Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (5): 1171-1183.doi: 10.12307/2026.023
Previous Articles Next Articles
Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment
Liu Yu1, Lei Senlin2, Zhou Jintao3, Liu Hui4, Li Xianhui5
- 1College of Physical Education, Hubei University of Education, Wuhan 430205, Hubei Province, China; 2College of Physical Education, 5College of Medicine, Jishou University, Jishou 416000, Hunan Province, China; 3Physical Education Department, Wuhan University, Wuhan 430072, Hubei Province, China; 4College of Medicine, Changjiang University, Jingzhou 434000, Hubei Province, China
-
Received:2024-11-27Accepted:2025-01-17Online:2026-02-18Published:2025-06-25 -
Contact:Li Xianhui, MD, Professor, College of Medicine, Jishou University, Jishou 416000, Hunan Province, China -
About author:Liu Yu, MS, Lecturer, College of Physical Education, Hubei University of Education, Wuhan 430205, Hubei Province, China -
Supported by:the National Natural Science Foundation of China, Nos. 82271514 (to LH) and 81860636 (to LXH)
CLC Number:
Cite this article
Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
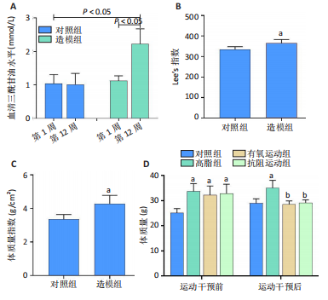
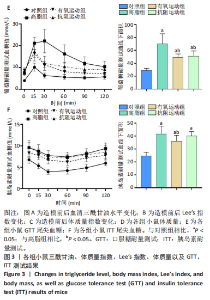
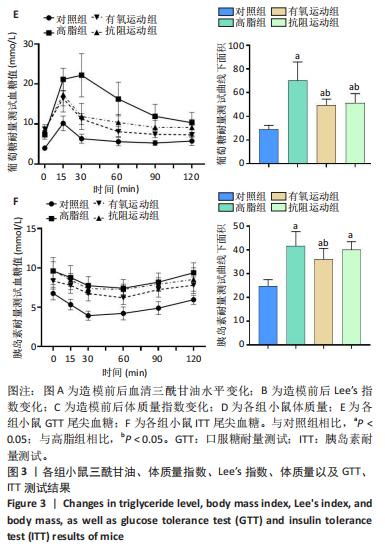
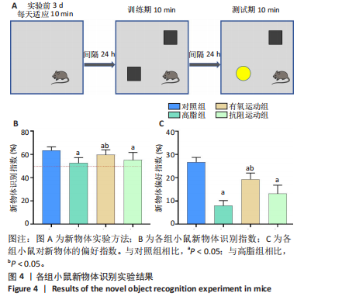
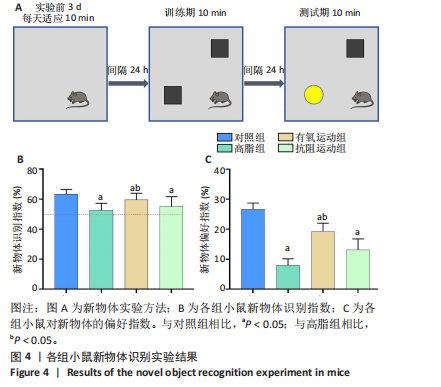
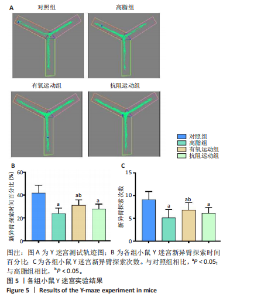
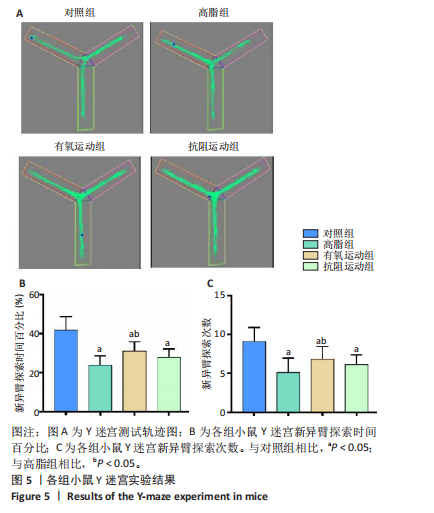
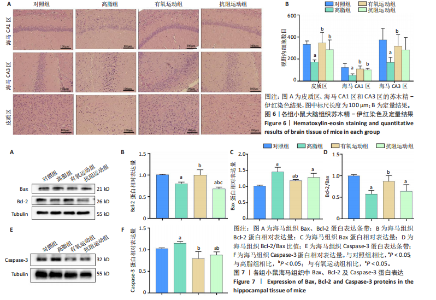
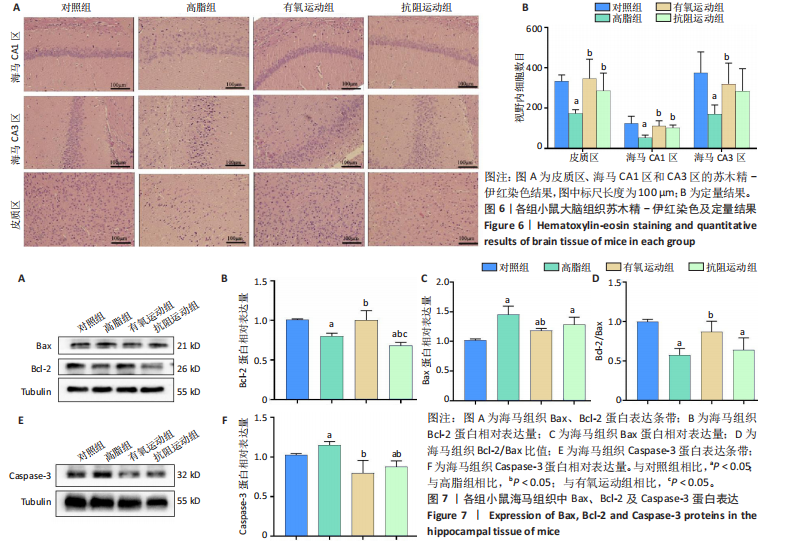
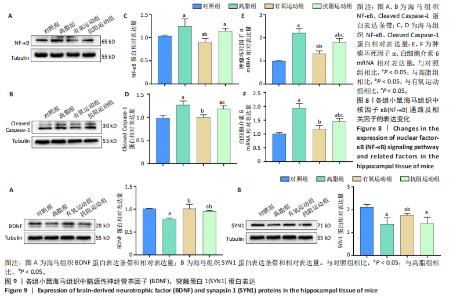
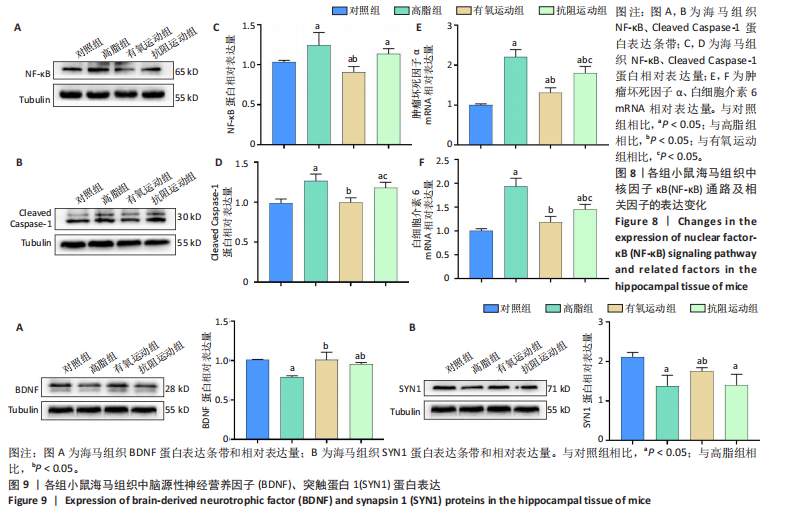
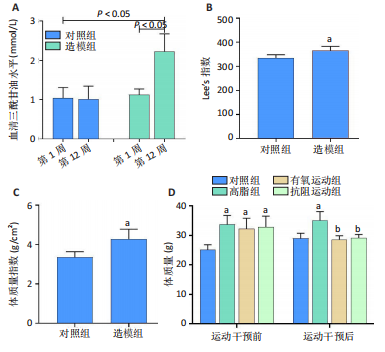
2.1 实验动物数量分析 实验过程中1只高脂+抗阻运动小鼠因无法完成训练而剔除,由同批次小鼠补足,其余小鼠在整个动物饲养、实验过程中均无异常死亡。 2.2 各组小鼠三酰甘油、Lee’s指数、体质量指数、体质量、葡萄糖耐量及胰岛素耐量测试结果 图3A为小鼠造模前后血清三酰甘油水平变化,结果表明高脂造模后小鼠血清三酰甘油水平显著高于对照组(P < 0.01);图3B为造模前后Lee’s指数变化,结果表明造模组Lee’s指数值显著高于对照组(P < 0.01);图3C为造模前后体质量指数变化,结果表明造模组体质量指数显著高于对照组(P < 0.01);图3D为运动前后体质量变化,结果表明运动干预前高脂组、有氧运动组和抗阻运动组体质量均显著高于对照组(P < 0.05),提示肥胖模型造模成功,在运动干预后高脂组体质量显著高于对照组(P < 0.05),有氧运动组和抗阻运动组体质量与对照组无显著差异,且均显著低于高脂组(P < 0.05);图3E为口服糖耐量测试结果,高脂组、有氧运动组和抗阻运动组曲线下面积显著高于对照组(P < 0.05),有氧运动组和抗阻运动组曲线下面积显著低于高脂组(P < 0.05),有氧运动组和抗阻运动组之间无显著差异(P=0.57);图3F为胰岛素耐量测试结果,高脂组、有氧运动组和抗阻运动组曲线下面积显著高于对照组(P < 0.05),有氧运动组曲线下面积显著低于高脂组(P < 0.05),抗阻运动组曲线下面积与高脂组无显著差异(P=0.53),有氧运动组和抗阻运动组之间无显著差异(P=0.067)。 2.3 各组小鼠新物体识别实验结果 图4A为新物体识别实验流程,图4B为各组小鼠对新物体的识别指数,高脂组识别指数显著低于对照组(P < 0.05),有氧运动组和抗阻运动组识别指数显著低于对照组(P < 0.05),有氧运动组识别指数显著高于高脂组(P < 0.05),抗阻运动组识别指数相对高于高脂组但无显著差异(P=0.35);图4C为各组小鼠对新物体的偏好指数,高脂组偏好指数显著低于对照组(P < 0.05),有氧运动组和抗阻运动组偏好指数显著低于对照组(P < 0.05),有氧运动组偏好指数显著高于高脂组(P < 0.05),抗阻运动组偏好指数相对高于高脂组但无显著差异(P=0.21)。结果表明,运动干预能有效缓解肥胖相关认知功能障碍,有氧运动干预效果更好。 2.4 各组小鼠Y迷宫实验结果 图5A为Y迷宫实验测试期各组小鼠移动轨迹图,测试期可见高脂组小鼠对新异臂的探索路径明显少于对照组,有氧运动组、抗阻运动组对新异臂的探索路径较高脂组明显增多;图5B为各组小鼠对新异臂的探索时间百分比,高脂组对新异臂的探索时间百分比显著低于对照组(P < 0.05),有氧运动组和抗阻运动组对新异臂的探索时间百分比显著低于对照组(P < 0.05),有氧运动组对新异臂的探索时间百分比显著高于高脂组(P < 0.05),抗阻运动组对新异臂的探索时间百分比与高脂组相比无显著差异(P=0.15);图5C为各组小鼠对新异臂的探索次数,与对照组相比,高脂组、有氧运动组和抗阻运动组对新异臂的探索次数显著减少(P < 0.05),与高脂组相比,有氧运动组对新异臂的探索次数增加且具有显著差异(P < 0.05),抗阻运动组新异臂的探索次数与高脂组相比无显著差异(P=0.29)。 2.5 各组小鼠大脑组织苏木精-伊红染色结果 苏木精-伊红染色显示(图6A),对照组海马和皮质组织脑细胞结构正常,胞核清晰,胞浆丰富,间质无水肿表现,海马锥体细胞排列紧密;与对照组相比,高脂组小鼠大脑海马和皮质部位的细胞丢失严重,细胞排列较为松散;而有氧运动和抗阻运动可明显提高皮质及海马区的神经元细胞密度,且细胞形态、排列均有明显改善。苏木精-伊红染色定量分析结果显示(图6B),高脂组海马CA1区、CA3区和皮质区细胞数量相比于对照组显著减少(P < 0.01),有氧运动组CA1区(P < 0.01)、CA3区(P < 0.05)和皮质区(P < 0.01)细胞数目相比于高脂组显著增多,抗阻运动组CA1区(P < 0.01)和皮质区(P < 0.05)细胞数目相比于高脂组显著增多,海马CA3区细胞数目与高脂组无显著差异(P=0.063)。 2.6 各组小鼠海马组织Bcl-2、Bax及Caspase-1 蛋白的表达变化 图7A为各组小鼠海马组织 Bax、Bcl-2蛋白表达条带;图7B为Bcl-2蛋白的相对表达量,与对照组相比,高脂组Bcl-2蛋白表达显著下调(P=0.007),与高脂组相比,有氧运动组Bcl-2蛋白表达显著上调(P < 0.05);图7C为Bax蛋白的相对表达量,与对照组相比,高脂组Bax蛋白表达显著上调(P=0.000 8),与高脂组相比,有氧运动组Bax蛋白表达显著下调(P=0.009 1);图7D为Bcl-2/Bax比值,与对照组相比,高脂组以及抗阻运动组Bcl-2/Bax 比值显著下调(P < 0.05);与高脂组相比,有氧运动组Bcl-2/Bax比值显著上调(P=0.01);抗阻运动组与高脂组Bcl-2/Bax比值无显著差异(P=0.48);图7E为各组小鼠海马组织Caspase-3蛋白条带;图7F为Caspase-3蛋白的相对表达量,与对照组相比,高脂组Caspase-3蛋白表达显著上调(P=0.012),而有氧运动组(P=0.021)和抗阻运动组(P=0.005 6)Caspase-3蛋白表达相比于高脂组显著下调。 2.7 各组小鼠海马组织NF-κB信号通路相关蛋白和基因的表达变化 图8A,B为各组小鼠海马组织NF-κB和Cleaved Caspase-1蛋白表达条带;图8C,D为NF-κB和Cleaved Caspase-1蛋白相对表达量;图8E,F为肿瘤坏死因子α和白细胞介素6的mRNA相对表达量。图8A,C显示,与对照组相比,高脂组、抗阻运动组NF-κB蛋白表达显著升高(P < 0.05);与高脂组相比,有氧运动组NF-κB蛋白表达显著下调(P < 0.05);抗阻运动组与高脂组NF-κB蛋白表达无显著差异(P=0.32)。图8B,D显示,与对照组相比,高脂组和抗阻运动组Cleaved Caspase-1蛋白表达显著升高(P < 0.05);有氧运动组与对照组Cleaved Caspase-1蛋白表达无显著差异(P=0.79);与高脂组、抗阻运动组相比,有氧运动组Cleaved Caspase-1蛋白表达显著下调(P < 0.05);抗阻运动组与高脂组Cleaved Caspase-1蛋白表达无显著差异(P=0.23)。图8E显示,高脂组、抗阻运动组和有氧运动组肿瘤坏死因子α mRNA表达显著高于对照组(P < 0.05);抗阻运动组和有氧运动组肿瘤坏死因子α mRNA表达显著低于高脂组(P < 0.05);有氧运动组肿瘤坏死因子α mRNA表达显著低于抗阻运动组(P < 0.05)。图8F显示,高脂组、抗阻运动组白细胞介素6 mRNA表达显著高于对照组(P < 0.05);有氧运动组与对照组白细胞介素6 mRNA表达无显著差异(P=0.069);抗阻运动组和有氧运动组白细胞介素6 mRNA表达显著低于高脂组(P < 0.05),有氧运动组白细胞介素6 mRNA表达显著低于抗阻运动组(P < 0.05)。 2.8 各组小鼠海马组织BDNF、突触蛋白1蛋白的表达变化 图9A,B为BDNF和突触蛋白1的蛋白条带及相对表达量,图9A显示,与对照组相比,高脂组和抗阻运动组BDNF蛋白表达显著下调(P < 0.05);有氧运动组与对照组BDNF蛋白表达无显著差异(P=0.98);与高脂组相比,有氧运动组、抗阻运动组BDNF蛋白表达显著上调(P < 0.05),有氧运动组与抗阻运动组BDNF蛋白表达无显著差异(P=0.36)。图9B显示,与对照组相比,高脂组、有氧运动组和抗阻运动组突触蛋白1蛋白表达显著下调 (P < 0.05);与高脂组相比,有氧运动组突触蛋白1蛋白表达显著上调(P < 0.05);抗阻运动组与高脂组突触蛋白1蛋白表达无显著差异(P=0.91),综上,有氧运动和抗阻运动均能有效增加高脂饲养小鼠海马BDNF的蛋白表达,有氧运动能有效增加高脂饲养小鼠海马区突触蛋白1的蛋白表达。"
| [1] GHOSH P, FONTANELLA RA, SCISCIOLA L, et al. Obesity-induced neuronal senescence: Unraveling the pathophysiological links. Ageing Res Rev. 2024;101:102533. [2] QI Y, WANG X. The Role of Gut Microbiota in High-Fat-Diet-Induced Diabetes: Lessons from Animal Models and Humans. Nutrients. 2023; 15(4):922. [3] TANG C, WANG Y, XU Z, et al. The relationships between high-fat diet and metabolic syndrome: Potential mechanisms. Food Bioscience. 2024; 59:104261. [4] BOWMAN K, THAMBISETTY M, KUCHEL GA, et al. Obesity and Longer Term Risks of Dementia in 65-74 Year Olds. Age Ageing. 2019;48(3): 367-373. [5] KULLMANN S, HENI M, HALLSCHMID M, et al. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol Rev. 2016;96(4):1169-1209. [6] TYAGI A, PUGAZHENTHI S. Targeting Insulin Resistance to Treat Cognitive Dysfunction. Mol Neurobiol. 2021;58(6):2672-2691. [7] CAI M, WAN J, CAI K, et al. The mitochondrial quality control system: a new target for exercise therapeutic intervention in the treatment of brain insulin resistance-induced neurodegeneration in obesity. Int J Obes (Lond). 2024;48(6):749-763. [8] 钟莎莎,刘奇,王强,等.肥胖引发低度炎症导致认知障碍的机制研究进展[J].中国医药导报,2024,21(8):32-35. [9] HUANG X, HUSSAIN B, CHANG J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27(1):36-47. [10] WANG X, GE A, CHENG M, et al. Increased hypothalamic inflammation associated with the susceptibility to obesity in rats exposed to high-fat diet. Exp Diabetes Res. 2012;2012:847246. [11] VADINI F, SIMEONE PG, BOCCATONDA A, et al. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes (Lond). 2020;44(6):1254-1263. [12] ZHANG Z, ZHANG B, WANG X, et al. Olfactory Dysfunction Mediates Adiposity in Cognitive Impairment of Type 2 Diabetes: Insights From Clinical and Functional Neuroimaging Studies. Diabetes Care. 2019;42(7): 1274-1283. [13] 李炎,刘鹏飞,刘学伟,等.开心散改善小鼠肥胖相关认知障碍的机制研究[J].中国中医基础医学杂志,2024,30(1):46-52. [14] SALMAN HB, SALMAN MA, YILDIZ AKAL E. The effect of omega-3 fatty acid supplementation on weight loss and cognitive function in overweight or obese individuals on weight-loss diet. Nutr Hosp. 2022;39(4):803-813. [15] 张鹏鹏.持续和间歇运动上调PGC-1α/Irisin/BDNF表达改善肥胖小鼠认知功能障碍[J].中国生物化学与分子生物学报,2023,39(9): 1346-1355. [16] LIM G, LEE H, LIM Y. Potential Effects of Resistant Exercise on Cognitive and Muscle Functions Mediated by Myokines in Sarcopenic Obese Mice. Biomedicines. 2022;10(10):2529. [17] ZHANG H, LIANG JL, WU QY, et al. Swimming Suppresses Cognitive Decline of HFD-Induced Obese Mice through Reversing Hippocampal Inflammation, Insulin Resistance, and BDNF Level. Nutrients. 2022; 14(12):2432. [18] HAO S, DEY A, YU X, et al. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun. 2016;51:230-239. [19] SHERAFATI-MOGHADAM M, PAHLAVANI HA, DARYANOOSH F, et al. The effect of high-intensity interval training (HIIT) on protein expression in Flexor Hallucis Longus (FHL) and soleus (SOL) in rats with type 2 diabetes. J Diabetes Metab Disord. 2022;21(2):1499-1508. [20] HORNBERGER TA JR, FARRAR RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29(1):16-31. [21] ZHENG Y, QIN Z, TSOI B, et al. Electroacupuncture on Trigeminal Nerve-Innervated Acupoints Ameliorates Poststroke Cognitive Impairment in Rats with Middle Cerebral Artery Occlusion: Involvement of Neuroprotection and Synaptic Plasticity. Neural Plast. 2020;2020: 8818328. [22] 李子木,梁维凤,常晶晶,等.白介素-1β基因敲除对小鼠行为的影响[J].合肥工业大学学报(自然科学版),2024,47(9):1224-1231. [23] 杨斌,张丽芝,陈琦芳,等.减重手术改善肥胖症认知功能障碍及其机制[J].生物化学与生物物理进展,2023,50(10):2373-2384. [24] MCLEAN FH, GRANT C, MORRIS AC, et al. Rapid and reversible impairment of episodic memory by a high-fat diet in mice. Sci Rep. 2018;8(1):11976. [25] ZHUANG H, YAO X, LI H, et al. Long-term high-fat diet consumption by mice throughout adulthood induces neurobehavioral alterations and hippocampal neuronal remodeling accompanied by augmented microglial lipid accumulation. Brain Behav Immun. 2022;100:155-171. [26] ELZINGA SE, HENN R, MURDOCK BJ, et al. cGAS/STING and innate brain inflammation following acute high-fat feeding. Front Immunol. 2022;13:1012594. [27] MARYAM K, ALI H. Aerobic and resistance exercises affect the BDNF/TrkB signaling pathway, and hippocampal neuron density of high-fat diet-induced obese elderly rats. Physiol Behav. 2023;264:114140. [28] PARK HS, PARK SS, KIM CJ, et al. Exercise Alleviates Cognitive Functions by Enhancing Hippocampal Insulin Signaling and Neuroplasticity in High-Fat Diet-Induced Obesity. Nutrients. 2019;11(7):1603. [29] SPITZ AZ, GAVATHIOTIS E. Physiological and pharmacological modulation of BAX. Trends Pharmacol Sci. 2022;43(3):206-220. [30] PANTIYA P, THONUSIN C, CHUNCHAI T, et al. Higher untrained fitness exerts a neuroprotection in Independence to caloric restriction or exercise in high-fat diet-induced obesity. Exp Neurol. 2023;365:114416. [31] YU H, LIN L, ZHANG Z, et al. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5(1):209. [32] NAKAHIRA K, HASPEL JA, RATHINAM VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222-230. [33] BAI J, LIU F. The cGAS-cGAMP-STING Pathway: A Molecular Link Between Immunity and Metabolism. Diabetes. 2019;68(6):1099-1108. [34] LI Y, BAI D, LU Y, et al. The crude guava polysaccharides ameliorate high-fat diet-induced obesity in mice via reshaping gut microbiota. Int J Biol Macromol. 2022;213:234-246. [35] SANG T, GUO C, GUO D, et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr Polym. 2021;256:117594. [36] ZHEN H, HU Y, LIU X, et al. The protease caspase-1: Activation pathways and functions. Biochem Biophys Res Commun. 2024;717:149978. [37] CAI X, CHEN J, XU H, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156(6):1207-1222. [38] 雷森林,谌晓安,陈平,等.脑源性神经营养因子介导帕金森病的运动防治:作用与机制[J].中国组织工程研究,2025,29(25):5454-5468. [39] 雷森林,陈平,谌晓安.运动调控BDNF表达改善阿尔茨海默病的潜在作用机制研究进展[J].中国细胞生物学学报,2024,46(6):1249-1262. [40] SEGUELLA L, PESCE M, CAPUANO R, et al. High-fat diet impairs duodenal barrier function and elicits glia-dependent changes along the gut-brain axis that are required for anxiogenic and depressive-like behaviors. J Neuroinflammation. 2021;18(1):115. [41] YAN P, LIU H, ZHOU T, et al. Crosstalk of Synapsin1 palmitoylation and phosphorylation controls the dynamicity of synaptic vesicles in neurons. Cell Death Dis. 2022;13(9):786. [42] MA W, LU K, LIANG HM, et al. Synapsin 1 Ameliorates Cognitive Impairment and Neuroinflammation in Rats with Alzheimer’s Disease: An Experimental and Bioinformatics Study. Curr Alzheimer Res. 2023;20(9):648-659. [43] 刘森,陈征,高欣冉,等.高脂饮食对大鼠海马形态及突触相关蛋白Synapsin-1表达的影响[J].安徽医科大学学报,2021,56(6):862-866. [44] HUDMON A, SCHULMAN H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473-510. |
| [1] | Yang Zhijie, Zhao Rui, Yang Haolin, Li Xiaoyun, Li Yangbo, Huang Jiachun, Lin Yanping, Wan Lei, HuangHongxing. Postmenopausal osteoporosis: predictive values of muscle mass, grip strength, and appendicular skeletal muscle index [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1073-1080. |
| [2] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [3] | Zhang Jiuxuan, Zhang Jinnan, Sui Xiaofan, Pei Xiaxia, Wei Jianhong, Su Qiang, Li Tian. Effects of ammonia poisoning on cognitive behavior and hippocampal synaptic damage in mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1122-1128. |
| [4] | Sun Yajie, Zhao Xinchen, Bo Shuangling. Spatiotemporal expression of bone morphologic protein 7 in mouse kidney development [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1156-1161. |
| [5] | Li Haojing, Wang Xin, Song Chenglin, Zhang Shengnan, Chen Yunxin. Therapeutic efficacy of extracorporeal shock wave therapy in the upper trapezius muscle area combined with exercise control training in patients with chronic non-specific neck pain [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1162-1170. |
| [6] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [7] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Advance in the mechanisms underlying miRNAs in steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1207-1214. |
| [8] | Bu Yangyang, Ning Xinli, Zhao Chen. Intra-articular injections for the treatment of osteoarthritis of the temporomandibular joint: different drugs with multiple combined treatment options [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1215-1224. |
| [9] | Wen Fan, Xiang Yang, Zhu Huan, Tuo Yanfang, Li Feng. Exercise improves microvascular function in patients with type 2 diabetes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1225-1235. |
| [10] | Liu Xinyue, Li Chunnian, Li Yizhuo, Xu Shifang. Regeneration and repair of oral alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1247-1259. |
| [11] | Chen Qiang, Wu Wenjuan, Jiang Shuhua, Huang Da. Physical exercise improves physical function in burn patients: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1269-1281. |
| [12] | Leng Xiaoxuan, Zhao Yuxin, Liu Xihua. Effects of different neuromodulatory stimulation modalities on non-motor symptoms in Parkinson’s patients: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1282-1293. |
| [13] | Wen Xiaolong, Weng Xiquan, Feng Yao, Cao Wenyan, Liu Yuqian, Wang Haitao. Effects of inflammation on serum hepcidin and iron metabolism related parameters in patients with type 2 diabetes mellitus: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1294-1301. |
| [14] | Yang Zeyu, Zhi Liang, Wang Jia, Zhang Jingyi, Zhang Qingfang, Wang Yulong, Long Jianjun. A visualized analysis of research hotspots in high-frequency repetitive transcranial magnetic stimulation from the macroscopic perspective [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1320-1330. |
| [15] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||