA60P和nHA70P支架的水接触角分别为(79.09±1.80)°,(81.12± 1.26)°,(78.36±2.44)°,3组间比较差异无显著性意义(P > 0.05)。nHA50P、nHA60P和nHA70P支架的水接触角均大于nHA50、nHA60和nHA70支架(P < 0.05)。 综合微观形貌、力学性能与亲水性检测结果,选择nHA60、nHA60P支架负载阿仑膦酸钠。 "
| [1] ZHANG L, YANG G, JOHNSON BN, et al. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16-33. [2] KOUSHIK TM, MILLER CM, ANTUNES E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv Healthc Mater. 2023;12(9):e2202766. [3] YANG Y, ZHOU B, LI M, et al. GO/Cu Nanosheet-Integrated Hydrogel Platform as a Bioactive and Biocompatible Scaffold for Enhanced Calvarial Bone Regeneration. Int J Nanomedicine. 2024;19:8309-8336. [4] O’BRIEN FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88-95. [5] QU M, WANG C, ZHOU X, et al. Multi-Dimensional Printing for Bone Tissue Engineering. Adv Healthc Mater. 2021;10(11):e2001986. [6] LEE J, BYUN H, MADHURAKKAT PERIKAMANA SK, et al. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv Healthc Mater. 2019;8(4):e1801106. [7] BEDAIR TM, HEO Y, RYU J, et al. Biocompatible and functional inorganic magnesium ceramic particles for biomedical applications. Biomater Sci. 2021;9(6):1903-1923. [8] SHIRZAEI SANI I, REZAEI M, BARADAR KHOSHFETRAT A, et al. Preparation and characterization of polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds for bone tissue engineering. Int J Biol Macromol. 2021;182:1638-1649. [9] WUBNEH A, TSEKOURA EK, AYRANCI C, et al. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018;80:1-30. [10] ALONZO M, PRIMO FA, KUMAR SA, et al. Bone tissue engineering techniques, advances and scaffolds for treatment of bone defects. Curr Opin Biomed Eng. 2021;17:100248. [11] STAFIN K, ŚLIWA P, PIĄTKOWSKI M. Towards Polycaprolactone-Based Scaffolds for Alveolar Bone Tissue Engineering: A Biomimetic Approach in a 3D Printing Technique. Int J Mol Sci. 2023;24(22):16180. [12] SUN T, FENG Z, HE W, et al. Novel 3D-printing bilayer GelMA-based hydrogel containing BP,β-TCP and exosomes for cartilage-bone integrated repair. Biofabrication. 2023;16(1). doi: 10.1088/1758-5090/ad04fe. [13] MURPHY SV, ATALA A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773-785. [14] YANG X, WANG Y, ZHOU Y, et al. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers (Basel). 2021;13(16):2754 [15] PARK JW, KANG HG. Application of 3-dimensional printing implants for bone tumors. Clin Exp Pediatr. 2022;65(10):476-482. [16] XUE N, DING X, HUANG R, et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals (Basel). 2022;15(7):879. [17] DEUERLING S, KUGLER S, KLOTZ M, et al. A Perspective on Bio-Mediated Material Structuring. Adv Mater. 2018;30(19):e1703656. [18] ELKHOULY H, MAMDOUH W, EL-KORASHY DI. Electrospun nano-fibrous bilayer scaffold prepared from polycaprolactone/gelatin and bioactive glass for bone tissue engineering. J Mater Sci Mater Med. 2021;32(9):111. [19] HUANG B, CHEN M, TIAN J, et al. Oxygen-Carrying and Antibacterial Fluorinated Nano-Hydroxyapatite Incorporated Hydrogels for Enhanced Bone Regeneration. Adv Healthc Mater. 2022;11(12):e2102540. [20] LIN H, LI Z, XIE Z, et al. An anti-infection and biodegradable TFRD-loaded porous scaffold promotes bone regeneration in segmental bone defects: experimental studies. Int J Surg. 2024;110(6):3269-3284. [21] WU X, NI S, DAI T, et al. Biomineralized tetramethylpyrazine-loaded PCL/gelatin nanofibrous membrane promotes vascularization and bone regeneration of rat cranium defects. J Nanobiotechnology. 2023;21(1):423. [22] ORYAN A, HASSANAJILI S, SAHVIEH S. Effectiveness of a biodegradable 3D polylactic acid/poly(ɛ-caprolactone)/hydroxyapatite scaffold loaded by differentiated osteogenic cells in a critical-sized radius bone defect in rat. J Tissue Eng Regen Med. 2021;15(2):150-162. [23] LONG J, YAO Z, ZHANG W, et al. Regulation of Osteoimmune Microenvironment and Osteogenesis by 3D-Printed PLAG/black Phosphorus Scaffolds for Bone Regeneration. Adv Sci (Weinh). 2023; 10(28):e2302539. [24] WANG Z, WANG Y, YAN J, et al. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev. 2021;174:504-534. [25] SALTZ A, KANDALAM U. Mesenchymal stem cells and alginate microcarriers for craniofacial bone tissue engineering: A review. J Biomed Mater Res A. 2016;104(5):1276-1284. [26] SILVA-BARROSO AS, CABRAL CSD, FERREIRA P, et al. Lignin-enriched tricalcium phosphate/sodium alginate 3D scaffolds for application in bone tissue regeneration. Int J Biol Macromol. 2023;239:124258. [27] NOROOZI R, SHAMEKHI MA, MAHMOUDI R, et al. In vitrostatic and dynamic cell culture study of novel bone scaffolds based on 3D-printed PLA and cell-laden alginate hydrogel. Biomed Mater. 2022;17(4). doi: 10.1088/1748-605X/ac7308. [28] SIVAKUMAR M, RAO KP. Preparation, characterization, and in vitro release of gentamicin from coralline hydroxyapatite-alginate composite microspheres. J Biomed Mater Res A. 2003;65(2):222-228. [29] ZHANG J, WANG Q, WANG A. In situ generation of sodium alginate/hydroxyapatite nanocomposite beads as drug-controlled release matrices. Acta Biomater. 2010;6(2):445-454. [30] SAMADIAN H, SALEHI M, FARZAMFAR S, et al. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif Cells Nanomed Biotechnol. 2018;46(sup1):964-974. [31] ZHANG L, YANG G, JOHNSON BN, et al. Three-dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater. 2018;84:16-33. [32] NALLA RK, KINNEY JH, RITCHIE RO. Effect of orientation on the in vitro fracture toughness of dentin: the role of toughening mechanism. Biomaterials. 2003;24(22):3955-3968. [33] WIRIA FE, LEONG KF, CHUA CK, et al. Poly-epsilon-caprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta Biomater. 2007;3(1):1-12. [34] NALLA RK, KINNEY JH, RITCHIE RO. Mechanistic fracture criteria for the failure of human cortical bone. Nat Mater. 2003;2(3):164-168. [35] MARTÍNEZ-VÁZQUEZ FJ, MIRANDA P, GUIBERTEAU F, et al. Reinforcing bioceramic scaffolds with in situ synthesized ε-polycaprolactone coatings. J Biomed Mater Res A. 2013;101(12):3551-3559. [36] WANG L, PANG Y, TANG Y, et al. A biomimetic piezoelectric scaffold with sustained Mg(2+) release promotes neurogenic and angiogenic differentiation for enhanced bone regeneration. Bioact Mater. 2023; 25:399-414. [37] MCCLUNG M, HARRIS ST, MILLER PD, et al. Bisphosphonate Therapy for Osteoporosis: Benefits, Risks, and Drug Holiday. Am J Med. 2013; 126(1):13-20. [38] RODAN GA, RESZKA AA. Bisphosphonate mechanism of action. Curr Mol Med. 2002;2(6):571-577. [39] RUSSELL RG, MÜHLBAUER RC, BISAZ S, et al. The influence of pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatite in vitro and on bone resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomised rats. Calcif Tissue Res. 1970;6(3):183-196. [40] TARAFDER S, BOSE S. Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesi. ACS Appl Mater Interfaces. 2014;6(13):9955-9965. [41] PARK KW, YUN YP, KIM SE, et al. The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model. Int J Mol Sci. 2015;16(11):26738-26753. [42] FRANCESCHETTI P, BONDANELLI M, CARUSO G, et al. Risk factors for development of atypical femoral fractures in patients on long-term oral bisphosphonate therapy. Bone. 2013;56(2):426-431. [43] LIN CH, SU JJ, LEE SY, et al. Stiffness modification of photopolymerizable gelatin-methacrylate hydrogels influences endothelial differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med. 2018; 12(10):2099-2111. [44] CHIMENE D, KAUNAS R, GAHARWAR AK. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv Mater. 2020;32(1):e1902026. [45] ROOHANI-ESFAHANI SI, NOURI-KHORASANI S, LU ZF, et al. Effects of bioactive glass nanoparticles on the mechanical and biological behavior of composite coated scaffolds. Acta Biomater. 2011;7(3):1307-1318. [46] KUMAR G, WATERS MS, FAROOQUE TM, et al. Freeform fabricated scaffolds with roughened struts that enhance both stem cell proliferation and differentiation by controlling cell shape. Biomaterials. 2012;33(16):4022-4030. [47] KUMAR G, TISON CK, CHATTERJEE K, et al. The determination of stem cell fate by 3D scaffold structures through the control of cell shape. Biomaterials. 2011;32(35):9188-9196. [48] Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474-5491. [49] AMINI AR, LAURENCIN CT, NUKAVARAPU SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5): 363-408. [50] ASGHARI F, RABIEI FARADONBEH D, MALEKSHAHI ZV, et al. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr Polym. 2022;278:118926. [51] VYAS C, ZHANG J, ØVREBØ Ø, et al. 3D printing of silk microparticle reinforced polycaprolactone scaffolds for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2021;118:111433. [52] MA J, XIA M, ZHU S, et al. A new alendronate doped HAP nanomaterial for Pb(2+), Cu(2+) and Cd(2+) effect absorption. J Hazard Mater. 2020; 400:123143. [53] WILLIAMS DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941-2953. |
| [1] | Sun Lei, Zhang Qi, Zhang Yu. Pro-osteoblastic effect of chlorogenic acid protein microsphere/polycaprolactone electrospinning membrane [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1877-1884. |
| [2] | Li Qingbin, Lin Jianhui, Huang Wenjie, Wang Mingshuang, Du Jiankai, Lao Yongqiang. Bone cement filling after enlarged curettage of giant cell tumor around the knee joint: a comparison of subchondral bone grafting and non-grafting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1896-1902. |
| [3] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [4] | Min Changqin, Huang Ying. Construction of pH/near-infrared laser stimuli-responsive drug delivery system and its application in treatment of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1940-1951. |
| [5] | . Effect of mussel-derived antimicrobial peptide-coated modified prosthesis on prevention of early periprosthetic joint infection and regulation of bone transfer [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 278-287. |
| [6] | Guo Jingwen, Wang Qingwei, He Zijun, Hu Zihang, Chen Zhi, Zhu Rong, Wang Yuming, Liu Wenfei, Luo Qinglu. Intra-articular injection of different concentrations of silicon-based bioceramics in treatment of knee osteoarthritis in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 288-295. |
| [7] | Wang Ying, Wang Yawen, Xu Yingjie, Wang Yuanfei, Wu Tong. Preparation of polycaprolactone/low molecular weight fucoidan nanofibers by emulsion electrospinning and assessment of their biocompatibility [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 433-442. |
| [8] | Wang Yu, Fan Minjie, Zheng Pengfei. Application of multistimuli-responsive hydrogels in bone damage repair: special responsiveness and diverse functions [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 469-479. |
| [9] | Bai Xiangyu, Huo Feng, Hao Yan, Wang Zecheng, Guo Xiaoyu. Platelet-derived growth factor BB-loaded chitosan/reduced graphene oxide scaffold for repairing alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 329-337. |
| [10] | Zhou Shibo, Yu Xing, Chen Hailong, Xiong Yang. Nanocrystalline collagen-based bone combined with Bushen Zhuangjin Decoction repairs bone defects in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 354-361. |
| [11] | Yan Qiquan, Yang Libin, Li Mengjun, Ni Yazhuo, Chen Keying, Xu Bo, Li Yaoyang, Ma Shiqing, Li Rui, Li Jianwen. Preparation and antibacterial properties of porcine small intestinal submucosal composite nanohydroxyapatite bioscaffold loaded with antimicrobial peptide KR-12-a5 [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 384-394. |
| [12] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||