Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (6): 1348-1358.doi: 10.12307/2026.552
Previous Articles Next Articles
Effects and mechanisms of isoginkgetin on osteoclastogenesis
Wen Guangwei1, 2, 3, Zhen Yinghao2, 4, Zheng Taikeng2, 4, Zhou Shuyi2, Mo Guoye2, Zhou Tengpeng2, 3, Li Haishan2, 4, Lai Yiyi2, 3
- 1Guangzhou Panyu District Hualong Hospital, Guangzhou 510405, Guangdong Province, China; 2Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 3Guangzhou Liwan District Orthopedic and Traumatology Hospital, Guangzhou 510405, Guangdong Province, China; 4Lingnan Medical Research Center, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China
-
Received:2024-11-14Accepted:2025-01-25Online:2026-02-28Published:2025-07-12 -
Contact:Lai Yiyi, MS, Associate chief physician, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; Guangzhou Liwan District Orthopedic and Traumatology Hospital, Guangzhou 510405, Guangdong Province, China Co-corresponding author: Li Haishan, PhD, Physician, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; Lingnan Medical Research Center, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
About author:Wen Guangwei, Attending physician, Guangzhou Panyu District Hualong Hospital, Guangzhou 510405, Guangdong Province, China; Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; Guangzhou Liwan District Orthopedic and Traumatology Hospital, Guangzhou 510405, Guangdong Province, China -
Supported by:Guangzhou Municipal Health and Wellness Science and Technology Project, No. 20241A010093 (to WGW); Liwan District Livelihood Science and Technology Project, No. 20230713 (to WGW); Guangdong Provincial Bureau of Traditional Chinese Medicine Scientific Research Project, No. 20241091 (to MGY); Guangzhou Municipal Health and Wellness Science and Technology Project, No. 20251A011106 (to ZTP)
CLC Number:
Cite this article
Wen Guangwei, Zhen Yinghao, Zheng Taikeng, Zhou Shuyi, Mo Guoye, Zhou Tengpeng, Li Haishan, Lai Yiyi. Effects and mechanisms of isoginkgetin on osteoclastogenesis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1348-1358.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
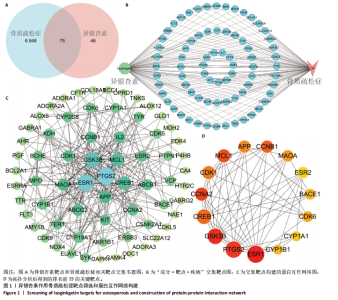
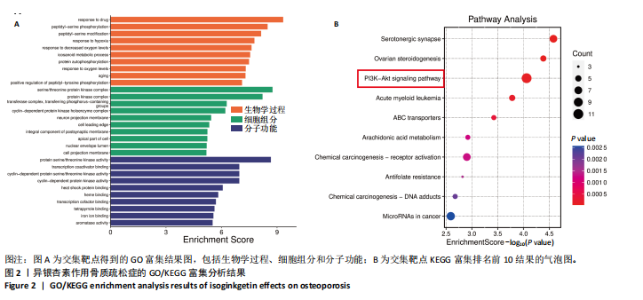
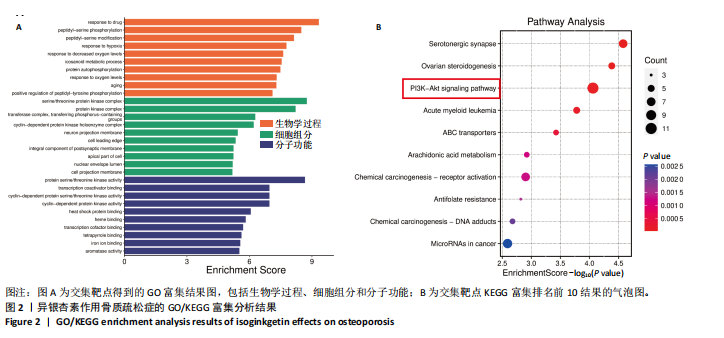
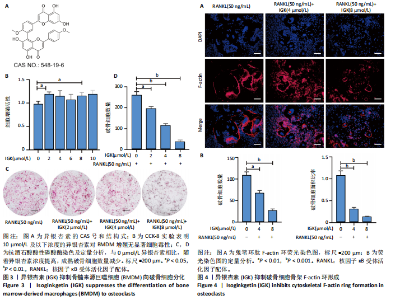
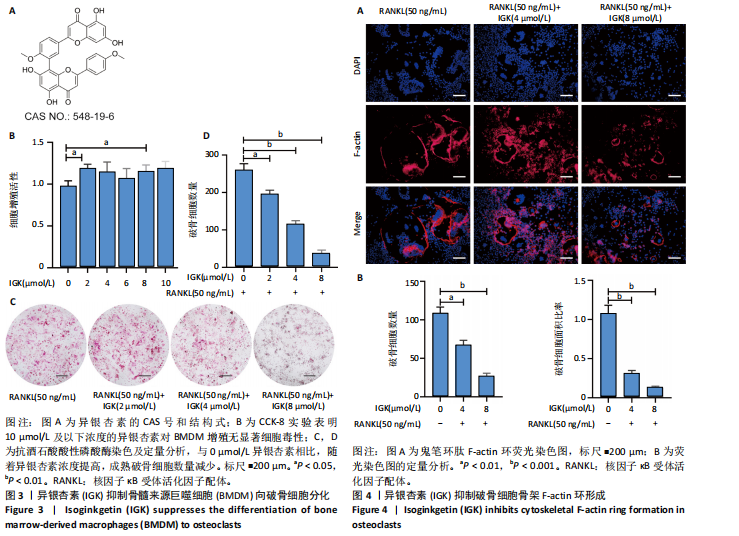
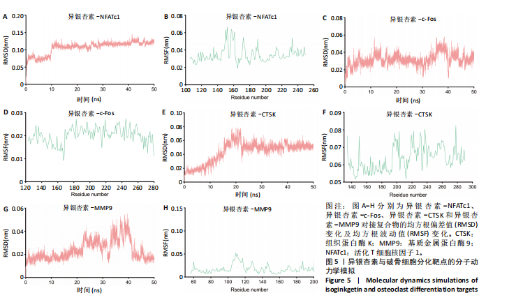
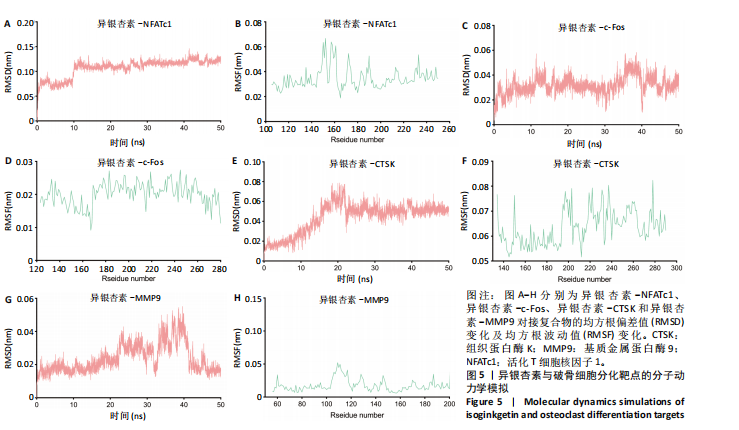
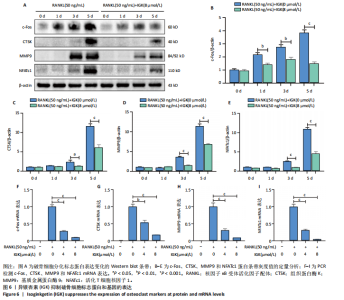
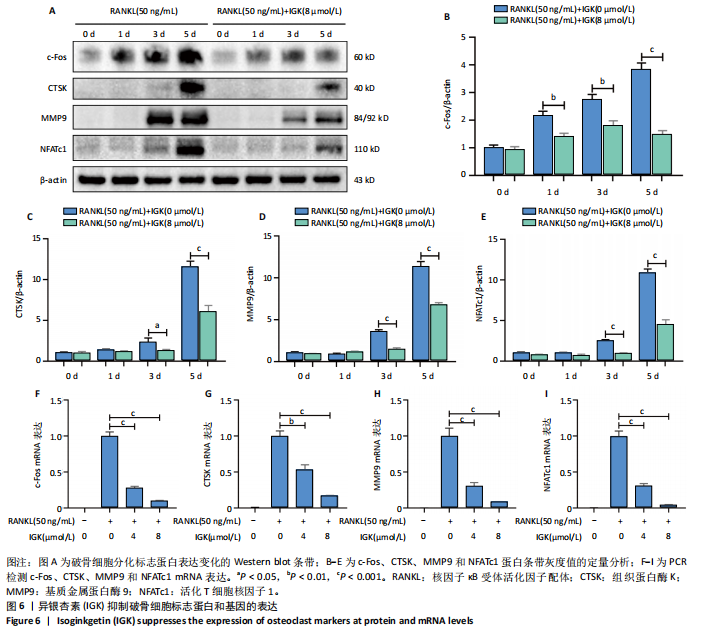
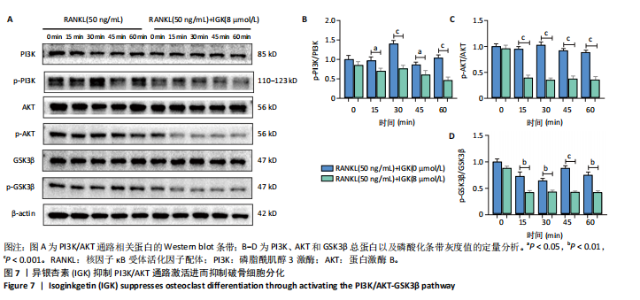
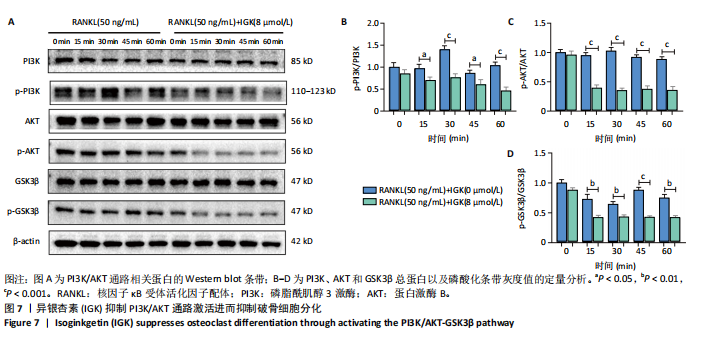
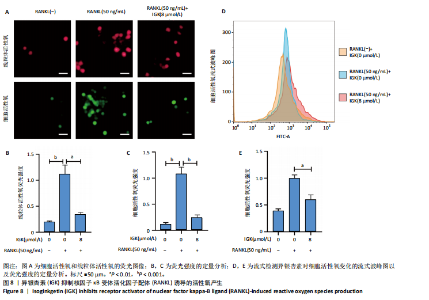
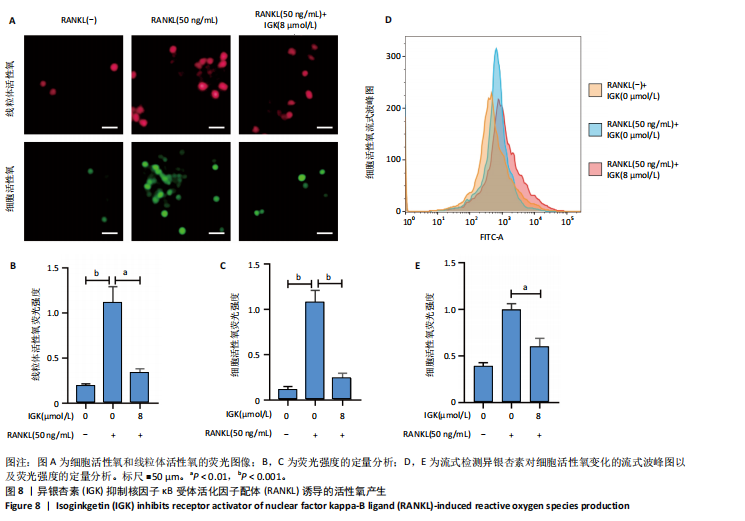
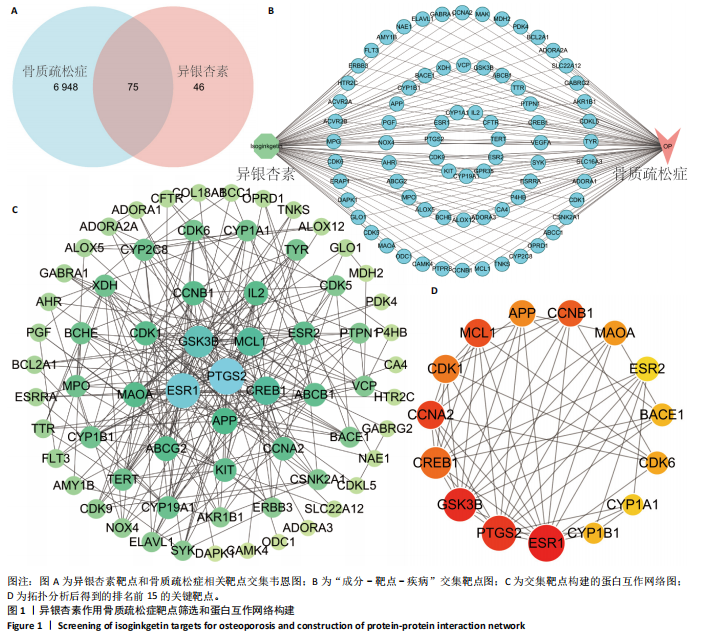
2.1 异银杏素作用骨质疏松症靶点筛选和蛋白互作网络构建 通过检索Swiss Target Prediction数据库共获得骨质疏松症潜在靶点7 023个;通过Genecards、OMIM、TTD和DisGeNET数据库检索去重后得到121个破骨细胞分化相关靶点,韦恩图取交集后得到75个异银杏素干预骨质疏松症的潜在靶点(图1A)。将75个潜在的交叉靶点导入STRING数据库,获得蛋白互作网络图(图1B,C);使用Cytoscape软件进行可视化,获得GSK3β、ESR1、MCL1及CCNA2等关键靶点(图1D)。 2.2 GO/KEGG富集分析 功能富集分析结果发现异银杏素作用骨质疏松症在生物学过程主要涉及药物反应、肽基丝氨酸磷酸化和对缺氧的反应等,细胞组分主要涉及丝氨酸/苏氨酸蛋白激酶复合物和蛋白激酶复合物等,分子功能主要涉及蛋白丝氨酸/苏氨酸激酶活性和转录激活子结合等(图2A)。异银杏素作用骨质疏松症关联的通路主要是PI3K/AKT信号通路等(图2B),这些结果表明异银杏素可能通过激活PI3K/AKT通路和细胞对药物、缺氧的反应影响骨质疏松症。 2.3 异银杏素抑制BMDM向破骨细胞分化 图3A展示了异银杏素的CAS号和结构式。CCK-8结果显示异银杏素在10 μmol/L以下对BMDM增殖无明显细胞毒性(图3B)。用不同浓度的异银杏素干预BMDM并诱导其向破骨细胞分化,破骨细胞特异性抗酒石酸酸性磷酸酶染色结果显示在没有异银杏素影响时,BMDM向破骨细胞分化显著,随着异银杏素浓度增加,BMDM向破骨细胞分化受到明显抑制,当异银杏素浓度为8 μmol/L时抑制效果最显著(图3C,D)。综上可见,异银杏素对BMDM增殖无明显细胞毒性影响,并且呈浓度依赖性抑制BMDM向破骨细胞分化。 2.4 异银杏素抑制细胞骨架F-actin环形成 破骨细胞分化过程中伴随着细胞核的聚集和细胞形态的巨大化,F-actin环帮助重组细胞骨架,为破骨细胞的融合分化提供结构支持[5,11]。使用罗丹明鬼笔环肽染液探讨异银杏素对F-actin环的影响,荧光染色结果显示与4 μmol/L异银杏素相比,8 μmol/L异银杏素更加明显抑制F-actin环面积的扩大和环内细胞核的聚集(图4A,B),从而抑制BMDM向破骨细胞分化。 2.5 异银杏素与破骨细胞分化靶点的分子动力学模拟 众所周知,NFATc1是破骨细胞分化过程中的关键转录因子,NFATc1通过结合自身启动子来自我扩增,引起下游基因(如c-fos、CTSK和MMP9)表达上升[9]。通过分子动力学模拟探讨异银杏素对破骨细胞分化标志蛋白的直接结合情况,结果显示异银杏素-NFATc1和异银杏素-c-Fos复合物在整个分子动力学模拟过程中处于较稳定状态,而异银杏素-CTSK和异银杏素-MMP9复合物的结合稳定性较差(图5A-H)。分子动力学模拟结果表明异银杏素与NFATc1、c-fos、CTSK和MMP9均能直接结合,可能通过调控破骨细胞分化相关蛋白和基因来抑制破骨细胞分化。 2.6 异银杏素抑制破骨细胞标志蛋白和基因的表达 NFATc1的表达是破骨细胞发生的关键,而c-Fos能与NFATc1相互作用,引起破骨细胞基因的转录与表达,促进破骨细胞分化。CTSK和MMP9参与骨基质的降解,其表达在破骨细胞分化中明显升高[20]。通过Western blot检测异银杏素对破骨细胞分化相关蛋白(NFATc1、c-Fos、CTSK和MMP9)表达的影响,结果显示异银杏素在破骨细胞分化全程(第1-5天)抑制c-fos的表达(图6A,B),在破骨细胞分化的中后期(第3-5天)抑制NFATc1、CTSK和MMP9的表达(图6C-E)。PCR检测结果以仅RANKL诱导为阳性对照,异银杏素呈浓度依赖性抑制c-fos、CTSK、MMP9和NFATc1的mRNA表达(图6F-I)。以上结果表明异银杏素通过降低破骨细胞分化蛋白和基因的表达抑制破骨细胞分化成熟。 2.7 异银杏素抑制PI3K/AKT通路激活进而抑制破骨细胞分化 当RANKL与受体RANK结合时,会激活下游的PI3K/AKT信号通路,促进破骨细胞前体细胞的分化和成熟,而下游GSK3β的活性变化与破骨细胞分化相关蛋白MMP9、CTSK和NFATc1的表达水平有关[12]。结合2.1和2.2的网络药理学结果,采用Western blot检测异银杏素对PI3K/AKT通路及下游GSK3β的激活情况,结果显示异银杏素在RANKL激活破骨细胞早期分化的15-60 min内显著抑制了PI3K、AKT和GSK3β的磷酸化水平(图7A-D)。综上可见,PI3K/AKT通路确实是影响异银杏素抑制破骨细胞分化的主要信号通路。 2.8 异银杏素抑制RANKL诱导的活性氧产生 在RANKL诱导破骨细胞分化中,细胞利用活性氧作为第二信使传递信号促进破骨细胞分化[21],而线粒体是细胞产生活性氧的主要场所。通过使用细胞活性氧H2DCFDA探针和线粒体MitoSOX Red探针检测细胞活性氧和线粒体活性氧的产生情况[4]。荧光结果"
| [1] CLYNES MA, HARVEY NC, CURTIS EM, et al. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105-117. [2] YOSHIDA S, IKEDO A, YANAGIHARA Y, et al. Bub1 suppresses inflammatory arthritis-associated bone loss in mice through inhibition of TNFα-mediated osteoclastogenesis. J Bone Miner Res. 2024;39(3):341-356. [3] CLÉZARDIN P, COLEMAN R, PUPPO M, et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol Rev. 2021;101(3):797-855. [4] LI H, DENG W, YANG J, et al. Corylifol A suppresses osteoclastogenesis and alleviates ovariectomy-induced bone loss via attenuating ROS production and impairing mitochondrial function. Biomed Pharmacother. 2024;171:116166. [5] HE J, ZHENG L, LI X, et al. Obacunone targets macrophage migration inhibitory factor (MIF) to impede osteoclastogenesis and alleviate ovariectomy-induced bone loss. J Adv Res. 2023;53:235-248. [6] HÄNDEL MN, CARDOSO I, VON BÜLOW C, et al. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ. 2023;381:e068033. [7] GU Q, YANG H, SHI Q. Macrophages and bone inflammation. J Orthop Translat. 2017;10:86-93. [8] LI H, DENG W, QIN Q, et al. Isoimperatorin attenuates bone loss by inhibiting the binding of RANKL to RANK. Biochem Pharmacol. 2023; 211:115502. [9] FANG C, GUO JW, WANG YJ, et al. Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion. Acta Pharmacol Sin. 2022;43(5):1299-1310. [10] CHEN X, ZHI X, WANG J, et al. RANKL signaling in bone marrow mesenchymal stem cells negatively regulates osteoblastic bone formation. Bone Res. 2022;10(1):54. [11] LI Y, ZHUANG Q, TAO L, et al. Urolithin B suppressed osteoclast activation and reduced bone loss of osteoporosis via inhibiting ERK/NF-κB pathway. Cell Prolif. 2022;55(10):e13291. [12] XU H, JIA Y, LI J, et al. Niloticin inhibits osteoclastogenesis by blocking RANKL-RANK interaction and suppressing the AKT, MAPK, and NF-κB signaling pathways. Biomed Pharmacother. 2022;149:112902. [13] YUE H, TIAN Y, LI Y, et al. Comparative study of holothurin A and echinoside A on inhibiting the high bone turnover via downregulating PI3K/AKT/β-catenin and OPG/RANKL/NF-κB signaling in ovariectomized mice. Food Funct. 2022;13(8):4748-4756. [14] MOON JB, KIM JH, KIM K, et al. Akt induces osteoclast differentiation through regulating the GSK3β/NFATc1 signaling cascade. J Immunol. 2012;188(1):163-169. [15] WU X, HUANG J, TANG J, et al. Isoginkgetin, a bioactive constituent from Ginkgo Biloba, protects against obesity-induced cardiomyopathy via enhancing Nrf2/ARE signaling. Redox Biol. 2022;57:102485. [16] KESTI S, MACAR O, KALEFETOĞLU MACAR T, et al. Investigation of the protective role of Ginkgo biloba L. against phytotoxicity, genotoxicity and oxidative damage induced by Trifloxystrobin. Sci Rep. 2024;14(1):19937. [17] WANG R, SHAO X, YANG J, et al. Ginkgo biloba Extract Mechanism Inhibits Hepatocellular Carcinoma through the Nuclear Factor-κB/p53 Signaling Pathway. J Environ Pathol Toxicol Oncol. 2020;39(2):179-189. [18] CAO F, QIN KR, KANG K, et al. Ginkgo biloba L. extract prevents steroid-induced necrosis of the femoral head by rescuing apoptosis and dysfunction in vascular endothelial cells via the PI3K/AKT/eNOS pathway. J Ethnopharmacol. 2022;296:115476. [19] WANG A, YANG Q, LI Q, et al. Ginkgo Biloba L. Extract Reduces H2O2-Induced Bone Marrow Mesenchymal Stem Cells Cytotoxicity by Regulating Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways and Oxidative Stress. Med Sci Monit. 2018;24:3159-3167. [20] DENG W, DING Z, WANG Y, et al. Dendrobine attenuates osteoclast differentiation through modulating ROS/NFATc1/MMP9 pathway and prevents inflammatory bone destruction. Phytomedicine. 2022; 96:153838. [21] AN Y, ZHANG H, WANG C, et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019;33(11):12515-12527. [22] CHENG C, WENTWORTH K, SHOBACK DM. New Frontiers in Osteoporosis Therapy. Annu Rev Med. 2020;71:277-288. [23] LEBOFF MS, GREENSPAN SL, INSOGNA KL, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022;33(10):2049-2102. [24] KHANDELWAL S, LANE NE. Osteoporosis: Review of Etiology, Mechanisms, and Approach to Management in the Aging Population. Endocrinol Metab Clin North Am. 2023;52(2):259-275. [25] YU B, WANG CY. Osteoporosis and periodontal diseases - An update on their association and mechanistic links. Periodontol 2000. 2022; 89(1):99-113. [26] WEI L, CHEN W, HUANG L, et al. Alpinetin ameliorates bone loss in LPS-induced inflammation osteolysis via ROS mediated P38/PI3K signaling pathway. Pharmacol Res. 2022;184:106400. [27] HU M, YAN H, LI H, et al. Use of network pharmacology and molecular docking to explore the mechanism of action of curcuma in the treatment of osteosarcoma. Sci Rep. 2023;13(1):9569. [28] LU J, SHI X, FU Q, et al. New mechanistic understanding of osteoclast differentiation and bone resorption mediated by P2X7 receptors and PI3K-Akt-GSK3β signaling. Cell Mol Biol Lett. 2024;29(1):100. [29] WANG L, SHI X, ZHAO R, et al. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-kappaB activation, osteoclastogenesis and bone resorption. Bone. 2010;46(5):1369-1379. [30] CHEN ZH, WU JJ, GUO DY, et al. Physiological functions of podosomes: From structure and function to therapy implications in osteoclast biology of bone resorption. Ageing Res Rev. 2023;85:101842. [31] ZENG XZ, HE LG, WANG S, et al. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression. Acta Pharmacol Sin. 2016;37(2):255-263. [32] WU L, LUO Z, LIU Y, et al. Aspirin inhibits RANKL-induced osteoclast differentiation in dendritic cells by suppressing NF-κB and NFATc1 activation. Stem Cell Res Ther. 2019;10(1):375. [33] PANG M, RODRÍGUEZ-GONZALEZ M, HERNANDEZ M, et al. AP-1 and Mitf interact with NFATc1 to stimulate cathepsin K promoter activity in osteoclast precursors. J Cell Biochem. 2019;120(8):12382-12392. [34] CHEN K, QIU P, YUAN Y, et al. Pseurotin A Inhibits Osteoclastogenesis and Prevents Ovariectomized-Induced Bone Loss by Suppressing Reactive Oxygen Species. Theranostics. 2019;9(6):1634-1650. [35] LI J, WEI JJ, WU CH, et al. Epimedin A inhibits the PI3K/AKT/NF-κB signalling axis and osteoclast differentiation by negatively regulating TRAF6 expression. Mol Med. 2024;30(1):125. [36] IANTOMASI T, ROMAGNOLI C, PALMINI G, et al. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int J Mol Sci. 2023;24(4):3772. [37] AGIDIGBI TS, KIM C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int J Mol Sci. 2019;20(14):3576. [38] PENG Q, WANG J, HAN M, et al. Tanshinone IIA inhibits osteoclastogenesis in rheumatoid arthritis via LDHC-regulated ROS generation. Chin Med. 2023;18(1):54. [39] HUANG J, SONG D, XU M, et al. Trifolirhizin reduces osteoclast formation and prevents inflammatory osteolysis by inhibiting RANKL-induced activation of NF-kappaB and MAPK signaling pathways and ROS. Phytother Res. 2024;38(9):4650-4666. [40] REID IR, BILLINGTON EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080-1092. |
| [1] | Chen Huiting, Zeng Weiquan, Zhou Jianhong, Wang Jie, Zhuang Congying, Chen Peiyou, Liang Zeqian, Deng Weiming. Tail anchoring technique of vertebroplasty in treatment of osteoporotic vertebral compression fractures with intravertebral cleft: a finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2145-2152. |
| [2] | Zeng Xuan, Weng Rui, Ye Shicheng, Tang Jiadong, Mo Ling, Li Wenchao. Two lumbar rotary manipulation techniques in treating lumbar disc herniation: a finite element analysis of biomechanical differences [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2153-2161. |
| [3] | Cheng Qisheng, Julaiti·Maitirouzi, Xiao Yang, Zhang Chenwei, Paerhati·Rexiti. Finite element analysis of novel variable-diameter screws in modified cortical bone trajectory of lumbar vertebrae [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2162-2171. |
| [4] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [5] | Liu Dawei, Cui Yingying, Wang Fanghui, Wang Zixuan, Chen Yuhan, Li Yourui, Zhang Ronghe. Epigallocatechin gallate-mediated bidirectional regulation of reactive oxygen species and its application in nanomaterials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2101-2112. |
| [6] | Hu Xiongke, Liu Shaohua, Tan Qian, Liu Kun, Zhu Guanghui. Shikonin intervention with bone marrow mesenchymal stem cells improves microstructure of femur in aged mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1609-1615. |
| [7] | Han Nianrong, Huang Yifei, Akram · Osman, Liu Yanlu, Hu Wei . Programmed cell death receptor-1 suppresses osteogenic differentiation of rat bone marrow mesenchymal stem cells in a high-glucose microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1649-1657. |
| [8] | Zou Yulian, Chen Chaopei, Huang Haixia, Lan Yuyan, Liu Min, Huang Ting. Resveratrol promotes osteogenic differentiation of bone marrow mesenchymal stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1669-1678. |
| [9] | Chen Ju, Zheng Jinchang, Liang Zhen, Huang Chengshuo, Lin Hao, Zeng Li. Effect and mechanism of beta-caryophyllene in mice with osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1341-1347. |
| [10] | Wu Zhilin, , He Qin, Wang Pingxi, Shi Xian, Yuan Song, Zhang Jun, Wang Hao . DYRK2: a novel therapeutic target for rheumatoid arthritis combined with osteoporosis based on East Asian and European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1569-1579. |
| [11] | Zhang Haiwen, Zhang Xian, Xu Taichuan, Li Chao. Bibliometric and visual analysis of the research status and trends of senescence in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1580-1591. |
| [12] | Huang Jie, Zeng Hao, Wang Wenchi, Lyu Zhucheng, Cui Wei. Visualization analysis of literature on the effect of lipid metabolism on osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1558-1568. |
| [13] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [14] | Yang Zhijie, Zhao Rui, Yang Haolin, Li Xiaoyun, Li Yangbo, Huang Jiachun, Lin Yanping, Wan Lei, HuangHongxing. Postmenopausal osteoporosis: predictive values of muscle mass, grip strength, and appendicular skeletal muscle index [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1073-1080. |
| [15] | Zhou Jian, Zhang Tao, Zhou Weili, Zhao Xingcheng, Wang Jun, Shen Jie, Qian Li, Lu Ming. Effects of resistance training on quadriceps mass and knee joint function in patients with osteoporosis and sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1081-1088. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||