Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (4): 892-900.doi: 10.12307/2025.960
Previous Articles Next Articles
Construction and evaluation of a neuralized intestinal mucosal tissue engineering model in vitro
Wang Mingqi, Feng Shiya, Han Yinhe, Yu Pengxin, Guo Lina, Jia Zixuan, Wang Xiuli
- Dalian Medical University, Dalian 116044, Liaoning Province, China
-
Received:2024-10-10Accepted:2024-11-30Online:2026-02-08Published:2025-05-20 -
Contact:Wang Xiuli, PhD, Professor, Doctoral supervisor, Dalian Medical University, Dalian 116044, Liaoning Province, China -
About author:Wang Mingqi, PhD, Lecturer, Dalian Medical University, Dalian 116044, Liaoning Province, China -
Supported by:National Key Research & Development Program of China - Intergovernmental International Cooperation Project, No. 2019YFE0117700 (to WXL)
CLC Number:
Cite this article
Wang Mingqi, Feng Shiya, Han Yinhe, Yu Pengxin, Guo Lina, Jia Zixuan, Wang Xiuli. Construction and evaluation of a neuralized intestinal mucosal tissue engineering model in vitro[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 892-900.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
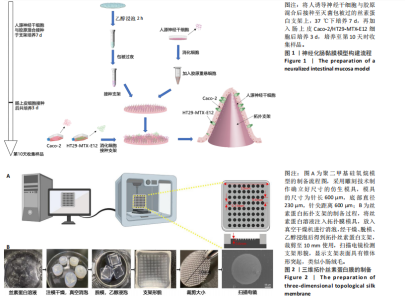
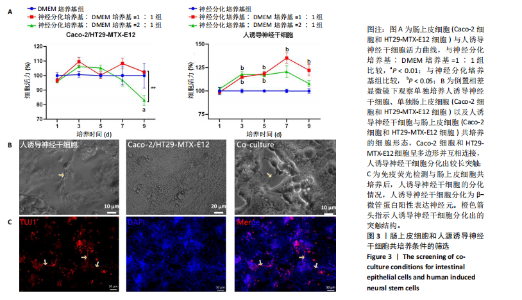
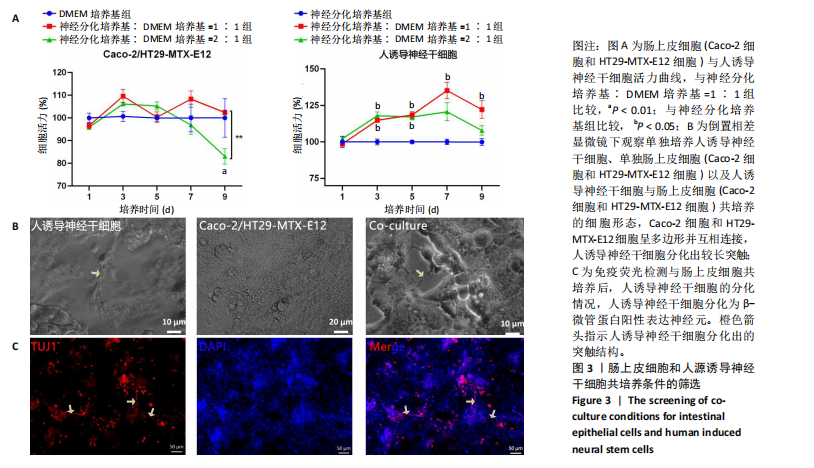
2.1 丝素蛋白支架的制备 依据人肠黏膜上皮绒毛结构和生理特性进行设计,采用聚二甲基硅氧烷制作模具,旨在模拟体内人小肠上皮的解剖形状。结合生理条件下的肠绒毛的尺寸、间距和制作工艺的精度,模具的尺寸为针长600 μm,底部直径230 μm,针尖间距600 μm。绒毛高度与绒毛宽度比约为2.6,与WANG 研究团队[14]所制作的模型比例相一致(图2A)。将可溶性丝素蛋白颗粒溶于超纯水中,均匀浇注在模具上,真空干燥箱中排出气泡、通风橱中干燥,得到具有三维结构的拓扑丝素蛋白支架。经乙醇浸泡后诱导β-折叠结构的形成,裁剪至直径为10 mm的圆形备用。扫描电镜检测拓扑丝素蛋白支架的表面形貌,支架表面具有锥体形突起,类似小肠绒毛,见图2B。 2.2 肠黏膜上皮细胞-神经干细胞共培养基的筛选 采用CCK-8法检测肠上皮细胞增殖情况,结果显示A组和B组间细胞活力无显著差异,C组培养第9天的细胞活力显著低于B组(P < 0.01);采用CCK-8法检测人诱导神经干细胞增殖情况,结果显示,E组、F组培养3,5 d的细胞活力高于D组(P < 0.05),E组培养7,9 d的细胞活力高于D组(P < 0.05),见图3A。根据CCK-8检测结果,采用神经分化培养基与DMEM培养基体积比1∶1作为共培养基,进一步共培养肠上皮细胞和人诱导神经干细胞。 倒置相差显微镜观察共培养基中各细胞随着时间而产生的形态变化,发现肠上皮细胞的形态规整,细胞伸"

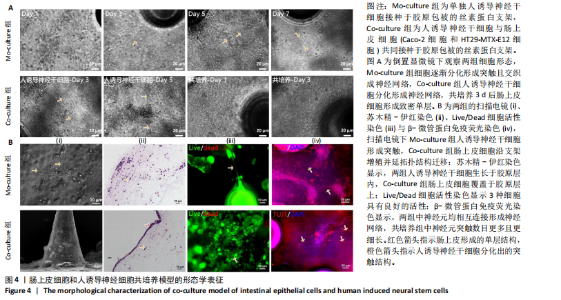
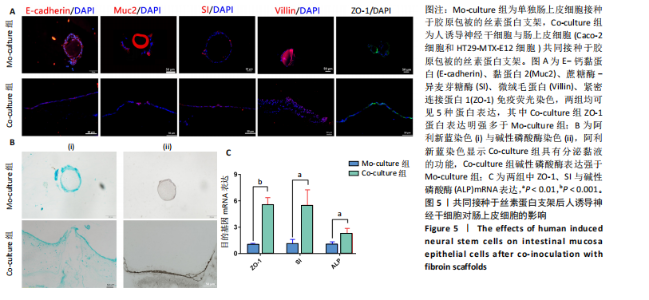
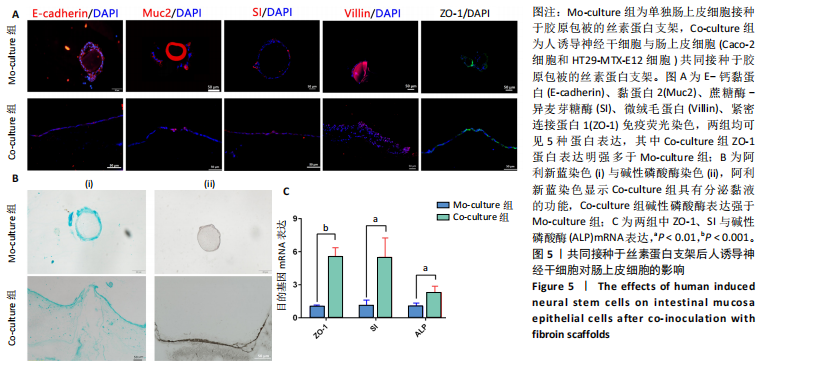
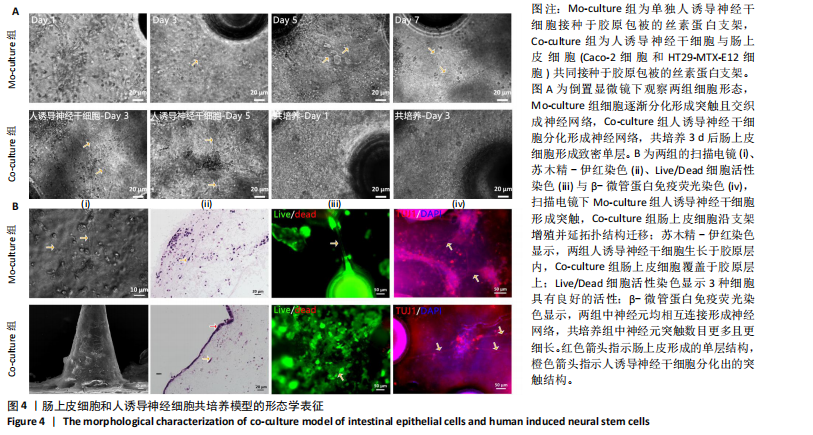
展呈多角形,培养至第3天细胞紧密排列形成铺路石状;单独培养的人诱导神经干细胞和与肠上皮细胞共培养的人诱导神经干细胞均有突触伸出,见图3B。进一步采用免疫荧光染色检测共培养基条件下,人诱导神经干细胞中β-微管蛋白表达,结果显示人诱导神经干细胞分化为β-微管蛋白阳性表达的神经元,并可见梭形或圆形的神经元胞体和细长的突触,见图3C。上述结果说明,在共培养基条件下,3种细胞生长、神经干细胞分化状态良好,最终确定共培养基为神经分化培养基与DMEM培养基等体积混合。 2.3 肠黏膜上皮-神经细胞共培养模型的形态学表征 将人诱导神经干细胞接种于支架表面,倒置相差显微镜下可见人诱导神经干细胞分化,并且在胶原内伸出突触,培养至第5天时突触逐渐变长交织形成神经网络,并且随着培养时间的增加,人诱导神经干细胞突触增多且增长(图4A)。将人诱导神经干细胞培养至第7天,加入Caco-2和HT29-MTX-E12细胞共培养3 d,可见肠上皮细胞在人诱导神经干细胞上密集排列,形成连续完整的单层(图4A)。 进一步对接种于丝素蛋白支架中的细胞进行形态学表征。扫描电镜观察结果显示,单独人诱导神经干细胞培养组和肠上皮细胞-人诱导神经干细胞共培养组中的人诱导神经干细胞形成突触,共培养组中肠上皮细胞呈鹅卵石状,沿支架增殖并延拓扑结构迁移(图4B-i)。苏木精-伊红染色显示,单独人诱导神经干细胞培养组人诱导神经干细胞生长于胶原层内,肠上皮细胞-人诱导神经干细胞共培养组中肠上皮细胞增殖形成单层覆盖于胶原层之上(图4B-ii),模拟人体生理状态下黏膜下神经丛和肠上皮细胞的解剖学位置。进一步采用Live/Dead细胞活性染色检测两组中的细胞活性,结果显示3种细胞活性良好(绿色荧光标记),少见凋亡细胞(红色荧光标记),并且人诱导神经干细胞形成细长的突触(图4B-iii)。为了进一步评估人诱导神经干细胞的分化情况,对单独人诱导神经干细胞培养组和肠上皮细胞-人诱导神经干细胞共培养组进行β-微管蛋白免疫荧光染色,结果显示两组中神经元均相互连接形成神经网络,共培养组中神经元突触数目更多且更细长(图4B-iv),提示与肠上皮细胞共培养可能促进神经元的分化。以上结果表明,实验成功构建了小肠黏膜下神经丛模型,肠上皮细胞和人诱导神经干细胞生长状态良好,并且人诱导神经干细胞可分化为β-微管蛋白阳性表达的神经元。 2.4 肠黏膜上皮-神经细胞共培养对上皮细胞的影响 为明确与人源诱导神经干细胞共培养后,肠上皮细胞功能的改变,结果见图5。 采用免疫荧光染色检测单独肠上皮细胞培养组与肠上皮细胞-人诱导神经干细胞共培养组肠上皮细胞中微绒毛蛋白、蔗糖酶-异麦芽糖酶、紧密连接蛋白1、E-钙黏蛋白以及杯状细胞的标志性黏蛋白2表达情况,见图5A。结果显示,两组均能表达微绒毛蛋白、蔗糖酶-异麦芽糖酶、紧密连接蛋白1、E-钙黏蛋白和黏蛋白,但相较于单独肠上皮细胞培养组,肠上皮细胞-人诱导神经干细胞共培养组中紧密连接蛋白1蛋白表达增加。 紧密连接蛋白1检测能够反映肠上皮细胞的屏障功能,其表达越高说明肠上皮细胞屏障功能越完整。碱性磷酸酶是一种表达于肠纹状缘的酶,可水解单磷酸盐,用于评估肠上皮细胞分化过程[15]。蔗糖酶-异麦芽糖酶为肠上皮细胞中负责将蔗糖和异麦芽糖分解为葡萄糖和麦芽糖的关键酶,该酶表达升高有助于肠上皮细胞功能成熟。RT-qPCR检测结果显示,肠上皮细胞-人诱导神经干细胞共培养组中紧密连接蛋白1、蔗糖酶-异麦芽糖酶与碱性磷酸酶mRNA表达均高于单独肠上皮细胞培养组(P < 0.001,P < 0.01,P < 0.01),见图5C。阿利新蓝染色结果显示,肠上皮细胞-人诱导神经干细胞共培养组肠上皮细胞具有分泌黏液的功能,见图5B-i。碱性磷酸酶染色结果显示,两组均具有碱性磷酸酶活性,其中肠上皮细胞-人诱导神经干细胞共培养组碱性磷酸酶表达强于单独肠上皮细胞培养组,见图5B-ii。 以上结果说明,相较于单独肠上皮细胞培养组,肠上皮细胞-人诱导神经干细胞共培养模型可以有效促进肠上皮细胞紧密连接蛋白1的表达,形成更紧密的上皮屏障,并促进肠上皮细胞黏液分泌及肠黏膜功能成熟。"
| [1] FURNESS JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286-294. [2] SASSELLI V, PACHNIS V, BURNS AJ. The enteric nervous system. Dev Biol. 2012;366(1):64-73. [3] RAO M, GERSHON MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13(9):517-528. [4] NGUYEN TT, BAUMANN P, TÜSCHER O, et al.The Aging Enteric Nervous System. Int J Mol Sci. 2023;24(11):9471. [5] ANLAUF M, SCHÄFER MK, EIDEN L, et al. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol. 2003;459(1):90-111. [6] ZAKHEM E, RAGHAVAN S, BITAR KN. Neo-innervation of a bioengineered intestinal smooth muscle construct around chitosan scaffold. Biomaterials. 2014;35(6):1882-1889. [7] WORKMAN MJ, MAHE MM, TRISNO S, et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23(1):49-59. [8] MANOUSIOUTHAKIS E, CHEN Y, CAIRNS DM, et al. Bioengineered in vitro enteric nervous system. J Tissue Eng Regen Med. 2019;13(9): 1712-1723. [9] KIM R, SUNG JH. Recent Advances in Gut- and Gut-Organ-Axis-on-a-Chip Models. Adv Healthc Mater. 2024;13(21):e2302777. [10] YILMAZ EG, HACıOSMANOĞLU N, JORDI SBU, et al. Revolutionizing IBD research with on-chip models of disease modeling and drug screening. Trends Biotechnol. 2024:S0167-7799(24)00284-1. doi: 10.1016/j.tibtech.2024.10.002. [11] RUDOLPH SE, LONGO BN, TSE MW, et al. Crypt-Villus Scaffold Architecture for Bioengineering Functional Human Intestinal Epithelium. ACS Biomater Sci Eng. 2022;8(11):4942-4955. [12] STENLING R, FREDRIKZON B, NYHLIN H, et al. Surface ultrastructure of the small intestine mucosa in healthy children and adults: a scanning electron microscopic study with some methodological aspects. Ultrastruct Pathol. 1984;6(2-3):131-140. [13] BENJAMIN J, MAKHARIA G, AHUJA V, et al. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: a randomized controlled trial. Dig Dis Sci. 2012; 57(4):1000-1012. [14] WANG Y, GUNASEKARA DB, REED MI, et al. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials. 2017;128:44-55. [15] SHIN J, CARR A, CORNER GA, et al. The intestinal epithelial cell differentiation marker intestinal alkaline phosphatase (ALPi) is selectively induced by histone deacetylase inhibitors (HDACi) in colon cancer cells in a Kruppel-like factor 5 (KLF5)-dependent manner. J Biol Chem. 2014;289(36):25306-25316. [16] CHEN WLK, SUTER E, MIYAZAKI H, et al. Synergistic Action of Diclofenac with Endotoxin-Mediated Inflammation Exacerbates Intestinal Injury in Vitro. ACS Infect Dis. 2021;7(4):838-848. [17] ZHANG X, CHEN X, WANG Z, et al. Goblet cell-associated antigen passage: A gatekeeper of the intestinal immune system. Immunology. 2023;170(1):1-12. [18] YANG S, CAI J, SU Q, et al. Human milk oligosaccharides combine with Bifidobacterium longum to form the “golden shield” of the infant intestine: metabolic strategies, health effects, and mechanisms of action. Gut Microbes. 2024;16(1):2430418. [19] ALLAIRE JM, CROWLEY SM, LAW HT, et al. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018; 39(9):677-696. [20] GAO P, MORITA N, SHINKURA R. Role of mucosal IgA antibodies as novel therapies to enhance mucosal barriers. Semin Immunopathol. 2024;47(1):1. [21] TULLIE L, JONES BC, DE COPPI P, et al. Building gut from scratch - progress and update of intestinal tissue engineering. Nat Rev Gastroenterol Hepatol. 2022;19(7):417-431. [22] YANG S, LI Y, ZHANG Y, et al. Impact of chronic stress on intestinal mucosal immunity in colorectal cancer progression. Cytokine Growth Factor Rev. 2024;80:24-36. [23] CHEESEMAN BL, ZHANG D, BINDER BJ, et al. Cell lineage tracing in the developing enteric nervous system: superstars revealed by experiment and simulation. J R Soc Interface. 2014;11(93):20130815. [24] HAN W, TELLEZ LA, PERKINS MH, et al. A Neural Circuit for Gut-Induced Reward. Cell. 2018;175(3):665-678.e23. [25] SCHNEIDER KM, BLANK N, ALVAREZ Y, et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023;186(13):2823-2838.e20. [26] PUZAN M, HOSIC S, GHIO C, et al. Enteric Nervous System Regulation of Intestinal Stem Cell Differentiation and Epithelial Monolayer Function. Sci Rep. 2018;8(1):6313. [27] SIWCZAK F, LOFFET E, KAMINSKA M, et al. Intestinal Stem Cell-on-Chip to Study Human Host-Microbiota Interaction. Front Immunol. 2021; 12:798552. [28] BAYER F, DREMOVA O, KHUU MP, et al. The Interplay between Nutrition, Innate Immunity, and the Commensal Microbiota in Adaptive Intestinal Morphogenesis. Nutrients. 2021;13(7):2198. [29] CHEN Y, RUDOLPH SE, LONGO BN, et al. Bioengineered 3D Tissue Model of Intestine Epithelium with Oxygen Gradients to Sustain Human Gut Microbiome. Adv Healthc Mater. 2022;11(16):e2200447. [30] CARDENAS D, BHALCHANDRA S, LAMISERE H, et al. Two- and Three-Dimensional Bioengineered Human Intestinal Tissue Models for Cryptosporidium. Methods Mol Biol. 2020;2052:373-402. [31] BAGHDADI MB, AYYAZ A, COQUENLORGE S, et al. Enteric glial cell heterogeneity regulates intestinal stem cell niches. Cell Stem Cell. 2022;29(1):86-100.e6. [32] WALLRAPP A, YANG D, CHIU IM. Enteric glial cells mediate gut immunity and repair. Trends Neurosci. 2022;45(4):251-253. [33] NAGY N, GOLDSTEIN AM. Enteric nervous system development: A crest cell’s journey from neural tube to colon. Semin Cell Dev Biol. 2017;66:94-106. [34] MERAN L, TULLIE L, EATON S, et al. Bioengineering human intestinal mucosal grafts using patient-derived organoids, fibroblasts and scaffolds. Nat Protoc. 2023;18(1):108-135. [35] POLING HM, SUNDARAM N, FISHER GW, et al. Human pluripotent stem cell-derived organoids repair damaged bowel in vivo. Cell Stem Cell. 2024;31(10):1513-1523.e7. [36] RAKHILIN N, BARTH B, CHOI J, et al. Simultaneous optical and electrical in vivo analysis of the enteric nervous system. Nat Commun. 2016;7:11800. [37] GOTO K, KATO G, KAWAHARA I, et al. In vivo imaging of enteric neurogenesis in the deep tissue of mouse small intestine. PLoS One. 2013;8(1):e54814. [38] GRUBIŠIĆ V, MCCLAIN JL, FRIED DE, et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020;32(10):108100. [39] STAKENBORG M, ABDURAHIMAN S, DE SIMONE V, et al. Enteric glial cells favor accumulation of anti-inflammatory macrophages during the resolution of muscularis inflammation. Mucosal Immunol. 2022;15(6):1296-1308. [40] LLORENTE C. The Imperative for Innovative Enteric Nervous System-Intestinal Organoid Co-Culture Models: Transforming GI Disease Modeling and Treatment. Cells. 2024;13(10):820. [41] LIN X, GAUDINO SJ, JANG KK, et al.IL-17RA-signaling in Lgr5(+) intestinal stem cells induces expression of transcription factor ATOH1 to promote secretory cell lineage commitment. Immunity. 2022;55(2):237-253.e8. |
| [1] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [2] | Pan Hongfei, Zhuang Zhenbing, Xu Baiyun, Yang Zhangyang, Lin Kairui, Zhan Bingqing, Lan Jinghan, Gao Heng, Zhang Nanbo, Lin Jiayu. Inhibitory effects of different concentrations of auranofin on M1 macrophage function and its therapeutic potential in diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1390-1397. |
| [3] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [4] | Sun Yajie, Zhao Xinchen, Bo Shuangling. Spatiotemporal expression of bone morphologic protein 7 in mouse kidney development [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1156-1161. |
| [5] | Yang Zeyu, Zhi Liang, Wang Jia, Zhang Jingyi, Zhang Qingfang, Wang Yulong, Long Jianjun. A visualized analysis of research hotspots in high-frequency repetitive transcranial magnetic stimulation from the macroscopic perspective [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1320-1330. |
| [6] | Wang Jie, Huang Rui, Zhang Ye, Shou Zhaoxi, Yao Jie, Liu Chenxi, Liao Jian. Role and mechanism of probiotics in peri-implantitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 901-907. |
| [7] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [8] | Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan. Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 936-948. |
| [9] | Yang Hu, Zheng Yu, Jia Chengming, Wang Tong, Zhang Guangfei, Ji Yaoyao. Immune microenvironment regulates bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 701-710. |
| [10] | Gu Jianmei, Yuan Kunshan, Zhou Qiang, Zhang Haijun, , . Application of laser microporous decellularized scaffolds in tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 499-507. |
| [11] | Yang Fengli, Zhou Chao, Xiong Wei, Zhou Yuxiang, Li Dengshun, Wang Xin, Li Zhanzhen. 3D printed poly-L-lactic acid bone scaffolds in repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 507-515. |
| [12] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [13] | Yan Qiquan, Yang Libin, Li Mengjun, Ni Yazhuo, Chen Keying, Xu Bo, Li Yaoyang, Ma Shiqing, Li Rui, Li Jianwen. Preparation and antibacterial properties of porcine small intestinal submucosal composite nanohydroxyapatite bioscaffold loaded with antimicrobial peptide KR-12-a5 [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 384-394. |
| [14] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [15] | Wang Zhuo, Sun Panpan, Cheng Huanzhi, Cao Tingting. Application of chitosan in repair and regeneration of oral hard and soft tissues [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 459-468. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||