Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (2): 384-394.doi: 10.12307/2025.582
Previous Articles Next Articles
Preparation and antibacterial properties of porcine small intestinal submucosal composite nanohydroxyapatite bioscaffold loaded with antimicrobial peptide KR-12-a5
Yan Qiquan1, Yang Libin2, Li Mengjun1, Ni Yazhuo1, Chen Keying1, Xu Bo1, Li Yaoyang1, Ma Shiqing3, Li Rui1, 2, Li Jianwen4
- 1Department of Prosthodontics, Hospital of Stomatology, Tianjin Medical University, Tianjin 300070, China; 2Department of Stomatology, 4Department of Radiology, Shizuishan Second People's Hospital, Shizuishan 753000, Ningxia Hui Autonomous Region, China; 3Department of Stomatology, Second Hospital of Tianjin Medical University, Tianjin 300211, China
-
Received:2024-07-09Accepted:2024-11-09Online:2026-01-18Published:2025-06-17 -
Contact:Li Rui, PhD, Associate professor, Department of Prosthodontics, Hospital of Stomatology, Tianjin Medical University, Tianjin 300070, China; Department of Stomatology, Shizuishan Second People's Hospital, Shizuishan 753000, Ningxia Hui Autonomous Region, China Li Jianwen, Chief physician, Department of Radiology, Shizuishan Second People's Hospital, Shizuishan 753000, Ningxia Hui -
About author:Yan Qiquan, Master candidate, Department of Prosthodontics, Hospital of Stomatology, Tianjin Medical University, Tianjin 300070, China Yang Libin, Associate chief physician, Department of Stomatology, Shizuishan Second People's Hospital, Shizuishan 753000, Ningxia Hui Autonomous Region, China -
Supported by:Special Clinical Research Project of Wu Jieping Medical Foundation, No. 320.6750.2021-07-21 (to MSQ)
CLC Number:
Cite this article
Yan Qiquan, Yang Libin, Li Mengjun, Ni Yazhuo, Chen Keying, Xu Bo, Li Yaoyang, Ma Shiqing, Li Rui, Li Jianwen. Preparation and antibacterial properties of porcine small intestinal submucosal composite nanohydroxyapatite bioscaffold loaded with antimicrobial peptide KR-12-a5[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 384-394.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
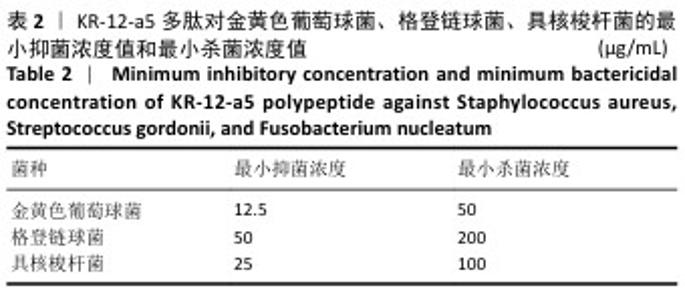
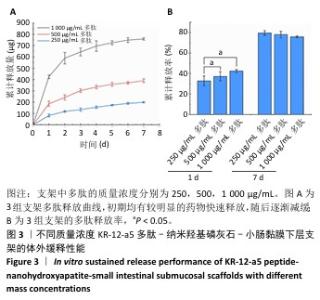
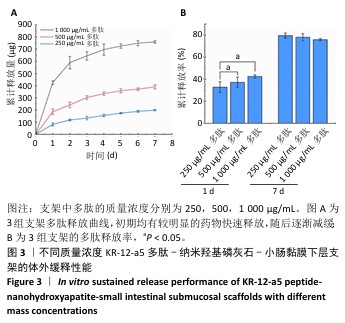
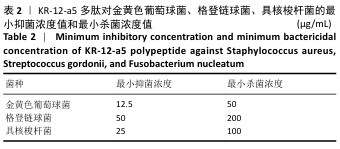
2.3 KR-12-a5多肽的抗菌性能检测结果 最小抑菌浓度和最小杀菌浓度是评估各种抗菌化合物抗菌功效的常用参数,表2为KR-12-a5多肽的最小抑菌浓度值和最小杀菌浓度检测结果。KR-12-a5多肽对金黄色葡萄球菌、格登链球菌、具核梭杆菌的最小抑菌浓度值分别为12.5,50,25 μg/mL,并且最小杀菌浓度值均为最小抑菌浓度值的4倍,表明KR-12-a5多肽作为广谱抗菌肽对骨缺损常见菌群(金黄色葡萄球菌、格登链球菌、具核梭杆菌)均具有较强的抗菌活性;此外,KR-12-a5多肽对金黄色葡萄球菌的最小抑菌浓度值最接近最小杀菌浓度值,说明KR-12-a5多肽对金黄色葡萄球菌的杀菌效果最为显著。 "
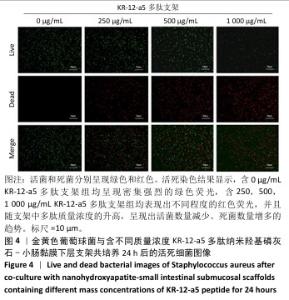
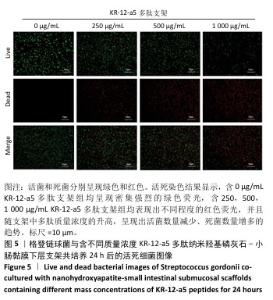
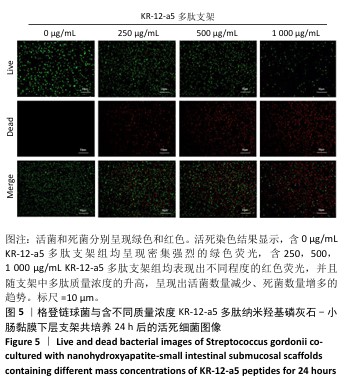
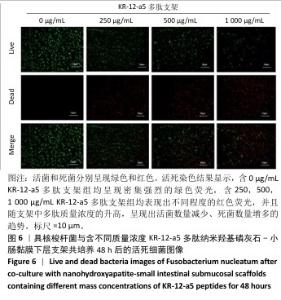
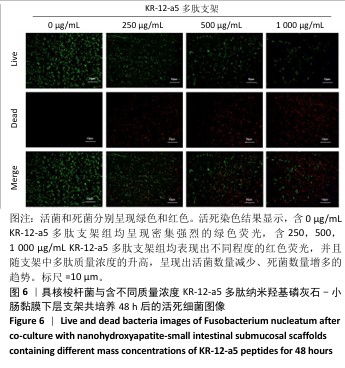
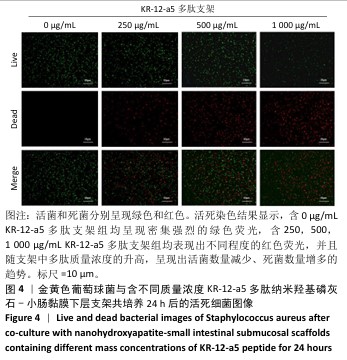
2.5 P-nHA-SIS支架抗菌性能检测结果 2.5.1 活死细菌染色 骨缺损区菌群经过AO/EB荧光染色后,在荧光显微镜下观察可见活菌表现出绿色荧光,死菌呈现红色荧光。金黄色葡萄球菌、格登链球菌、具核梭杆菌与交联nHA-SIS支架、P-nHA-SIS支架共培养后的AO/EB荧光染色结果,见图4-6。交联nHA-SIS支架组均呈现密集强烈的绿色荧光,说明nHA-SIS支架自身并不具备抗菌活性;250,500,1 000 μg/mL P-nHA-SIS支架组均表现出不同程度的红色荧光,并且随支架中多肽质量浓度的升高,呈现出活菌数量减少、死菌数量增多的趋势,证实KR-12-a5多肽对金黄色葡萄球菌、格登链球菌、具核梭杆菌有较强的抑制作用。 量化分析结果显示,200 μg/mL P-nHA-SIS支架组格登链球菌死亡率为25.36%,金黄色葡萄球菌和具核梭杆菌死亡率分别为27.54%和21.33%;500 μg/mL P-nHA-SIS支架组格登链球菌死亡率为57.23%,金黄色葡萄球菌和具核梭杆菌死亡率分别为84.13%和74.63%;1 000 μg/mL P-nHA-SIS支架组格登链球菌死亡率上升至77.96%,金黄色葡萄球菌和具核梭杆菌死亡率分别为86.13%和81.23%。 "
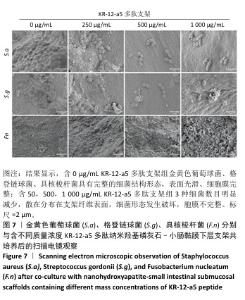
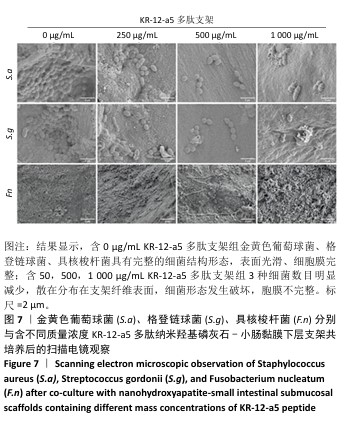
2.5.2 扫描电镜观察 金黄色葡萄球菌、格登链球菌、具核梭杆菌分别与交联nHA-SIS和不同质量浓度P-nHA-SIS支架共同培养后的扫描电镜观察结果,见图7。交联nHA-SIS组中金黄色葡萄球菌、格登链球菌、具核梭杆菌具有完整的细菌结构形态,表面光滑、细胞膜完整,金黄色葡萄球菌与格登链球菌均呈链状排列,具核梭杆菌相互堆叠。50,500,1 000 μg/mL P-nHA-SIS支架组细菌数目明显减少,散在分布在支架纤维表面,说明KR-12-a5多肽有着较强的抑菌效果,能够减少黄色葡萄球菌、格登链球菌、具核梭杆菌对支架材料的黏附;此外,细菌形态发生破坏,胞膜不完整,这可能是KR-12-a5多肽作用于细胞膜改变胞膜通透性造成的。 以上结果显示500 μg/mL P-nHA-SIS支架组已达到较满意的抑菌效果,因此后续实验选择500 μg/mL P-nHA-SIS支架。 "
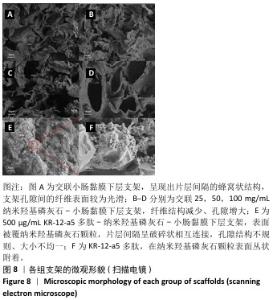
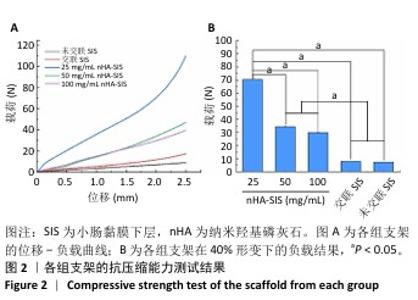
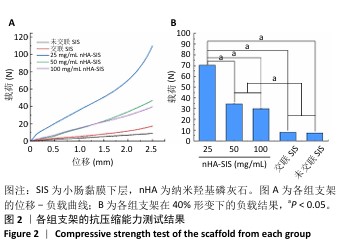
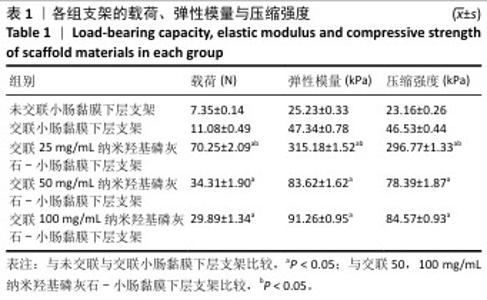
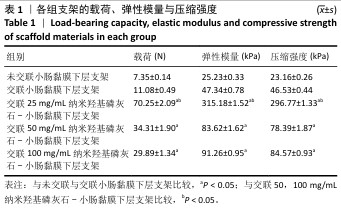
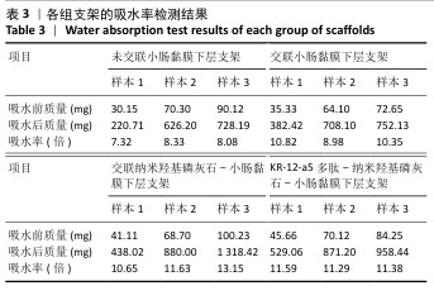
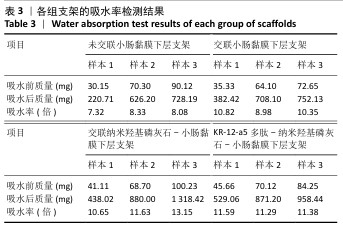
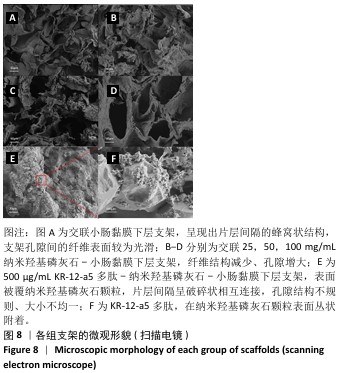
2.6 P-nHA-SIS支架表征实验结果 2.6.1 各组支架的扫描电镜观察 各组支架的微观结构见图8。交联小肠黏膜下层支架呈现出片层间隔的蜂窝状结构,支架孔隙直径为(118.40±25.41) μm,由于不含纳米羟基磷灰石颗粒,支架孔隙间的纤维表面较为光滑;随着纳米羟基磷灰石含量的增加,纤维结构逐渐减少、孔隙增大,交联25,50,100 mg/mL nHA-SIS支架的孔隙直径分别为(185.50±30.34),(212.65±35.82),(357±40.73) μm。交联nHA-SIS和P-nHA-SIS支架纤维表面被覆纳米羟基磷灰石颗粒,可见大量聚集成团的纳米羟基磷灰石颗粒,片层间隔呈破碎状相互连接,孔隙结构不规则、大小不均一;此外,P-nHA-SIS支架中纳米羟基磷灰石颗粒表面可见丛状堆积物附着,这是nHA-SIS支架所没有的,这些颗粒可能是多肽在电镜下的表现。"
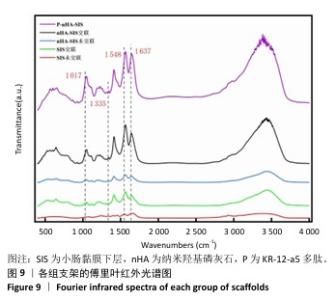
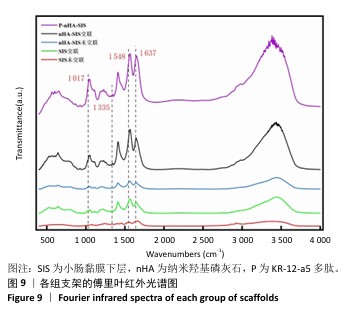
2.6.2 各组支架的傅里叶红外光谱分析 傅里叶红外光谱图主要显示了酰胺基团振动产生的特征峰,各组支架傅里叶红外光谱见图9。交联小肠黏膜下层支架在酰胺Ⅰ(1 637 cm-1)、酰胺Ⅱ(1 548 cm-1)和酰胺Ⅲ(1 335 cm-1)处相比于未交联小肠黏膜下层支架表现出更高的吸收峰,主要由C=O振动、N-H弯曲和C-N拉伸以及C–N拉伸和N-H平面内弯曲引起,表明1-乙基-3-(3-二甲氨丙基)碳二亚胺盐酸盐/N-羟基琥珀酰亚胺交联剂成功地在胶原之间形成了酰胺键结合。P-nHA-SIS支架中酰胺键的吸收峰显著高于交联nHA-SIS支架,这可能是纳米羟基磷灰石表面多肽链中肽键的原因。与交联小肠黏膜下层支架相比,交联nHA-SIS支架和P-nHA-SIS支架在 1 017 cm-1处出现纳米羟基磷灰石的特征峰,说明共混后复合支架依然保有纳米羟基磷灰石的化学结构特征。 "
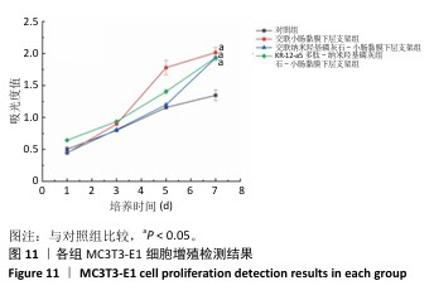
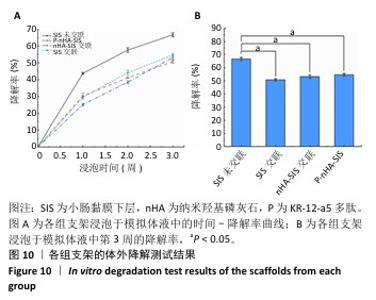
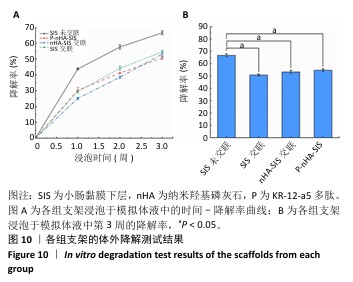
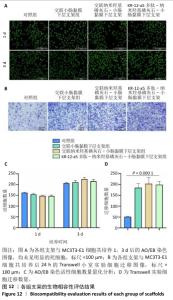
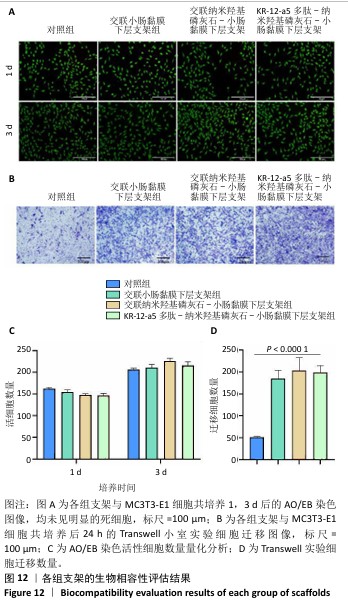
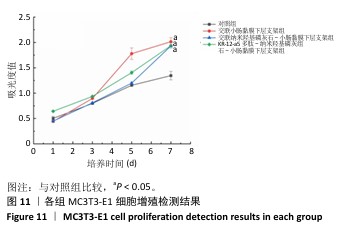
2.7 P-nHA-SIS支架生物相容性评估结果 通过CCK-8法检测各组支架对MC3T3-E1细胞增殖的影响[19],结果显示:随着培养时间的延长,各组细胞吸光度值均增加,交联小肠黏膜下层支架组、交联nHA-SIS支架组和P-nHA-SIS支架组培养第7天的吸光度值均高于对照组(P < 0.05),见图11,表明P-nHA-SIS支架具备良好的生物相容性,并且可以促进细胞增殖。 各组细胞AO/EB荧光染色结果见图12。各组均未见明显的死细胞,均保持了较好的细胞活力,并且各实验组活细胞数量多于对照组,可能是因为支架本身含有多种细胞生长因子,可促进MC3T3-E1细胞的生长增殖。 如图12所示,各实验组迁移细胞数量均多于对照组,表明交联小肠黏膜下层支架组、交联nHA-SIS支架组和P-nHA-SIS支架均可以促进MC3T3-E1细胞的迁移,具有较好的生物相容性。"
| [1] MAJIDINIA M, SADEGHPOUR A, YOUSEFI B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2017;233(4): 2937-2948. [2] XIE Y, ZHANG F, AKKUS O, et al. A collagen/PLA hybrid scaffold supports tendon‐derived cell growth for tendon repair and regeneration. J Biomed Mater Res. 2022;110(12):2624-2635. [3] KIM HD, AMIRTHALINGAM S, KIM SL, et al. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv Healthcare Mater. 2017;6(23):1700612. [4] ROCHA CV, GONÇALVES V, DA SILVA MC, et al. PLGA-Based Composites for Various Biomedical Applications. Int J Mol Sci. 2022;23(4):2034. [5] SU Y, YE B, ZENG L, et al. Small Intestinal Submucosa Biomimetic Periosteum Promotes Bone Regeneration. Membranes (Basel). 2022; 12(7):719. [6] ZAFAR F, HINTON RB, MOORE RA, et al. Physiological Growth, Remodeling Potential, and Preserved Function of a Novel Bioprosthetic Tricuspid Valve. J Am Coll Cardio. 2015;66(8):877-888. [7] PAJOR K, MICHALICHA A, BELCARZ A, et al. Antibacterial and Cytotoxicity Evaluation of New Hydroxyapatite-Based Granules Containing Silver or Gallium Ions with Potential Use as Bone Substitutes. Int J Mol Sci. 2022;23(13):7102. [8] 刘岩,郑雪新.3D打印聚乳酸-纳米羟基磷灰石/壳聚糖/多西环素抗菌支架的性能[J].中国组织工程研究,2024,28(22):3532-3538. [9] 阿曼台都曼别克,何惠宇,韩祥祯.羟基磷灰石-氧化石墨烯复合涂层促进大鼠骨缺损的修复[J].中国组织工程研究,2025,29(1): 2030-2037. [10] ATAK BH, BUYUK B, HUYSAL M, et al. Preparation and characterization of amine functional nano-hydroxyapatite/chitosan bionanocomposite for bone tissue engineering applications. Carbohydr Polym. 2017;164: 200-213. [11] CHINIPARDAZ Z, ZHONG JM, YANG S. Regulation of LL-37 in Bone and Periodontium Regeneration. Life. 2022;12(10):1533. [12] LIU J, LIU Y, ZHANG CY, et al. Research on the construction and properties of waste cotton cellulose/carbon dots composite antibacterial hydrogel. J Phys Conf Ser. 2024. doi:10.1088/1742-6596/2686/1/012017 [13] HANEY EF, MANSOUR SC, HANCOCK RE. Antimicrobial Peptides: An Introduction. Methods Mol Biol. 2017;1548:3-22. [14] WEI S, XU P, YAO Z, et al. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021;124:205-218. [15] ZHUO H, ZHANG X, LI M, et al. Antibacterial and Anti-Inflammatory Properties of a Novel Antimicrobial Peptide Derived from LL-37. Antibiotics(Basel). 2022;11(6):754. [16] LAKSHMAIAH NARAYANA J, GOLLA R, MISHRA B, et al. Short and Robust Anti-Infective Lipopeptides Engineered Based on the Minimal Antimicrobial Peptide KR12 of Human LL-37. ACS Infect Dis. 2021;7(6): 1795-1808. [17] JACOB B, PARK IS, BANG JK, et al. Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity. J Pept Sci. 2013;19(11):700-707. [18] RAHMANPANAH S, SEIFI M, GHARAVI Z, et al. Evaluation of shear bond strength and enamel remineralizing effect of experimental orthodontic composite containing nano-hydroxyapatite: An in vitro study. Int Orthod. 2023;21(1):100725. [19] MACHADO A, PEREIRA I, SILVA V, et al. Injectable hydrogel as a carrier of vancomycin and a cathelicidin-derived peptide for osteomyelitis treatment. J Biomedical Materials Res. 2022;110(11):1786-1800. [20] ZHAO R, YANG R, COOPER PR, et al. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules. 2021;26(10):3007. [21] HUIWEN W, SHUAI L, JIA X, et al. 3D-printed nanohydroxyapatite/methylacrylylated silk fibroin scaffold for repairing rat skull defects. J Biol Eng. 2024;18(1):22. [22] LEBLANC LATOUR M, PELLING AE. Mechanosensitive osteogenesis on native cellulose scaffolds for bone tissue engineering. J Biomech. 202;135:111030. [23] LI J, WANG C, GAO G, et al. MBG/ PGA-PCL composite scaffolds provide highly tunable degradation and osteogenic features. Bioact Mater. 2022;15:53-67. [24] ZHOU Y, DENG G, SHE H, et al. Polydopamine-coated biomimetic bone scaffolds loaded with exosomes promote osteogenic differentiation of BMSC and bone regeneration. Regen Ther. 2023;23:25-36. [25] KOHLI N, SHARMA V, ORERA A, et al. Pro-angiogenic and osteogenic composite scaffolds of fibrin, alginate and calcium phosphate for bone tissue engineering. J Tissue Eng. 2021;12:20417314211005610. [26] REN L, ZHANG Z, DENG C, et al. Antibacterial and pro-osteogenic effects of β-Defensin-2-loaded mesoporous bioglass. Dent Mater J. 2021;40(2):464-471. [27] TOMLINSON RE, SILVA MJ. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013;1(4):311-322. [28] DAY NB, DALHUISEN R, LOOMIS NE, et al. Tissue-adhesive hydrogel for multimodal drug release to immune cells in skin. Acta Biomater. 2022;150:211-220. [29] MENG S, HU H, QIAO Y, et al. A Versatile Hydrogel with Antibacterial and Sequential Drug-Releasing Capability for the Programmable Healing of Infectious Keratitis. ACS Nano. 2023;17(23): 24055-24069. [30] WANG L, LI J, XIONG Y, et al. Ultrashort Peptides and Hyaluronic Acid-Based Injectable Composite Hydrogels for Sustained Drug Release and Chronic Diabetic Wound Healing. ACS Appl Mater Interfaces. 2021;13(49):58329-58339. [31] BLASI-ROMERO A, ÅNGSTRÖM M, FRANCONETTI A, et al. KR-12 Derivatives Endow Nanocellulose with Antibacterial and Anti-Inflammatory Properties: Role of Conjugation Chemistry. ACS Appl Mater Interfaces. 2023;15(20):24186-24196. [32] MUHAMMAD T, STRÖMSTEDT AA, GUNASEKERA S, et al. Transforming Cross-Linked Cyclic Dimers of KR-12 into Stable and Potent Antimicrobial Drug Leads. Biomedicines. 2023;11(2):504. [33] CHANCI K, DIOSA J, GIRALDO MA, et al. Physical behavior of KR-12 peptide on solid surfaces and Langmuir-Blodgett lipid films: Complementary approaches to its antimicrobial mode against S. aureus. Biochim Biophys Acta Biomembr. 2022;1864(1):183779. |
| [1] | Zhang Qiya, Tong Yixiang, Yang Shijiao, Zhang Yumeng, Deng Ling, Wu Wei, Xie Yao, Liao Jian, Mao Ling. In vitro biocompatibility of graded glass infiltrated ultra-translucent zirconia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 443-450. |
| [2] | Shi Chunrong, He Jiaxu, Deng Lishan, Wang Hailan, Zhao Aimin, Yu Yiling, Geng Haixia, Song Weijun. Application of graphene oxide in field of oral implant restoration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6118-6126. |
| [3] | Yang Yuqian, Li Wenjun, Zhao Jian, Chen Gang. Injectable dental pulp extracellular matrix prepared by grinding treatment for dental pulp regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4663-4670. |
| [4] | Yang Wenjing, Gegentana. Finite element analysis of the mechanical properties of the tooth in endodontic root canal treatment for endodontic periapical disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4295-4304. |
| [5] | Qin Jingjie, Guo Zige, Li Rui, Ma Shiqing, Lu Ruijie, Li Mengjun. Modification with bone forming peptide 1 and polydopamine coating to improve bioactivity of polyetheretherketone surface [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3318-3325. |
| [6] | Yan Xinghua, Wang Xinyu, Liu Miao, Han Zekui, Song Yihan, Zhang Yan, Sun Zihui. Preparation and mechanical property analysis of hydrophilic Gyroid structure implant [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3343-3350. |
| [7] | Wang Huan, Lu Jing, Li Ying, Meng Maohua, Shu Jiayu, Luo Yuncai, Li Wenjie, Dong Qiang. Manufacture and mechanical property on zirconia abutments with a titanium base in dental implant restoration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2171-2177. |
| [8] | Zhao Shuai, Li Dongyao, Wei Suiyan, Cao Yijing, Xu Yan, Xu Guoqiang. Biocompatibility of poly(vinylidene fluoride) piezoelectric bionic periosteum prepared by electrospinning [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 730-737. |
| [9] | Chen Mingxue, Niu Jianhua, Lin Haiyan, Wu Gang, Wan Ben. Physicochemical properties and cytocompatibility of biomimetically precipitated nanocrystalline calcium phosphate granules [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3502-3508. |
| [10] | Wei Suiyan, Cao Yijing, Zhao Shuai, Li Dongyao, Wei Qin, Xu Yan, Xu Guoqiang. Cytocompatibility of electrospun polyvinylidene fluoride piezoelectric bionic periosteum [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2351-2357. |
| [11] | Cao Yijing, Wei Suiyan, Zhao Shuai, Li Dongyao, Wei Qin, Zhang Xujing, Xu Yan, Xu Guoqiang. Evaluation of antibacterial properties of uniaxial and coaxial minocycline hydrochloride-loaded bone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1547-1553. |
| [12] | Wei Yuxue, Wang Di, Liu Xiaoqiu. Design, synthesis and properties of oral composite resin monomers with different photoinitiators [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 731-735. |
| [13] | He Fan, Shan Xianfeng, Xiong Xiuli, Wang Xuejin. Biocompatibility and in vitro osteogenic differentiation of alginates/chitosan/58S bioglass composite membrane for guided bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3984-3991. |
| [14] | Li Shaoping, Yang Yuqing, Xiao Wenyundeng, Yin Lulu, Liu Libo, Liu Ying, Sun Yifan, Chen Zhiyu. Preparation and characterization of nano-hydroxyapatite/aspirin/polyvinyl alcohol/gelatin/sodium alginate hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3956-3963. |
| [15] | Zeng Jindi, Song Jingjing, Zhang Yuhang, Yang Zhengyi, Nie Ermin, Zhang Chunyuan, Jiang Rui. Surface roughness and bacteria adhesion of full zirconia restoration after different polishing treatment [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(21): 3320-3324. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||