Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (3): 561-569.doi: 10.12307/2025.877
Previous Articles Next Articles
Finite element analysis of stress distribution of anchors at different implantation depths under different bone density conditions in rotator cuff tears
Wang Meng, Lu Tan, Li Minjie, Liu Zhicheng, Guo Xiaoyong
- First Affiliated Hospital of Xinxiang Medical College, Weihui 453100, Henan Province, China
-
Received:2024-11-27Accepted:2025-03-15Online:2026-01-28Published:2025-07-03 -
Contact:Lu Tan, MD, Associate chief physician, First Affiliated Hospital of Xinxiang Medical College, Weihui 453100, Henan Province, China -
About author:Wang Meng, Master candidate, Physician, First Affiliated Hospital of Xinxiang Medical College, Weihui 453100, Henan Province, China -
Supported by:2024 Henan Medical Science and Technology Project, No. LHGJ20240491 (to LT); Ministry of Education Industry-University Cooperation and Collaborative Education Project, No. 231105079300854 (to LT)
CLC Number:
Cite this article
Wang Meng, Lu Tan, Li Minjie, Liu Zhicheng, Guo Xiaoyong. Finite element analysis of stress distribution of anchors at different implantation depths under different bone density conditions in rotator cuff tears[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 561-569.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
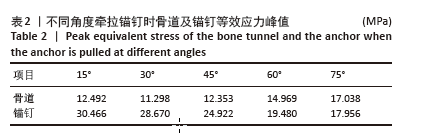
2.1 模型有效性验证 此次研究模型重点关注区域体网格分别划分为0.2 mm和0.4 mm,其余条件均设置为相同,导入ANSYS分析应力结果。网格划分为0.4 mm得出的锚钉最大等效应力、肱骨最大等效应力分别为25.451 MPa和19.972 MPa;网格划分为0.2 mm得出的锚钉最大等效应力、肱骨最大等效应力分别为26.574 MPa和19.195 MPa。2个相邻网格之间应力相对变化结果均在5%以内[33],证明此次研究模型网格划分可收敛。 将锚钉以45°插入肱骨大结节足印区,锚钉近端与骨表面平齐,给予锚钉15°,30°,45°,60°,75° 等方向100 N的牵拉,得出锚钉等效应力峰值,见表2。等效应力峰值与与包呼日查等[34]在相同条件下锚钉应力峰值分别为34.63,33.01,28.30,28.00,26.41 MPa及变化趋势类似,锚钉应力主要分布在锚钉下方锚孔及近端螺纹,证明此次研究模型构建真实可信,可进行后续研究。"
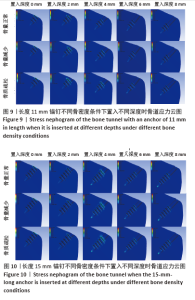
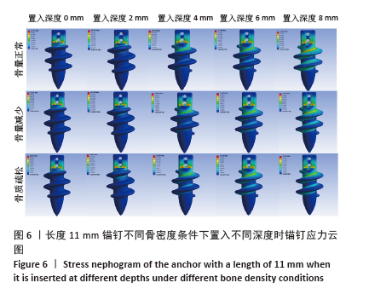
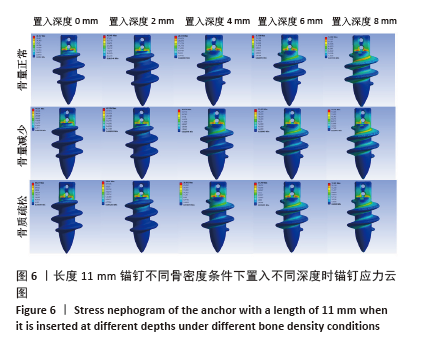
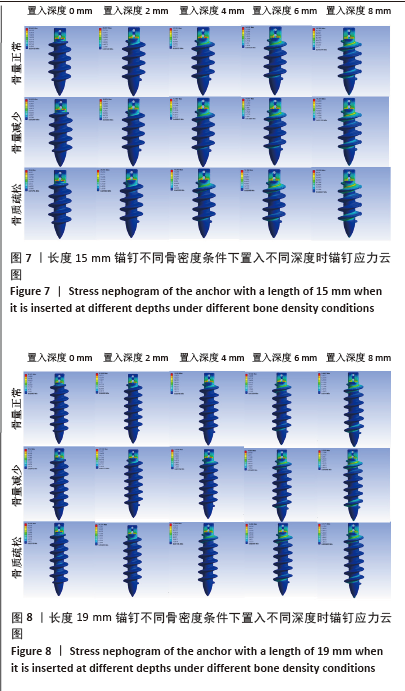
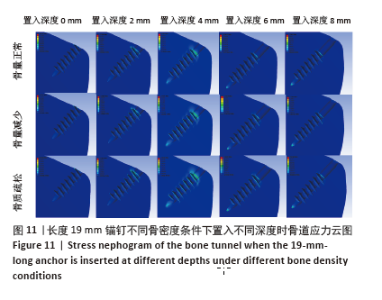
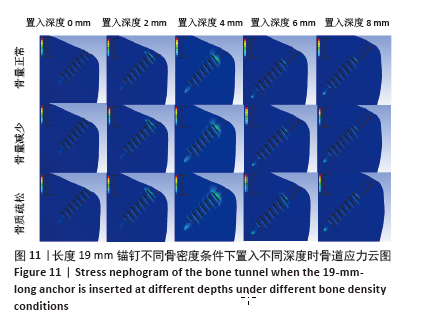
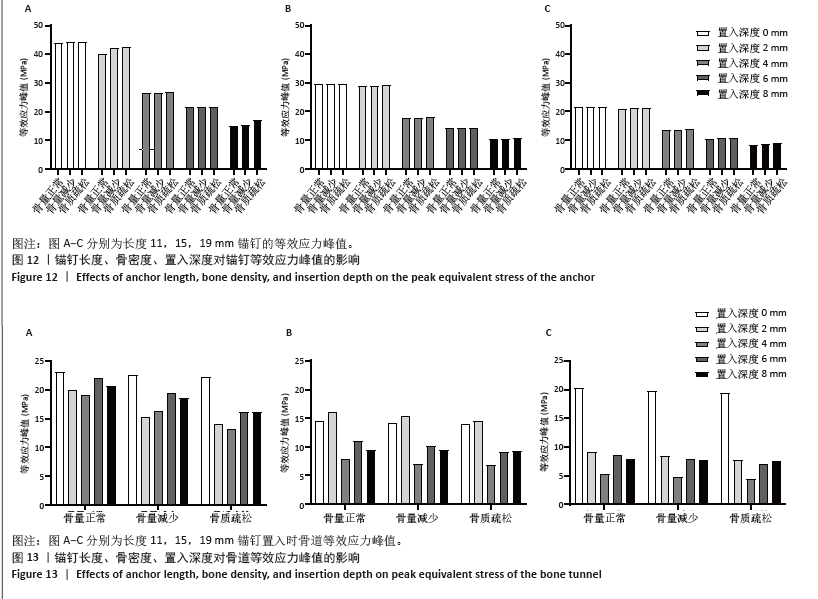
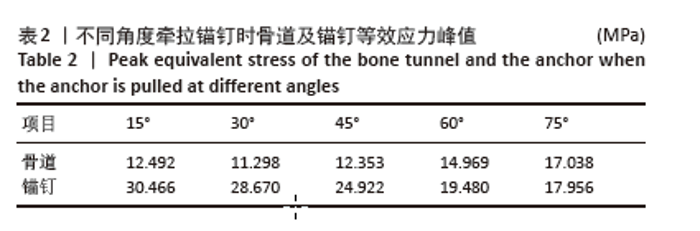
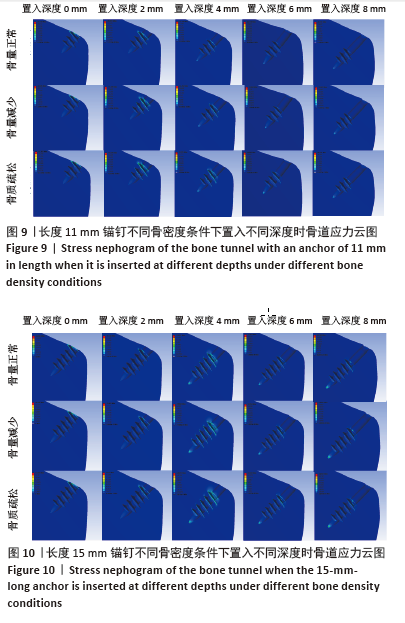
相同骨密度条件下锚钉置入不同深度时,骨道应力分布范围及均匀性出现先增加后减小的情况,从锚钉置入深度 0 mm到锚钉置入深度4 mm时,应力分布范围明显增加,尤其在与锚钉螺纹边缘接触部分;从锚钉置入深度4 mm到锚钉置入深度8 mm时,骨道应力分布范围及均匀性均减小,在锚钉置入深度4 mm时应力分布较均匀且范围最大;而骨密度对骨道应力分布模式影响较小,见图9-11。 2.3 锚钉及骨道等效应力峰值 长度11,15,19 mm的锚钉在不同骨密度条件下置入不同深度时骨道及锚钉等效应力峰值不同,见表3,4。当置入深度相同时,随着骨密度的降低,锚钉等效应力峰值逐渐增加;骨道周围等效应力峰值逐渐降低。当骨密度相同时,随着锚钉置入深度增加,锚钉等效应力峰值均逐渐减小;骨道等效应力峰值随锚钉置入深度增加出现先减小后增加的趋势,在锚钉置入深度为4 mm时最小,置入深度6,8 mm时的骨道等效应力峰值均高于此。见图12,13。"
| [1] YOO TH, KIM SJ, CHOI YR, et al. Age, tear size, extent of retraction, and fatty infiltration associated with a high chance of a similar rotator cuff tear in the contralateral shoulder regardless of symptoms in patients undergoing cuff repair in the index shoulder. Arthroscopy. 2023;39(7): 1611-1617. [2] DEWAN EMH, DHAKAL RO, HOUGHTON AJ, et al. Treatment options for massive irreparable rotator cuff tears: a review of arthroscopic surgical options. EFORT Open Rev. 2023;8(1): 11-10. [3] MORAN TE, WERNER BC. Surgery and rotator cuff disease: a review of the natural history, indications, and outcomes of nonoperative and operative treatment of rotator cuff tears. Clin Sports Med. 2023;42(1): 21-24. [4] BEDI A, BISHOP J, KEENER J, et al. Rotator cuff tears. Nat Rev Dis Primers. 2024;10(1): 8. [5] LANZAFAME GU, MARCOLONGO A, MARCHETTI F, et al. Histological, radiological and clinical analysis of the supraspinatus tendon and muscle in rotator cuff tears. BMC Musculoskelet Disord. 2023;24(1): 127-127. [6] GUPTA A, MIGLIORINI F, MAFFULLI N. Management of rotator cuff injuries using allogenic platelet - rich plasma. J Orthop Surg Res. 2024; 19(1):165. [7] DEMETRACOPOULOS A, DILLINGHAM M. Rotator Cuff Disease: Treatment Options and Considerations. Sports Med Arthrosc Rev. 2018; 26(3): 129-133. [8] MENEKSE S. Comparison of Outcomes between Open and Arthroscopic Rotator Cuff Repair. Adv Orthop. 2024;2024(1):5575404. [9] HSU KL, KUAN FC, GARCIA AV, et al. Factors association with reparability of rotator cuff tears: A systematic review and meta - analysis. J Shoulder Elbow Surg. 2024;33(9):465-477. [10] MACLEAN IS, BROCKMEIER SF. Failed and revision rotator cuff repair. Clin Sports Med. 2023;42(1):141-155. [11] PIATTI M, GORLA M, ALBERIO F. Comparison of all - suture anchors with metallic anchors in arthroscopic cuff repair: Structural and functional properties and clinical suitability. J Orthop. 2023;39:66-69. [12] HOFFMAN TR, LAMPLOT JD, MCCLISH SJ, et al. Three medial all suture anchors improves contact force compared to two hard body anchors in a biomechanical two-tendon rotator cuff tear model. Arthrosc Sports Med Rehabil. 2022;4(5):e1601-e1607. [13] CLEVENGER TA, BEEBE MJ, STRAUSS EJ. The effect of insertion angle on the pullout strength of threaded suture anchors: a validation of the deadman theory. Arthroscopy. 2014;30(8):900-905. [14] KAWAKAMI J, YAMAMOTO N, NAGAMOTO H, et al. Minimum Distance of Suture Anchors Used for Rotator Cuff Repair Without Decreasing the Pullout Strength: A Biomechanical Study. Arthroscopy. 2018;34(2): 377-385. [15] 周恩昌,殷浩,唐萍,等.锚钉数量及位置对肩关节Bankart损伤修复强度影响的有限元分析[J].中国骨伤,2018,31(12):1136-1139. [16] LI XW, XU YJ, HAN S, et al. Risk Factors and Corresponding Management for Suture Anchor Pullout during Arthroscopic Rotator Cuff Repair. J Clin Med. 2022;11(22):6870-6870. [17] HONG JP, HUANG SW, LEE CH, et al. Osteoporosis increases the risk of rotator cuff tears: a population-based cohort study. J Bone Miner Metab. 2022;40(2):348-356. [18] KAWASHIMA I, ISHIZUKA S, OBA H, et al. Prevalence and Treatment Rates of Osteoporosis Among Individuals with Rotator Cuff Tears. J Shoulder Elbow Surg. 2024;33(11):e606-e609. [19] CHUNG SW, OH JH, GONG HS, et al. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39(10):2099-2107. [20] KLAUE C, KLAUE S, SATTLER MC, et al. X-treme CT analysis of cancellous bone at the rotator cuff insertion in human individuals with osteoporosis: superficial versus deep quality. Arch Orthop Trauma Surg. 2013;133(3):381-387. [21] BUENO C, SACRISTÁN H, PÉREZ G, et al. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears. A systematic review. Rev Fac Cien Med Univ Nac Cordoba. 2023;80(3):252-274. [22] BURKHART SS. The deadman theory of suture anchors: observations along a south texas fence line. Arthroscopy. 1995;11(1):119-123. [23] ABEDI A, FARAHMAND F, ZANJANI OL, et al. Effect of geometrical design variables on implantation configuration and fixation stiffness of titling bone anchors: A parametric finite element study. Med Eng Phys. 2024;129:104191. [24] HITCHON S, SOLTANMOHAMMADI P, MILNER SJ, et al. Porous versus solid shoulder implants in humeri of different bone densities: A finite element analysis. J Orthop Res. 2024;42(9):1897-1906. [25] 王伟斌,袁欣华,扶青松,等.骨质疏松性肱骨近端骨折PMMA骨水泥强化螺钉钢板固定的有限元分析[J].中国骨伤,2023,36(3): 262-267. [26] DAHAN G, TRABELSI N, SAFRAN O, et al. Finite element analyses for predicting anatomical neck fractures in the proximal humerus. Clin Biomech (Bristol). 2019;68:114-121. [27] RHO JY, HOBATHO MC, ASHMAN RB. Relations of mechanical properties to density and CT numbers in human bone. Med Eng Phys. 1995;17(5): 347-355. [28] WANG H, CHEN L, XU G, et al. Biomechanical effects of deltoid muscle atrophy on rotator cuff tissue: a finite element study. Sci Rep. 2024;14(1):17592-17592. [29] 刘蒙飞,马鹏程,尹灿,等.不同骨密度对膝关节单髁置换后关节内各结构影响的三维有限元分析[J].中国组织工程研究,2024, 28(24):3801-3806. [30] POLIKEIT A, NOLTE LP, FERGUSON SJ. The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: finite - element analysis. Spine (Phila Pa 1976). 2003;28(10): 991-996. [31] 郭文文,刘静,曹慧,等.正常与骨质疏松肱骨的三维重建及有限元分析[J].中国医疗设备,2018,33(4):34-37. [32] SANO H, IMAGAWA K, YAMAMOTO N, et al. Predicting failures of suture anchors used for rotator cuff repair: A CT-based 3-dimensional finite element analysis. Biomed Mater Eng. 2015; 25(4):371-380. [33] REDEPENNING DH, LUDEWIG PM, LOOFT JM. Finite element analysis of the rotator cuff: A systematic review. Clin Biomech (Bristol). 2020;71: 73-85. [34] 包呼日查, 齐岩松, 陶立元, 等. 肩袖损伤修补金属锚钉置入角度-有限元分析[J]. 中华肩肘外科电子杂志,2019,7(1):56-62. [35] YAMAURA K, FUJIBAYASHI I, KUROSAWA T, et al. Timing of retears after arthroscopic rotator cuff repair and associated factors: a retrospective analysis. J Shoulder Elbow Surg. 2023;32(9):1929-1936. [36] LEE JS, SUH KT, SHIN WC, et al. Socioeconomic and Other Risk Factors for Retear after Arthroscopic Surgery for Nontraumatic Rotator Cuff Tear. Medicina (Kaunas). 2024;60(4):640. [37] ZHAO J, ZENG L, LIANG G, et al. Risk factors for symptomatic rotator cuff tears: a retrospective case - control study. Front Med (Lausanne). 2024;10:1321939-1321939. [38] TINGART MJ, APRELEVA M, LEHTINEN J, et al. Anchor design and bone mineral density affect the pull-out strength of suture anchors in rotator cuff repair: which anchors are best to use in patients with low bone quality? Am J Sports Med. 2004;32(6):1466-1473. [39] PATTERSON BM, BOZOGHLIAN MF. Modifiable and Nonmodifiable Risk Factors Associated with the Development of Recurrent Rotator Cuff Tears. Orthop Clin North Am. 2023;54(3):319-326. [40] YAMAUCHI S, TSUKADA H, SASAKI E, et al. Biomechanical analysis of bioabsorbable suture anchors for rotator cuff repair using osteoporotic and normal bone models. J Orthop Sci. 2022;27(1):115-121. [41] NAGAMOTO H, YAMAMOTO N, SANO H, et al. A biomechanical study on suture anchor insertion angle: Which is better, 90 degrees or 45 degrees? J Orthop Sci. 2017;22(1): 56-62. [42] OH JH, JEONG HJ, YANG SH, et al. Pullout strength of all - suture anchors: effect of the insertion and traction angle - a biomechanical study. Arthroscopy. 2018;34(10):2784-2795. [43] MENG M, WANG J, HUANG H, et al. 3D printing metal implants in orthopedic surgery: Methods, applications and future prospects. J Orthop Translat. 2023;42:94-112. [44] OMER S, BEDRI K, SOHEIL A, et al. Investigation of lattice infill parameters for additively manufactured bone fracture plates to reduce stress shielding. Comput Biol Med. 2023;161:107062-107062. [45] YAKACKI CM, POUKALOVA M, GULDBERG RE. The effect of the trabecular microstructure on the pullout strength of suture anchors. J Biomech. 2010;43(10):1953-1959. [46] MAHAR TA, TUCKER SB, UPASANI VV. Increasing the insertion depth of suture anchors for rotator cuff repair does not improve biomechanical stability. J Shoulder Elbow Surg. 2005;14(6):626-630. |
| [1] | Yang Zhijie, Zhao Rui, Yang Haolin, Li Xiaoyun, Li Yangbo, Huang Jiachun, Lin Yanping, Wan Lei, HuangHongxing. Postmenopausal osteoporosis: predictive values of muscle mass, grip strength, and appendicular skeletal muscle index [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1073-1080. |
| [2] | Zhou Jian, Zhang Tao, Zhou Weili, Zhao Xingcheng, Wang Jun, Shen Jie, Qian Li, Lu Ming. Effects of resistance training on quadriceps mass and knee joint function in patients with osteoporosis and sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1081-1088. |
| [3] | Cao Wenqi, Feng Xiuzhi, Zhao Yi, Wang Zhimin, Chen Yiran, Yang Xiao, Ren Yanling. Effect of macrophage polarization on osteogenesis-angiogenesis coupling in type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 917-925. |
| [4] | Zeng Hao, Sun Pengcheng, Chai Yuan, Huang Yourong, Zhang Chi, Zhang Xiaoyun. Association between thyroid function and osteoporosis: genome-wide data analysis of European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1019-1027. |
| [5] | Yu Xinlin, Chen Huiyu, Wang Yingying, Guo Weizhong, Feng Bin Lin Chengshou, Lin Wang. Finite element analysis of internal fixation with new retrograde intramedullary nail on lateral femur condyle for distal type A2 femur fractures [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 546-552. |
| [6] | Zhao Jingang, Liu Liping, Chen Jianwei, . Finite element analysis comparing lumbar fusion and artificial intervertebral disc replacement [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 553-560. |
| [7] | Ma Jingbo, Yang Guangnan, Liu Jiang, Jiang Qiang, Zhang Hanshuo, Han Jiaheng, Ding Yu. Endoscopic lumbar canal decompression for upper lumbar spinal stenosis: a comparison of biomechanical stability of three surgical models [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 577-585. |
| [8] | Abudusalamu·Tuoheti, Xiao Yang, Wang Yixi, Musitapa·Mijiti, Chen Qihao, Maimaitiming·Saiyiti, Guo Hailong, Paerhati·Rexiti. Effects of three internal fixation techniques on biomechanics of adjacent segment degeneration in lumbar interbody fusion [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 586-595. |
| [9] | Xu Jiamu, Yang Cheng, Li Weimin, Wang Chunqing. Role and pathogenesis of pyroptosis and inflammatory factors in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 691-700. |
| [10] | Shang Depeng, Wei Haiyu, Yang Fan. Finite element analysis for three different types of internal screw fixation in treatment of severe lumbar 1 vertebral body fractures [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 537-545. |
| [11] | Yang Fengli, Zhou Chao, Xiong Wei, Zhou Yuxiang, Li Dengshun, Wang Xin, Li Zhanzhen. 3D printed poly-L-lactic acid bone scaffolds in repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 507-515. |
| [12] | Cheng Yanan, Yu Jiazhi, Liu Yinchang, Wu Jie, Yu Tong, Wang Lu, Li Xiaoguang. Three-dimensional finite element analysis of molar distalization with clear aligners with different thicknesses and edges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 310-318. |
| [13] | Xu Hao, Ding Lu, Li Xiao. Investigating the effect of the mechanical wear on abutment screw in Morse taper connection implant implant system by using finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [14] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [15] | Li Liangkui, Huang Yongcan, Wang Peng, Yu Binsheng. Effect of anterior controllable anteriodisplacement and fusion on vertebrae-ossification of posterior longitudinal ligament complex and implants: a finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1761-1767. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||