Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (4): 901-907.doi: 10.12307/2026.504
Previous Articles Next Articles
Role and mechanism of probiotics in peri-implantitis
Wang Jie1, 2, Huang Rui1, 2, Zhang Ye1, 2, Shou Zhaoxi1, 2, Yao Jie1, 2, Liu Chenxi1, 2, Liao Jian1, 2
- 1School of Stomatology, Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Stomatological Hospital, Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Received:2024-11-18Accepted:2025-01-14Online:2026-02-08Published:2025-05-20 -
Contact:Liao Jian, MD, Chief physician, Doctoral supervisor, School of Stomatology, Guizhou Medical University, Guiyang 550004, Guizhou Province, China; Stomatological Hospital, Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Wang Jie, Master candidate, School of Stomatology, Guizhou Medical University, Guiyang 550004, Guizhou Province, China; Stomatological Hospital, Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 8226030370 (to LJ)
CLC Number:
Cite this article
Wang Jie, Huang Rui, Zhang Ye, Shou Zhaoxi, Yao Jie, Liu Chenxi, Liao Jian. Role and mechanism of probiotics in peri-implantitis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 901-907.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
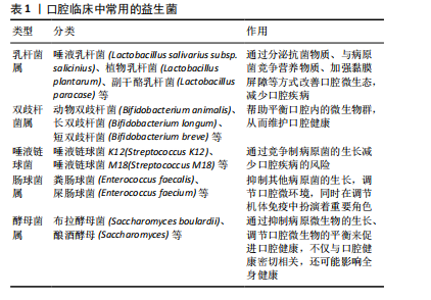
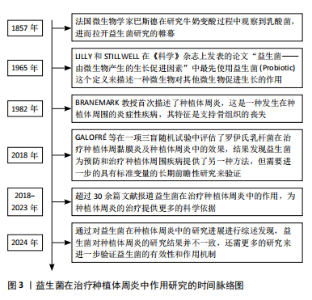
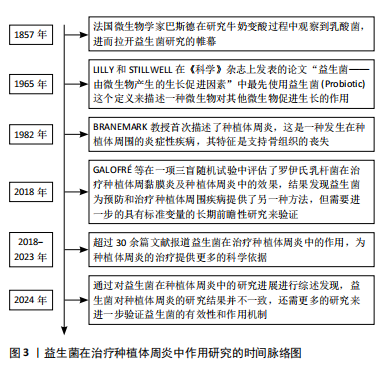
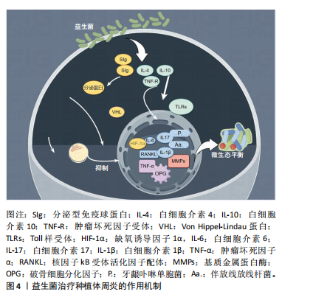
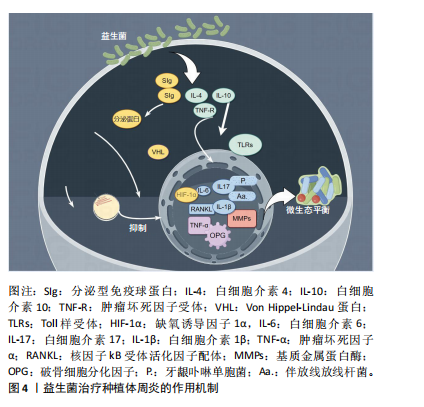
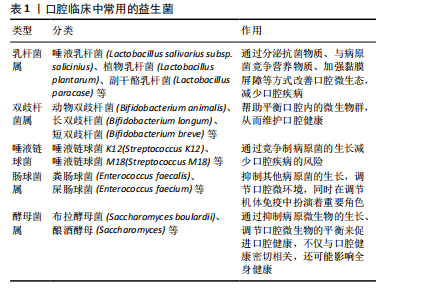
2.2 益生菌概述 益生菌被定义为活的微生物,具有抗菌和抗炎特性,给予适当的益生菌可以给宿主带来益处[16]。有益于健康的益生菌主要有以下几类:乳酸杆菌、双歧杆菌、酵母菌、肠球菌、链球菌、片球菌、明串珠菌、芽孢杆菌、大肠杆菌等[17]。益生菌最初被用于治疗肠道疾病,有证据表明益生菌可以积极影响肠道微生物群,减少抗生素相关性腹泻的持续时间[18]。事实上,许多益生菌已被证明可以预防和改善全身性疾病,包括改善高血压[19]、降低血糖水平以及提高老年人的免疫力[20-21]。 口腔临床中常用的益生菌主要分为3类:第1类是完全厌氧的菌双歧杆菌属,第2类是厌氧乳杆菌属,第3类是兼性厌氧的球菌属[22-23],详见表1。已有大量研究对益生菌在口腔卫生保健和口腔疾病治疗中的应用进行了深入探索,目前益生菌可改善龋病、牙周炎等[24-25]。SARMENTO等[26]发现了益生菌具有影响儿童口腔微生物群的潜力,可减少唾液中变形链球菌的数量,从而减少儿童口腔常见疾病的发生。罗伊氏乳杆菌已被证明对牙龈炎和牙周病具有显著的疗效[27],一项随机对照研究认为在个人口腔卫生功效减弱的情况下,食用罗伊氏乳杆菌含片可以改善和维持牙周健康[28]。种植体周炎与牙周炎有及其相似的临床症状[29],治疗目标是减轻病原微生物的负担,从而恢复种植体表面周围的共生菌群[30]。对于种植体周炎,单纯的牙周非手术治疗似乎不足以恢复种植体周围组织,在大多数情况下需要进行外科再生手术[31]。为了克服传统治疗方法的局限性,越来越多学者对益生菌作为种植体周炎治疗的辅助疗法进行了探索。 2.3 益生菌治疗种植体周炎的作用机制 益生菌治疗种植体周炎的机制尚未完全阐述,然而,多项研究结果表明,益生菌通过多种机制调节种植体周微生物群、免疫反应及炎症反应[32-33],见图4。"

2.3.1 调节口腔微生态的平衡 有研究表明,与健康的种植体周围相比,种植体周炎相关的微生物群更为复杂,微生物多样性和致病性增加与微生物群落的生态失调有关[34],种植体周炎微生物群主要由厌氧革兰阴性菌组成[35],例如牙龈卟啉单胞菌、连翘坦纳菌、具核梭杆菌和中间卟啉单胞菌等[36]。益生菌是有益微生物,能够通过分泌微生物的复合物和细菌素来破坏致病菌的细胞壁、降低生物膜的pH值、改善微生态内微生物的组成[37],以诱导细菌群落结构的变化,这可能导致细菌间相互作用,从而减少毒性最强的牙周病原体并恢复口腔生态系统的平衡[38];还可以有效抑制病原菌在种植体周围组织中的定植,平衡口腔微生物群,从而发挥抗感染作用[39-40]。 2.3.2 抑制炎症反应 当植入体内后,种植体表面暴露并形成唾液来源的薄膜,从而形成口腔细菌生物膜,由于细菌组成和毒力的定量和定性变化,这些生物膜在种植体周围组织中诱导免疫炎症反应,最终导致种植体周炎的发生[41]。据报道,白细胞介素、肿瘤坏死因子α、基质金属蛋白酶和参与骨代谢的生长因子可能与种植体周炎和骨质流失的风险增加有关[42],值得注意的是,这些促炎细胞因子可以诱导破骨细胞形成[43]。益生菌能够通过多种途径抑制炎症因子的分泌,促进抗炎因子的产生,从而激活抗炎机制[44-47]。 乳酸菌菌株具有诱导炎症细胞合成细胞因子的能力,它们影响促炎细胞因子的产生;此外,它们诱导sIgA类抗体的合成-分泌型免疫球蛋白A,从而间接减轻炎症程度[48]。一项随机对照研究表明,益生菌组在给予罗伊氏乳杆菌2周后牙龈指数和细菌斑块数量显著低于安慰剂组[49]。GRUNER等[50]发现乳酸杆菌可显著降低牙龈指数、探诊出血和牙周袋深度。核因子κB是一种炎症信号通路[51],种植体周围组织受到外界刺激因素干扰时会通过核因子κB信号通路释放炎症因子,从而导致周围骨组织的炎症反应[52],有研究结果证实双歧杆菌可通过核因子κB途径抑制炎症反应[53]。一项横断面研究证明,种植体周围龈沟液中的短链脂肪酸与种植体周围疾病之间存在相关性[54],短链脂肪酸具有广谱的抗菌活性,可以促进益生菌的生长、抑制特定病原菌的繁殖,从而达到抗炎作用[55]。 2.3.3 增强免疫功能 与其他以细菌感染为主的疾病不同,种植体周炎引起的软组织和硬组织破坏目前被认为是由细菌破坏种植体-宿主免疫平衡引起的,主要由宿主自身的免疫细胞介导[56]。细菌通过释放内毒素、外毒素和免疫原性片段来激活并持续刺激免疫细胞[10],这种微弱的炎症信号被宿主免疫细胞层放大,在突破一定阈值后可表现出相应的临床症状[57-58]。 相关研究人员发现,益生菌对病原菌的直接干预并不能完全解释其在种植体周围疾病中的意义[59]。研究证实,益生菌具有抗病毒活性、免疫调节作用[60],可以增强宿主口腔黏膜对种植体周围区域致病菌的抵抗力[61],还能产生抗菌化合物抵消致病微生物的黏附,调节免疫功能[62]。益生菌的抑菌和杀菌活性是基于产生对病原体具有拮抗作用的代谢物以及肠壁定植竞争和刺激免疫系统加强抗体的产生[16,63]。也有报道称,益生菌通过减少促炎细胞因子的产生和促进调节性T细胞的发育和功能,从而对宿主免疫系统产生有益作用[64-65]。最常见的免疫识别受体是Toll样受体,Toll样家族中的Toll样受体4和Toll样受体2通常被认为是调节种植体周炎的关键免疫识别受体[66]。INVERNICI等[67]发现动物双歧杆菌可导致牙龈上皮中β-防御素3分泌增加,从而增强了对牙龈卟啉单胞菌等病原菌的抵抗力;此外,Toll样受体4和CD-4、CD-57阳性免疫细胞的局部表达上调,这些细胞在病原体识别和先天免疫激活中发挥作用。另有报道称,益生菌具有免疫刺激性的细胞外多糖,可以激活巨噬细胞和淋巴细胞,从而增强免疫功能[68]。有证据表明,巨噬细胞是免疫炎症过程的核心参与者[69],巨噬细胞与M1表达和牙周袋深度值之间存在显著相关性[70],这意味着巨噬细胞可能在种植体周围炎的发生和进展中发挥相当大的作用,而益生菌能够通过激活巨噬细胞分泌活性蛋白,增强免疫功能[71]。 2.4 益生菌在种植体周炎中的应用 益生菌在种植体周炎中的应用越来越受到人们的关注,体外实验和临床试验表明,益生菌疗法是非手术治疗种植体周炎的一种有效辅助手段。 2.4.1 体外研究 体外实验表明,罗伊氏乳杆菌可以抑制种植体周围菌群的定植,如牙龈卟啉单胞菌、放线菌、中间普雷沃氏菌、金黄色葡萄球菌,从而改善种植体周围的微生态环境,维持种植体周平衡[72]。MULLA等[73]通过体外实验发现多种致病菌对50 mg/mL的唾液乳杆菌敏感,因此认为唾液乳杆菌在该剂量下可以有效对抗种植体周炎中的牙龈卟啉单胞菌、中间普氏菌、金黄色葡萄球菌和唾液链球菌等主要致病菌。VACCA等[74]发现唾液链球菌是口腔微生物组的成员,在体外表现出抑制种植体上病原生物膜形成的能力,表明唾液链球菌可用于预防或治疗种植体周围疾病。唾液乳酸菌具有一定黏附能力,可能在种植体周围龈下菌斑的形成过程中与致病菌发生共聚,影响致病菌的黏附和聚集,产生位点竞争而发挥抑菌性[75-76],这种竞争性抑制作用有助于减少口腔中的致病菌数量,进而改善种植体周围的微生态环境,并且益生菌能直接对种植体周炎病原体生物膜产生抑制作用,其抑制作用可能来自特定物质的产生。例如,各种乳杆菌科和双歧杆菌,包括发酵柠檬球菌和嗜酸乳杆菌,可通过产生过氧化氢、细菌素和有机酸对种植体周围疾病病原体产生直接抑制作用[77]。 2.4.2 临床研究 研究人员设计了一项随访3个月的随机、对照、平行三盲前瞻性试验,对纳入的种植体周炎患者进行非手术机械治疗后,将受试者随机分配每天服用1片益生菌锭剂或1片安慰剂锭剂,持续30 d,在基线及30,90 d时对受试者种植体部位进行临床测量及微生物学检查,结果表明,罗伊氏乳杆菌通过降低种植体周牙龈卟啉单胞菌浓度降低种植体周探诊出血和探查深度等临床参数[78]。 乳酸杆菌已被证明可以预防伴放线放线杆菌、牙龈卟啉单胞菌和中间普氏菌的生长[79-80]。TADA等[81]进行了双盲、随机、安慰剂对照的临床试验,对连续6个月服用含罗伊氏乳杆菌益生菌片剂的种植体周炎患者进行研究,在摄入益生菌后0,4,12和24周进行临床检查和细菌采样,发现益生菌组探诊深度在第4,24周显著低于第0周,牙龈卟啉单胞菌、齿垢密螺旋体等细菌数量在第12,24周时有所减少。植物乳杆菌对发酵食品中的病原体起拮抗作用,并表现出抗菌活性[82]。有益细菌可以产生抗菌肽(如细菌素),从而可以诱导对致病微生物的抑制作用[83]。 一项横断面研究显示,大量食用发酵和益生菌食品可能与种植体周围的健康有关,发酵和益生菌产品可能有助于预防种植体患者的种植体周围疾病[84]。 除此之外,多项荟萃分析中也评估了益生菌在种植体周炎中的辅助益处,阐述益生菌在控制临床炎症和病因因素方面的优势,例如:张艺庭[85]对13篇病例对照研究进行总结,结果显示实验组随访3个月时探诊出血明显改善,随访6个月时牙龈出血明显减少;BUTERA等[86]荟萃分析选定了6项研究、共212例患者,结果显示与对照组相比,实验组随访6周至3个月的探诊深度及探诊出血均有改善;LóPEZ-VALVERDE等[87]将9项研究纳入分析,发现益生菌在治疗种植体周围疾病方面的有益作用。 从现有的证据来看,益生菌为防止种植体周炎提供了另一种可供选择的治疗方法,但也需要进一步的长期前瞻性研究进行验证。"

| [1] BERGLUNDH T, ARMITAGE G, ARAUJO MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45(S20):S286-S291. [2] ASTOLFI V, RÍOS-CARRASCO B, GIL-MUR FJ, et al. Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study. Int J Environ Res Public Health. 2022;19(7):4147. [3] RODRIGO D, SANZ-SÁNCHEZ I, FIGUERO E, et al. Prevalence and risk indicators of peri-implant diseases in Spain. J Clin Periodontol. 2018;45(12): 1510-1520. [4] ROOS-JANSÅKER AM, LINDAHL C, RENVERT H, et al. Nine-to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. J Clin Periodontol. 2006;33(4):290-295. [5] DIAZ P, GONZALO E, VILLAGRA LJG, et al. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health. 2022;22(1):449. [6] BELIBASAKIS GN, MANOIL D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J Dent Res. 2021;100(1):21-28. [7] MOMBELLI A. Maintenance therapy for teeth and implants. Periodontol 2000. 2019;79(1):190-199. [8] AMATO M, DI SPIRITO F, D’AMBROSIO F, et al. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms. 2022;10(11):2289. [9] CHUN GIOK K, MENON RK. The Microbiome of Peri-Implantitis: A Systematic Review of Next-Generation Sequencing Studies. Antibiotics. 2023;12(11):1610. [10] LAFAURIE GI, SABOGAL MA, CASTILLO DM, et al. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J Periodontol. 2017;88(10):1066-1089. [11] LIÑARES A, SANZ-SÁNCHEZ I, DOPICO J, et al. Efficacy of adjunctive measures in the non‐surgical treatment of peri‐implantitis: A systematic review. J Clin Periodontol. 2023;50(S26):224-243. [12] RAMS TE, DEGENER JE, VAN WINKELHOFF AJ. Antibiotic resistance in human chronic periodontitis microbiota. J Periodontol. 2014;85(1): 160-169. [13] VAN WINKELHOFF AJ, HERRERA GONZALES D, WINKEL EG, et al. Antimicrobial resistance in the subgingival microflora in patients with adult periodontitis. A comparison between The Netherlands and Spain. J Clin Periodontol. 2000;27(2):79-86. [14] HAFFAJEE AD, TELES RP, SOCRANSKY SS. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontol 2000. 2006;42:219-258. [15] YADAV MK, KUMARI I, SINGH B, et al. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl Microbiol Biotechnol. 2022;106(2):505-521. [16] REID G; FOOD AND AGRICULTURAL ORGANIZATION OF THE UNITED NATION AND THE WHO. The importance of guidelines in the development and application of probiotics. Curr Pharm Des. 2005;11(1):11-16. [17] FIJAN S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11(5): 4745-4767. [18] SAVIANO A, PETRUZZIELLO C, CANCRO C, et al. The Efficacy of a Mix of Probiotics (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103) in Preventing Antibiotic-Associated Diarrhea and Clostridium difficile Infection in Hospitalized Patients: Single-Center, Open-Label, Randomized Trial. Microorganisms. 2024;12(1):198. [19] CHEN Z, LIANG W, LIANG J, et al. Probiotics: functional food ingredients with the potential to reduce hypertension. Front Cell Infect Microbiol. 2023;13:1220877. [20] KALLASSY J, GAGNON E, ROSENBERG D, et al. Strains of Faecalibacterium prausnitzii and its extracts reduce blood glucose levels, percent HbA1c, and improve glucose tolerance without causing hypoglycemic side effects in diabetic and prediabetic mice. BMJ Open Diab Res Care. 2023;11(3):e003101. [21] LIU Y, WANG J, WU C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-biotics, and Post-biotics. Front Nutr. 2021;8: 634897. [22] HOMAYOUNI RAD A, POURJAFAR H, MIRZAKHANI E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front Cell Infect Microbiol. 2023;13:1120995. [23] 晏子衡.益生菌对Er:YAG激光治疗种植体周围炎疗效影响的临床观察[D].南京:南京医科大学,2018. [24] Zaghloul SA, Hashem SN, El-Sayed SR, et al. Evaluation of the Cariogenic and Anti-Cariogenic Potential of Human Colostrum and Colostrum-Derived Probiotics: Impact on S. mutans Growth, Biofilm Formation, and L. rhamnosus Growth. Life. 2023;13(9):1869. [25] ZIDAR A, KRISTL J, KOCBEK P, et al. Treatment challenges and delivery systems in immunomodulation and probiotic therapies for periodontitis. Expert Opin Drug Deliv. 2021;18(9):1229-1244. [26] SARMENTO ÉG, CESAR DE, MARTINS ML, et al. Effect of probiotic bacteria in composition of children’s saliva. Food Res Int. 2019;116: 1282-1288. [27] INCHINGOLO F, MARTELLI FS, GARGIULO ISACCO C, et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines. 2020;8(5):115. [28] SCHLAGENHAUF U, REHDER J, GELBRICH G, et al. Consumption of Lactobacillus reuteri-containing lozenges improves periodontal health in navy sailors at sea: A randomized controlled trial. J Periodontol. 2020;91(10):1328-1338. [29] DARBY I. Risk factors for periodontitis & peri-implantitis. Periodontol 2000. 2022;90(1):9-12. [30] ROCCUZZO A, IMBER JC, SALVI GE, et al. Peri-implantitis as the consequence of errors in implant therapy. Periodontology 2000. 2023; 92(1):350-361. [31] CHALA M, ANAGNOSTAKI E, MYLONA V, et al. Adjunctive Use of Lasers in Peri-Implant Mucositis and Peri-Implantitis Treatment: A Systematic Review. Dent J (Basel). 2020;8(3):68. [32] CAI R, LIU Y, WANG X, et al. Influences of standardized clinical probing on peri-implant soft tissue seal in a situation of peri-implant mucositis: A histomorphometric study in dogs. J Periodontol.2024;95(3):233-243. [33] DI RAIMONDO R, SANZ-ESPORRIN J, MARTIN IS, et al. Hard tissue volumetric and soft tissue contour linear changes at implants with different surface characteristics after experimentally induced peri-implantitis: an experimental in vivo investigation. Clin Oral Invest. 2021;25(6):3905-3918. [34] DI SPIRITO F, GIORDANO F, DI PALO MP, et al. Reliability and Accuracy of YouTube Peri-Implantitis Videos as an Educational Source for Patients in Population-Based Prevention Strategies. Healthcare. 2023;11(14):2094. [35] DINI C, COSTA RC, BERTOLINI M, et al. In-vitro polymicrobial oral biofilm model represents clinical microbial profile and disease progression during implant-related infections. J Appl Microbiol. 2023;134(11):lxad265. [36] DI SPIRITO F, GIORDANO F, DI PALO MP, et al. Microbiota of Peri-Implant Healthy Tissues, Peri-Implant Mucositis, and Peri-Implantitis: A Comprehensive Review. Microorganisms. 2024;12(6):1137. [37] ROUTIER A, BLAIZOT A, AGOSSA K, et al. What do we know about the mechanisms of action of probiotics on factors involved in the pathogenesis of periodontitis? A scoping review of in vitro studies. Arch Oral Biol. 2021;129:105196. [38] BACA-CASTAÑÓN ML, DE LA GARZA-RAMOS MA, ALCÁZAR-PIZAÑA AG, et al. Antimicrobial Effect of Lactobacillus reuteri on Cariogenic Bacteria Streptococcus gordonii, Streptococcus mutans, and Periodontal Diseases Actinomyces naeslundii and Tannerella forsythia. Probiotics Antimicrob Proteins. 2015;7(1):1-8. [39] NGUYEN T, BRODY H, RADAIC A, et al. Probiotics for periodontal health-Current molecular findings. Periodontol 2000. 2021;87(1):254-267. [40] HAN N, JIA L, GUO L, et al. Balanced oral pathogenic bacteria and probiotics promoted wound healing via maintaining mesenchymal stem cell homeostasis. Stem Cell Res Ther. 2020;11(1):61. [41] SUN H, CHAN Y, LI X, et al. Multi-omics analysis of oral bacterial biofilm on titanium oxide nanostructure modified implant surface: In vivo sequencing-based pilot study in beagle dogs. Materials Today Bio. 2022;15:100275. [42] LAFUENTE-IBÁÑEZ DE MENDOZA I, SETIEN-OLARRA A, GARCÍA-DE LA FUENTE AM, et al. Role of proinflammatory mutations in peri-implantitis: systematic review and meta-analysis. Int J Implant Dent. 2022;8(1):2. [43] JIA Q, LIU L, YU Y, et al. Inhibition of EGFR Pathway Suppresses M1 Macrophage Polarization and Osteoclastogenesis, Mitigating Titanium Particle-Induced Bone Resorption. J Inflamm Res. 2024;17: 9725-9742. [44] VIRK MS, VIRK MA, HE Y, et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients. 2024;16(4):546. [45] MALKA O, MALISHEV R, BERSUDSKY M, et al. Tryptophol Acetate and Tyrosol Acetate, Small-Molecule Metabolites Identified in a Probiotic Mixture, Inhibit Hyperinflammation. J Innate Immun. 2023;15(1):531-547. [46] VINCENZI A, GOETTERT MI, VOLKEN DE SOUZA CF. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 2021;57:27-38. [47] HSU CY, MUSTAFA MA, MOATH OMAR T, et al. Gut instinct: harnessing the power of probiotics to tame pathogenic signaling pathways in ulcerative colitis. Front Med. 2024;11:1396789. [48] RADAIC A, BRODY H, CONTRERAS F, et al. Nisin and Nisin Probiotic Disrupt Oral Pathogenic Biofilms and Restore Their Microbiome Composition towards Healthy Control Levels in a Peri-Implantitis Setting. Microorganisms. 2022;10(7):1336. [49] KRASSE P, CARLSSON B, DAHL C, et al. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed Dent J. 2006;30(2):55-60. [50] GRUNER D, PARIS S, SCHWENDICKE F. Probiotics for managing caries and periodontitis: Systematic review and meta-analysis. J Dent. 2016; 48:16-25. [51] QU R, CHEN X, HU J, et al. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-α/NF-κB signaling pathways. Sci Rep. 2019;9(1):1348. [52] LASSERRE JF, BRECX MC, TOMA S. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials (Basel). 2018;11(10):1802. [53] RIEDEL CU, FOATA F, PHILIPPE D, et al. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-κB activation. World J Gastroenterol. 2006;12(23):3729-3735. [54] LIU Y, YANG H, WANG P, et al. Correlation between short-chain fatty acids and peri-implant disease: A cross-sectional study. J Periodontol. 2024. doi: 10.1002/JPER.23-0682. [55] FU Q, ZHOU S, YU M, et al. Portulaca oleracea Polysaccharides Modulate Intestinal Microflora in Aged Rats in vitro. Front Microbiol. 2022;13: 841397. [56] JAMALPOOR Z, ASGARI A, LASHKARI MH, et al. Modulation of Macrophage Polarization for Bone Tissue Engineering Applications. Iran J Allergy Asthma Immunol. 2018;17(5):398-408. [57] ZHU L, LIU X, NEMETH DP, et al. Interleukin-1 causes CNS inflammatory cytokine expression via endothelia-microglia bi-cellular signaling. Brain Behav Immun. 2019;81:292-304. [58] LIU LR, LIU JC, BAO JS, et al. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front Immunol. 2020;11:1024. [59] GALOFRÉ M, PALAO D, VICARIO M, et al. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. J Periodontal Res. 2018;53(3):378-390. [60] CAI R, LIU Y, WANG X, et al. Influences of standardized clinical probing on peri-implant soft tissue seal in a situation of peri-implant mucositis: A histomorphometric study in dogs. J Periodontol. 2024;95(3):233-243. [61] MATSUBARA VH, FAKHRUDDIN KS, NGO H, et al. Probiotic Bifidobacteria in Managing Periodontal Disease: A Systematic Review. Int Dent J. 2023;73(1):11-20. [62] AL-HABSI N, AL-KHALILI M, HAQUE SA, et al. Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics. Nutrients. 2024; 16(22):3955. [63] FULLER R. Probiotics in human medicine. Gut. 1991;32(4):439-442. [64] ENGEVIK MA, RUAN W, ESPARZA M, et al. Immunomodulation of dendritic cells by Lactobacillus reuteri surface components and metabolites. Physiol Rep. 2021;9(2):e14719. [65] MU Q, TAVELLA VJ, LUO XM. Role of Lactobacillus reuteri in Human Health and Diseases. Front Microbiol. 2018;9:757. [66] ANTHONEY N, FOLDI I, HIDALGO A. Toll and Toll-like receptor signalling in development. Development. 2018;145(9):dev156018. [67] INVERNICI MM, FURLANETO FAC, SALVADOR SL, et al. Bifidobacterium animalis subsp lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS One. 2020;15(9): e0238425. [68] KNACKSTEDT R, KNACKSTEDT T, GATHERWRIGHT J. The role of topical probiotics on wound healing: A review of animal and human studies. Int Wound J. 2020;17(6):1687-1694. [69] OISHI Y, MANABE I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018; 30(11):511-528. [70] GALARRAGA-VINUEZA ME, OBREJA K, RAMANAUSKAITE A, et al. Macrophage polarization in peri-implantitis lesions. Clin Oral Invest. 2021;25(4):2335-2344. [71] JEONG S, KWON A, JEONG H, et al. Synergistic Immunostimulatory Activities of Probiotic Strains, Leuconostoc lactis and Weissella cibaria, and the Prebiotic Oligosaccharides They Produce. Microorganisms. 2023;11(5):1354. [72] MULLA M, MULLA M, HEGDE S, et al. In vitro assessment of the effect of probiotic lactobacillus reuteri on peri-implantitis microflora. BMC Oral Health. 2021;21(1):408. [73] MULLA M, HEGDE S, KOSHY A, et al. Effect of Probiotic Lactobacillus salivarius on Peri-Implantitis Pathogenic Bacteria: An In Vitro Study. Cureus. 2021;13(12):e20808. [74] VACCA C, CONTU MP, ROSSI C, et al. In vitro Interactions between Streptococcus intermedius and Streptococcus salivarius K12 on a Titanium Cylindrical Surface. Pathogens. 2020;9(12):1069. [75] YANG Y, SONG X, WANG G, et al. Understanding Ligilactobacillus salivarius from Probiotic Properties to Omics Technology: A Review. Foods. 2024;13(6):895. [76] PALLOS D, SOUSA V, FERES M, et al. Salivary Microbial Dysbiosis Is Associated With Peri-Implantitis: A Case-Control Study in a Brazilian Population. Front Cell Infect Microbiol. 2022;11: 696432. [77] ZHU Y, XIAO L, SHEN D, et al. Competition between yogurt probiotics and periodontal pathogens in vitro. Acta Odontol Scand. 2010;68(5):261-268. [78] GALOFRÉ M, PALAO D, VICARIO M, et al. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. J Periodontal Res. 2018;53(3):378-390. [79] KAZEMI N, KHORASGANI MR, NOORBAKHSHNIA M, et al. Protective effects of a lactobacilli mixture against Alzheimer’s disease-like pathology triggered by Porphyromonas gingivalis. Sci Rep. 2024;14(1): 27283. [80] KÕLL-KLAIS P, MÄNDAR R, LEIBUR E, et al. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20(6):354-361. [81] TADA H, MASAKI C, TSUKA S, et al. The effects of Lactobacillus reuteri probiotics combined with azithromycin on peri-implantitis: A randomized placebo-controlled study. J Prosthodont Res. 2018;62(1): 89-96. [82] ZHAN H, HE Y, WANG Q, et al. Evaluation of Probiotic Strains Isolated from Artemisia argyi Fermentation Liquor and the Antagonistic Effect of Lactiplantibacillus plantarum against Pathogens. Fermentation. 2023;9(6):536. [83] SANCA FMM, BLANCO IR, DIAS M, et al. Antimicrobial Activity of Peptides Produced by Lactococcus lactis subsp. lactis on Swine Pathogens. Animals. 2023;13(15):2442. [84] SAHIN T. Fermented foods and probiotic consumption frequency as protective indicators for peri-implant diseases – a cross-sectional study. BMC Oral Health. 2024;24(1):849. [85] 张艺庭.在种植体周围疾病治疗中应用益生菌的临床疗效的Meta分析[D].济南:山东大学,2023. [86] BUTERA A, MAIORANI C, GALLO S, et al. Evaluation of Adjuvant Systems in Non-Surgical Peri-Implant Treatment: A Literature Review. Healthcare. 2022;10(5):886. [87] LÓPEZ-VALVERDE N, LÓPEZ-VALVERDE A, BLANCO RUEDA JA. The role of probiotic therapy on clinical parameters and human immune response in peri-implant diseases: a systematic review and meta-analysis of randomized clinical studies. Front Immunol. 2024;15:1371072. [88] SAYARDOUST S, JOHANSSON A, JÖNSSON D. Do Probiotics Cause a Shift in the Microbiota of Dental Implants-A Systematic Review and Meta-Analysis. Front Cell Infect Microbiol. 2022;12:823985. [89] EFSA PANEL ON BIOLOGICAL HAZARDS (BIOHAZ), RICCI A, ALLENDE A, et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 8: suitability of taxonomic units notified to EFSA until March 2018. EFSA J. 2018; 16(7):e05315. [90] LIU X, ZHAO H, WONG A. Accounting for the health risk of probiotics. Heliyon. 2024;10(6):e27908. |
| [1] | Pan Hongfei, Zhuang Zhenbing, Xu Baiyun, Yang Zhangyang, Lin Kairui, Zhan Bingqing, Lan Jinghan, Gao Heng, Zhang Nanbo, Lin Jiayu. Inhibitory effects of different concentrations of auranofin on M1 macrophage function and its therapeutic potential in diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1390-1397. |
| [2] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [3] | Sun Yajie, Zhao Xinchen, Bo Shuangling. Spatiotemporal expression of bone morphologic protein 7 in mouse kidney development [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1156-1161. |
| [4] | Yang Zeyu, Zhi Liang, Wang Jia, Zhang Jingyi, Zhang Qingfang, Wang Yulong, Long Jianjun. A visualized analysis of research hotspots in high-frequency repetitive transcranial magnetic stimulation from the macroscopic perspective [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1320-1330. |
| [5] | Wang Mingqi, Feng Shiya, Han Yinhe, Yu Pengxin, Guo Lina, Jia Zixuan, Wang Xiuli. Construction and evaluation of a neuralized intestinal mucosal tissue engineering model in vitro [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 892-900. |
| [6] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [7] | Deng Ran, Wei Yi, Ji Xiaowei. Induction of M1/M2 polarization of macrophages by lipopolysaccharides and titanium particles in peri-implant tissues [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7415-7422. |
| [8] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [9] | Wang Wanchun, , Yi Jun, Yan Zhangren, Yang Yue, Dong Degang, Li Yumei. 717 Jiedu Decoction remodels homeostasis of extracellular matrix and promotes repair of local injured tissues in rats after Agkistrodon halys bite [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6457-6465. |
| [10] | Hu Shujuan, Liu Dang, Ding Yiting, Liu Xuan, Xia Ruohan, Wang Xianwang. Ameliorative effect of walnut oil and peanut oil on atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6482-6488. |
| [11] | Xu Zhi, Chen Yundong, Sun Yujie, Gong Xiaonan, Li Yuwan. Data of spinal osteosarcoma patients in United States based on SEER database: construction and validation of a prediction model for treatment outcomes and prognosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6583-6590. |
| [12] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei. Causal association between metabolites and sarcopenia: a big data analysis of genome-wide association studies in the European population [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6369-6380. |
| [13] | Sun Yahui, Wang Yufeng, Guo Chao, Yao Junjie, Ji Yuanyuan, Li Zhongxu, Lou Huijuan, Jiang Jinglei, Sun Yiping, Xu Jing, Cong Deyu. Effect of massage on extracellular matrix collagen deposition in skeletal muscle of type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5549-5555. |
| [14] | Zhao Zhenning, , , Li Kaiying, , , Yang Nan, , , Sun Wenjing, , , Wei Xiaoge, , , Mu Jing, Ma Huisheng, , . Regulatory mechanism of electroacupuncture on hypothalamic-pituitary-testicular axis in oligospermic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5563-5571. |
| [15] | Tian Yong, Zhou Qing, Luo Chuanquan, Hu Hongmei, Ma Changlin, Yang Lei, Wei Lin. Emodin promotes autophagy to improve myocardial injury in septic model mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5572-5578. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||