Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (24): 5171-5178.doi: 10.12307/2025.724
Previous Articles Next Articles
Role of Siblings protein family in cardiovascular diseases
Chen Jinjie1, Li Geng1, Jiang Yefan2
- 1Department of Heart and Great Vessels Surgery, Wuhan Union Hospital, Wuhan 430022, Hubei Province, China; 2Department of Heart and Great Vessels Surgery, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China
-
Received:2024-08-13Accepted:2024-10-22Online:2025-08-28Published:2025-01-24 -
Contact:Li Geng, Doctoral candidate, Attending physician, Department of Heart and Great Vessels Surgery, Wuhan Union Hospital, Wuhan 430022, Hubei Province, China -
About author:Chen Jinjie, Doctoral candidate, Attending physician, Department of Heart and Great Vessels Surgery, Wuhan Union Hospital, Wuhan 430022, Hubei Province, China -
Supported by:the National Natural Science Foundation of China, No. 8170020715
CLC Number:
Cite this article
Chen Jinjie, Li Geng, Jiang Yefan. Role of Siblings protein family in cardiovascular diseases[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5171-5178.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
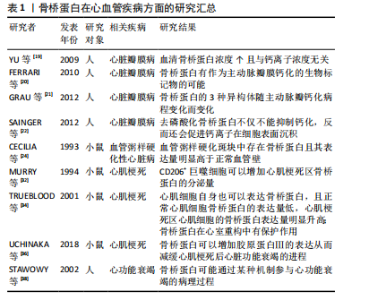
2.1 骨桥蛋白在心血管疾病方面的研究进展 骨桥蛋白也称为骨唾液酸蛋白,分子质量为34 kD[14],是一种分泌型 Siblings家族蛋白,也是Siblings蛋白家族中研究最为广泛及深入的蛋白,可经过O-和N-糖基化、硫酸化、磷酸化以及转谷氨酰胺进行不同的翻译后修饰。现阶段被公认为是一个抑制钙化结节形成的蛋白分子[15-16],可以辅助破骨细胞向骨组织迁移[4],C端的RGD肽使其可以与多种细胞表面的整合素受体结合,具有介导细胞增殖[4]、迁移、黏附等作用[14],在靠近RGD肽段的地方还存在着凝血酶切割靶点,骨桥蛋白可被凝血酶切割成含有RGD肽段的N端和含有肝素结合位点的N端[17]。凝血酶切割之后,骨桥蛋白通过RGD肽与整合素受体结合的能力明显增强[18]。骨桥蛋白可以在如上皮、肾、骨和牙齿等的各种组织中表达,也可以在包括血液和母乳在内的所有体液中检测出。 以往认为骨桥蛋白的主要作用是参与骨形成,近年来发现其在心血管系统特别是血管重塑过程中发挥重要调节作用,见表1。"

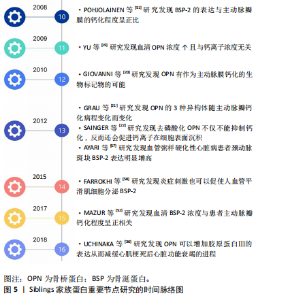
2.1.1 心脏瓣膜病 在心脏瓣膜钙化疾病中,现阶段认为病理发展过程为:①在机械力学、炎症等刺激下内皮细胞发生损伤,巨噬细胞、T淋巴细胞等炎症细胞和低密度脂蛋白从内皮细胞的间隙中进入瓣膜内部;②低密度脂蛋白氧化为氧化低密度脂蛋白,巨噬细胞吞噬低密度脂蛋白形成泡沫细胞,促使粥样斑块形成,炎症细胞外基质分泌肿瘤坏死因子α、转化生长因子β、白细胞介素1β加速瓣膜钙化形成;③基质金属蛋白酶及金属蛋白酶抑制剂共同作用下,使得瓣膜增厚、硬化;④钙化结节形成。 现阶段,在科研中骨桥蛋白常作为瓣膜钙化的指标,在钙化瓣膜中骨桥蛋白可代偿性增高。2009年YU等[19]第一次证明了在主动脉瓣钙化及狭窄患者血清中骨桥蛋白浓度升高了1.5倍,并且与血清钙离子浓度没有明显的关系,相似的结论在2010年被FERRARI等[20]再次证明,提示骨桥蛋白有作为主动脉瓣膜钙化的生物标记物的可能。2012年GRAU等[21]进行了更加细致的实验,发现了骨桥蛋白的3种异构体(分别为OPN-a、OPN-b及OPN-c)在主动脉瓣钙化病程中的变化,证明了OPN-a在病程初期达到峰值,在主动脉瓣严重钙化狭窄后期表达下降;OPN-b和OPN-c在病程全程持续升高,在瓣膜钙化后期表达达到峰值。2012年SAINGER等[22]发现主动脉瓣钙化患者血清中以去磷酸化骨桥蛋白为主,而正常人血清中则以磷酸化骨桥蛋白为主,并且发现去磷酸化骨桥蛋白不具有抑制钙化的作用,反而会促进钙离子在细胞表面沉积。骨桥蛋白除了具有作为主动脉瓣膜钙化生物标志物的潜力之外,还有可能通过其不同异构体的比值预测病程长短,还有预测主动脉瓣钙化狭窄患者行经导管主动脉瓣置入术后预后的作用,有研究指出经导管主动脉瓣置入术前患者血清骨桥蛋白较高的情况下,术后患者心脏事件发生概率会明显增加[23]。但目前尚缺乏有关骨桥蛋白对主动脉瓣膜钙化过程影响的相关研究。 2.1.2 动脉粥样硬化性疾病 骨桥蛋白首次于1993年被CECILIA在小鼠模型的血管粥样硬化斑块中发现并且其表达量明显高于正常血管壁[24]。后续有更多的研究集中探讨骨桥蛋白在血管粥样硬化/钙化病程中的作用。 现阶段公认的血管粥样硬化病理过程为:①血管内皮细胞受到刺激后,细胞表面增加对炎性细胞的黏附而促使炎性细胞浸润,形成泡沫细胞;②血管中间层的血管平滑肌细胞向内膜迁移、增殖并合成分泌细胞外基质大分子,且血管平滑肌细胞及巨噬细胞发生凋亡、死亡,参与斑块本身及其帽子形成[25];③血栓在斑块破损处形成[26]。而骨桥蛋白在其中各个部分均有参与。 首先,在动物实验中发现损伤的血管壁中骨桥蛋白表达明显高于未损伤的血管壁[24]。骨桥蛋白在血管受损状态下可以作为炎症介导因子,具有趋化巨噬细胞的能力[27],提示骨桥蛋白很有可能参与了血管粥样硬化的初始步骤。其次,骨桥蛋白在体外实验中被证实可以通过结合整合素受体αvβ3参与内皮细胞的迁移,而且在血栓酶剪切后骨桥蛋白促进细胞迁移的能力明显增强[28]。骨桥蛋白同时还具有促进粥样硬化斑块形成的能力,可以促进巨噬细胞的浸润而达到增加斑块稳定性的作用[29]。最后,研究表明骨桥蛋白可以通过αvβ3受体参与促进新生血管形成[30]。在血管钙化过程中,骨桥蛋白扮演了抑制剂的角色,减缓了血管钙化的进程[31]。 2.1.3 心肌梗死及心力衰竭 骨桥蛋白首次于1994年被MURRY报道在心肌梗死区由巨噬细胞分泌表达[32],后续有相关研究指出主要是CD206+巨噬细胞增加骨桥蛋白的分泌量[33]。2001年TRUEBLOOD等[34]发现心肌细胞自身也可以表达骨桥蛋白,而且正常心肌细胞骨桥蛋白的表达量低,而心肌梗死区心肌细胞的骨桥蛋白表达量明显升高[35]。在心肌梗死的1-3 d 骨桥蛋白表达量升高,在4-14 d时骨桥蛋白表达量则开始下降[17]。 心肌梗死后出现的持续的、不可逆的心肌重构是造成心力衰竭的主要原因,Trueblood等[34]发现OPN-/-小鼠心肌梗死后心室腔明显增大,提示骨桥蛋白在心室重构中有保护作用。2018年UCHINAKA等[36]通过进一步研究发现骨桥蛋白可以增加胶原蛋白Ⅲ的表达从而减缓心肌梗死后心力衰竭的进程。 体外实验发现骨桥蛋白在肥大心肌组织中表达明显增高[37]。2002年Stawowy等[38]通过心肌活检的方法检测了10例扩张型心肌病患者,发现心衰患者的心肌组织中骨桥蛋白表达明显升高,提示骨桥蛋白可能通过某种机制参与了心功能衰竭的病理过程。 心肌梗死后发生的炎症反应是诱导心肌细胞凋亡、心肌纤维化,促进心室重塑发生的主要原因之一。炎症反应促进心脏组织各种细胞中骨桥蛋白表达上调,从而破坏基质金属蛋白酶与金属蛋白酶抑制剂之间的平衡,影响细胞外基质的改变,引起左心室重塑,进一步发展为心力衰竭。另外,高表达的骨桥蛋白通过赖氨酰氧化酶参与Ⅰ型胶原的合成并促进其相互交联形成不溶性胶原蛋白,进而使左心室僵硬度增高,左心室机械性能及功能减低,最后演变成心力衰竭。 心室重构主要由心肌肥大、心脏成纤维细胞增殖和心肌纤维化引起。心肌梗死后可导致肾素-血管紧张素-醛固酮系统的过度激活,可导致心肌梗死后心室重构,进而导致心力衰竭的发生。在心肌纤维化的过程中,一氧化氮可以有效阻止这一过程[39],在小鼠的体内实验中发现,骨桥蛋白可以抑制一氧化氮合酶的活性从而加速心肌纤维化进程[40]。同时,骨桥蛋白也参与了血管紧张素Ⅱ/醛固酮系统的调节,肾素-血管紧张素-醛固酮系统的过度激活可导致血管紧张素Ⅱ水平明显升高,通过ERK和P38MAPK信号途径促进骨桥蛋白的表达,进一步促进心肌梗死后炎症反应及细胞外基质增多,参与心室重塑进程[41]。 在此之外,骨桥蛋白在体内被证实可以募集T细胞而造成慢性心肌病,引起心肌损伤,导致扩张型心肌病从而引起心力衰竭[42]。 2.1.4 动脉夹层 骨桥蛋白在胸主动脉夹层组织中表达升高,且血管平滑肌细胞表型发生改变[43]。骨桥蛋白可以促使血管平滑肌细胞表型从可收缩表型转变为合成表型[44]。 升高的骨桥蛋白可以刺激基质金属蛋白酶2的表达[45],从而参与主动脉夹层的病理过程。 2.2 骨涎蛋白2在心血管疾病方面的研究 骨涎蛋白2,分子质量32-33 kD,N端具有多聚谷氨酸肽段提供羟基磷灰石的结合位点[46-47],其位于136号位点的色氨酸磷酸化被认为是其促进羟基磷灰石形成的关键步骤[48],C端的RGD肽使其可以与细胞表面的整合素受体结合,可促进细胞间相互连接[47]。见表2[49-58]。"

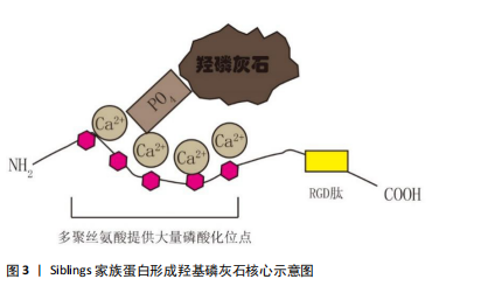
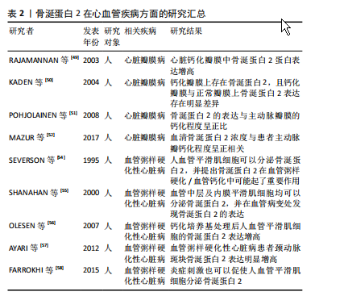
2.2.1 心脏瓣膜疾病 2003年首次报道骨涎蛋白2在心脏瓣膜钙化疾病中的作用,Rajamannan等[49]检测了22例主动脉钙化瓣膜,并与20例正常瓣膜比较,结果显示钙化瓣膜骨涎蛋白2蛋白表达增高。在2004年,Kaden等[50]通过免疫组化方法在16例钙化瓣膜上发现了骨涎蛋白2的存在,并证明钙化瓣膜与正常瓣膜上骨涎蛋白2表达存在明显差异。2008年Pohjolainen等[51]在113例正常及不同程度钙化的主动脉瓣膜中通过qPCR及免疫荧光证实了骨涎蛋白2的存在,而且证实了骨涎蛋白2的表达与主动脉瓣膜的钙化程度呈正比。Mazur等[52]在20例患者中检测到血清骨涎蛋白2浓度与患者主动脉瓣钙化程度呈正相关,提示血清骨涎蛋白2有成为主动脉瓣膜钙化生物标志物的潜力。现阶段已有骨涎蛋白2促进间充质干细胞成骨化分析的相关研究[53],并有相关报道提出骨涎蛋白2可以作为羟基磷灰石形成的核心及启动点[47]。但现阶段仍缺少骨涎蛋白2在主动脉瓣膜钙化中作用的研究,这里是未来研究主动脉瓣膜钙化的潜在位点。 2.2.2 血管粥样硬化性疾病 Severson等[54]于1995年首先通过体外实验证明了人血管平滑肌细胞可以分泌骨涎蛋白2,并提出骨涎蛋白2在血管粥样硬化/血管钙化中可能起了重要作用。2000年Shanahan等[55]证明了血管中层及内膜平滑肌细胞均可以分泌骨涎蛋白2,并在血管病变处发现骨涎蛋白2的表达。2007年Olesen等[56]发现钙化培养基处理后人血管平滑肌细胞的骨涎蛋白2表达增高。2012年Ayari等[57]通过微阵列分析检测了人颈动脉斑块,证明骨涎蛋白2表达明显增高。2017年Farrokhi等[58]发现了氧化低密度脂蛋白可以促使人血管平滑肌细胞骨涎蛋白2表达增加,表明了炎症刺激也可以促使人血管平滑肌细胞分泌骨涎蛋白2,并且提出RUNX2为骨涎蛋白2的上游,RUNX2升高可以介导骨涎蛋白2表达升高。可见,炎症、内环境紊乱等因素可促使人血管平滑肌细胞的钙化相关基因表达增加,其中RUNX2介导骨涎蛋白2表达升高,骨涎蛋白2因为其本身羟基磷灰石形成的“启动子”的身份,可促使血管粥样硬化/血管钙化的形成。 2.3 牙本质基质蛋白在心血管疾病的研究 现大多数研究认为牙本质基质蛋白为前体蛋白,在相关蛋白酶作用下被水解成37 kD大小的N端片段及57 kD大小的C端片段后发挥作用[11],57 kD大小的片段在钙化过程中起了决定性作用。C端片段在磷酸化后可扮演羟基磷灰石核心的角色[59],且该片段具有RGD肽段,可参与细胞黏附、迁移等过程。现阶段,牙本质基质蛋白的研究主要集中于牙齿及骨骼的形成。有研究表明,牙本质基质蛋白缺失的小鼠在软骨形成过程中存在障碍[14],提示了牙本质基质蛋白在骨化过程中起了重要作用。 牙本质基质蛋白在心血管疾病中的研究十分有限,只有少量研究指出在钙化培养基或低密度脂蛋白处理下人血管平滑肌细胞分泌的牙本质基质蛋白增加[60-61],但牙本质基质蛋白在骨化中的关键性角色、其“羟基磷灰石核心”的角色、其与骨涎蛋白2的同源性,表明了牙本质基质蛋白在瓣膜钙化疾病、动脉粥样硬化中可能扮演了关键作用。 2.4 基质细胞外磷酸糖蛋白及牙本质涎磷蛋白在心血管疾病中的研究 基质细胞外磷酸糖蛋白被认为是一个骨化过程的抑制蛋白,在基质细胞外磷酸糖蛋白过表达小鼠中骨重建过程减弱[62];而牙本质涎磷蛋白被认为是一个前体蛋白,在金属蛋白酶水解下分解为3个肽段,分别为牙本质磷蛋白(DPP)、牙本质涎蛋白(DSP)、牙本质糖蛋白(DGP)[14],其中牙本质磷蛋白具有很强的钙离子吸附力,且其含有的RGD肽可介导其与细胞黏附[63]。但现阶段并没有有关这两个蛋白在心血管疾病中的研究。 2.5 Siblings家族蛋白促进钙化的途径 2.5.1 蛋白本身结合Ca2+的特性 Siblings家族蛋白的N端处有多聚丝氨酸形成的大量磷酸化位点,使得蛋白呈酸性,这样使得其具有结合Ca2+的能力[11],Ca2+可与PO4继续结合,如此延展下去便形成了羟磷灰石核心并继续扩展下去[2](如图3)。"

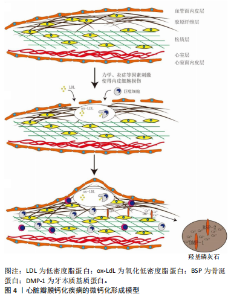
2.5.2 蛋白中的RGD肽段的作用 Siblings家族蛋白在其末端均有RGD肽段,RGD肽段可与整合素受体、CD44受体结合,参与细胞的迁移、黏附、钙化过程。RGD肽段可能通过以下几种方式促进细胞钙化:①Siblings家族蛋白可以通过RGD肽段与整合素αvβ3受体结合,激活MAPK通路刺激ERK1/2及P38发生磷酸化,促使下游RUNX2表达升高,从而促使细胞成骨样分化[1]。②Siblings家族蛋白的N端黏附于细胞外基质,通过RGD肽段与细胞整合素受体结合,最后通过LINC(细胞骨架及核骨架结连接子)传递到核内,引起染色体解螺旋从而调控相关基因表达[64-65]。也有实验表明力学刺激传递到整合素受体后是通过调控RasErk1/2,刺激Erk磷酸化后调控RUNX2表达上升,而使得细胞成骨样分化[66-67]。③Siblings家族蛋白中的骨桥蛋白可以促使巨噬细胞向M2型分化[68-69],而M2型巨噬细胞分泌的转化生长因子β增加[70-71],转化生长因子β可以促使瓣膜间质细胞成骨样分化[72-73]。而且骨桥蛋白有趋化巨噬细胞的作用[74-75]。所以,Siblings家族蛋白极有可能在趋化巨噬细胞、促使巨噬细胞极化后通过巨噬细胞介导钙化。 结合现在比较公认的心脏瓣膜钙化模型及各个文献中Siblings家族蛋白在成骨方面的作用,提出心脏瓣膜的微钙化形成模型(图4)。①完整的心脏瓣膜在力学、炎症的刺激下,内皮细胞损伤,巨噬细胞等炎症细胞、低密度脂蛋白浸入瓣膜,开始形成泡沫细胞、粥样硬化斑块[76]。②巨噬细胞等炎症细胞分泌大量炎症因子,如肿瘤坏死因子α、转化生长因子β等,其促使瓣膜间质细胞RUNX2表达升高,RUNX2可介导骨涎蛋白2、牙本质基质蛋白表达升高,打破Siblings家族蛋白成骨与抑制成骨的平衡。③骨涎蛋白2、牙本质基质蛋白通过RGD肽段结合瓣膜间质细胞,促进其RUNX2表达进一步升高,形成正反馈循环;骨涎蛋白2、牙本质基质蛋白自身结合Ca2+、"

| [1] HASSAN T, QIU Y, HASAN MR, et al. Effects of Dentin Phosphophoryn-Derived RGD Peptides on the Differentiation and Mineralization of Human Dental Pulp Stem Cells In Vitro. Biomedicines. 2022;10(11): 2781. [2] PAN B, CHENG X, TAN W, et al. Pan-cancer analysis shows that IBSP is a potential prognostic and immunotherapeutic biomarker for multiple cancer types including osteosarcoma. Front Immunol. 2023; 14:1188256. [3] ZHU Y, WANG Y, ZHANG S, et al. Association of polymicrobial interactions with dental caries development and prevention. Front Microbiol. 2023;14:1162380. [4] BARKAS GI, KOTSIOU OS. The Role of Osteopontin in Respiratory Health and Disease. J Pers Med. 2023;13(8):1259. [5] BAI RJ, LI YS, ZHANG FJ. Osteopontin, a bridge links osteoarthritis and osteoporosis. Front Endocrinol (Lausanne). 2022;13:1012508. [6] CHEN Y, QIN Y, DAI M, et al. IBSP, a potential recurrence biomarker, promotes the progression of colorectal cancer via Fyn/β-catenin signaling pathway. Cancer Med. 2021;10(12):4030-4045. [7] FISHER LW, FEDARKO NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44 Suppl 1:33-40. [8] CHEN Y, PETHÖ A, GANAPATHY A, et al. DPP promotes odontogenic differentiation of DPSCs through NF-κB signaling. Sci Rep. 2021;11(1): 22076. [9] GAO W, LIU D, SUN H, et al. SPP1 is a prognostic related biomarker and correlated with tumor-infiltrating immune cells in ovarian cancer. BMC Cancer. 2022;22(1):1367. [10] COURBON G, KENTRUP D, THOMAS JJ, et al. FGF23 directly inhibits osteoprogenitor differentiation in Dmp1-knockout mice. JCI Insight. 2023;8(24):e156850. [11] WANG Y, LYU J, QIAN X, et al. Involvement of Dmp1 in the Precise Regulation of Hair Bundle Formation in the Developing Cochlea. Biology (Basel). 2023;12(4):625. [12] KIM JH, IRFAN M, HOSSAIN MA, et al. LPS-induced inflammation potentiates dental pulp stem cell odontogenic differentiation through C5aR and p38. Connect Tissue Res. 2023;64(5):505-515. [13] TERASAKA K, GOHBARA M, ABE T, et al. Association between evolocumab use and slow progression of aortic valve stenosis. Heart Vessels. 2024. [14] SCHURMAN CA, KAYA S, DOLE N, et al. Aging impairs the osteocytic regulation of collagen integrity and bone quality. Bone Res. 2024; 12(1):13. [15] AIERKEN Y, HE H, LI R, et al. Inhibition of Slc39a14/Slc39a8 reduce vascular calcification via alleviating iron overload induced ferroptosis in vascular smooth muscle cells. Cardiovasc Diabetol. 2024;23(1):186. [16] ALKAISSI H, MCFARLANE SI. Hyperhomocysteinemia and Accelerated Aging: The Pathogenic Role of Increased Homocysteine in Atherosclerosis, Osteoporosis, and Neurodegeneration. Cureus. 2023;15(7):e42259. [17] TANG Z, XIA Z, WANG X, et al. The critical role of osteopontin (OPN) in fibrotic diseases. Cytokine Growth Factor Rev. 202;74:86-99. [18] ZHAO L, LEUNG LL. MORSER J. Methods to Investigate Thrombin Cleavage of Osteopontin (OPN). Methods Mol Biol. 2024;2747:95-117. [19] YU PJ, SKOLNICK A, FERRARI G, et al. Correlation between plasma osteopontin levels and aortic valve calcification: potential insights into the pathogenesis of aortic valve calcification and stenosis. J Thorac Cardiovasc Surg. 2009;138(1):196-199. [20] FERRARI G, SAINGER R, BECKMANN E, et al. Validation of plasma biomarkers in degenerative calcific aortic stenosis. J Surg Res. 2010; 163(1):12-17. [21] GRAU JB, POGGIO P, SAINGER R, et al. Analysis of osteopontin levels for the identification of asymptomatic patients with calcific aortic valve disease. Ann Thorac Surg. 2012;93(1):79-86. [22] SAINGER R, GRAU JB, POGGIO P, et al. Dephosphorylation of circulating human osteopontin correlates with severe valvular calcification in patients with calcific aortic valve disease. Biomarkers. 2012;17(2):111-118. [23] PATEL M, RODRIGUEZ D, YOUSEFI K, et al. Osteopontin and LDLR Are Upregulated in Hearts of Sudden Cardiac Death Victims With Heart Failure With Preserved Ejection Fraction and Diabetes Mellitus. Front Cardiovasc Med. 2020;7:610282. [24] PERVAIZ N, KATHURIA I, AITHABATHULA RV, et al. Matricellular proteins in atherosclerosis development. Matrix Biol. 2023;120:1-23. [25] YIN Z, ZHANG J, SHEN Z, et al. Regulated vascular smooth muscle cell death in vascular diseases. Cell Prolif. 2024:e13688. doi: 10.1111/cpr.13688. [26] JASPAN VN, GREENBERG GS, PARIHAR S, et al. The Role of Sleep in Cardiovascular Disease. Curr Atheroscler Rep. 2024;26(7):249-262. [27] HUANG K, CHEN S, YU LJ, et al. Serum secreted phosphoprotein 1 level is associated with plaque vulnerability in patients with coronary artery disease. Front Immunol. 2024;15:1285813. [28] HARK C, CHEN J, BLÖCK J, et al. RGD-coated polymeric microbubbles promote ultrasound-mediated drug delivery in an inflamed endothelium-pericyte co-culture model of the blood-brain barrier. Drug Deliv Transl Res. 2024 Mar 18. doi: 10.1007/s13346-024-01561-6. [29] KADOGLOU NPE, KHATTAB E, VELIDAKIS N, et al. The Role of Osteopontin in Atherosclerosis and Its Clinical Manifestations (Atherosclerotic Cardiovascular Diseases)-A Narrative Review. Biomedicines. 2023;11(12):3178. [30] ANAGNOSTIS P, FLORENTIN M, LIVADAS S, et al. Bone Health in Patients with Dyslipidemias: An Underestimated Aspect. Int J Mol Sci. 2022;23(3):1639. [31] HAO N, YONG H, ZHANG F, et al. Aortic calcification accelerates cardiac dysfunction via inducing apoptosis of cardiomyocytes. Int J Med Sci. 2024;21(2):306-318. [32] MURRY CE, GIACHELLI CM, SCHWARTZ SM, et al. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol. 1994;145(6):1450-1462. [33] SHIRAKAWA K, ENDO J, KATAOKA M, et al. IL (Interleukin)-10-STAT3-Galectin-3 Axis Is Essential for Osteopontin-Producing Reparative Macrophage Polarization After Myocardial Infarction. Circulation. 2018;138(18):2021-2035. [34] TRUEBLOOD NA, XIE Z, COMMUNAL C, et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001;88(10):1080-1087. [35] FERTALA J, WANG ML, RIVLIN M, et al. Extracellular Targets to Reduce Excessive Scarring in Response to Tissue Injury. Biomolecules. 2023;13(5):758. [36] UCHINAKA A, YOSHIDA M, TANAKA K, et al. Overexpression of collagen type III in injured myocardium prevents cardiac systolic dysfunction by changing the balance of collagen distribution. J Thorac Cardiovasc Surg. 2018;156(1):217-226.e3. [37] SHIRAKAWA K, SANO M. Osteopontin in Cardiovascular Diseases. Biomolecules. 2021;11(7):1047. [38] STAWOWY P, BLASCHKE F, PFAUTSCH P, et al. Increased myocardial expression of osteopontin in patients with advanced heart failure. Eur J Heart Fail. 2002;4(2):139-146. [39] ALQUDAH M, HALE TM, CZUBRYT MP. Targeting the renin-angiotensin-aldosterone system in fibrosis. Matrix Biol. 2020;91-92:92-108. [40] FASTRÈS A, ROELS E, TUTUNARU AC, et al. Osteopontin and fibronectin in lung tissue, serum, and bronchoalveolar lavage fluid of dogs with idiopathic pulmonary fibrosis and control dogs. J Vet Intern Med. 2023;37(6):2468-2477. [41] WALSH-WILKINSON É, DROLET MC, LE HOUILLIER C, et al. Sex differences in the response to angiotensin II receptor blockade in a rat model of eccentric cardiac hypertrophy. PeerJ. 2019;7:e7461. [42] CLAEYS L, ZHYTNIK L, WISSE LE, et al. Exploration of the skeletal phenotype of the Col1a1+/Mov13 mouse model for haploinsufficient osteogenesis imperfecta type 1. Front Endocrinol (Lausanne). 2023; 14:1145125. [43] GONG Y, LI T, LIU Q, et al. Analysis of differential metabolites in serum metabolomics of patients with aortic dissection. BMC Cardiovasc Disord. 2024;24(1):226. [44] ROMBOUTS KB, VAN MERRIENBOER TAR, KET JCF, et al. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. Eur J Clin Invest. 2022;52(4):e13697. [45] FAN F, ZHOU Q, XU Z, et al. Osteopontin in the Pathogenesis of Aortic Dissection by the Enhancement of MMP Expressions. Int Heart J. 2019;60(2):429-435. [46] WEST N, CHAPPLE I, CULSHAW S, et al. EFP workshop participants and methodological consultant. BSP Implementation of Prevention and Treatment of Peri-implant Diseases - The EFP S3 Level Clinical Practice Guideline. J Dent. 2024;149:104980. [47] MA Y, CHEN B, ZHANG B, et al. High expression of integrin-binding sialoprotein (IBSP) is associated with poor prognosis of osteosarcoma. Aging (Albany NY). 2023;16(1):28-42. [48] CHAVEZ MB, TAN MH, KOLLI TN, et al. Functional defects in cementoblasts with disrupted bone sialoprotein functional domains, in vitro. Bone. 2024;179:116961. [49] RAJAMANNAN NM, SUBRAMANIAM M, RICKARD D, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107(17):2181-2184. [50] KADEN JJ, BICKELHAUPT S, GROBHOLZ R, et al. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis. 2004;13(4):560-566. [51] POHJOLAINEN V, TASKINEN P, SOINI Y, et al. Noncollagenous bone matrix proteins as a part of calcific aortic valve disease regulation. Hum Pathol. 2008;39(11):1695-1701. [52] MAZUR P, WYPASEK E, GAWĘDA B, et al. Stenotic Bicuspid and Tricuspid Aortic Valves-Micro-Computed Tomography and Biological Indices of Calcification. Circ J. 2017;81(7):1043-1050. [53] KRIEGEL A, SCHLOSSER C, HABECK T, et al. Bone Sialoprotein Immobilized in Collagen Type I Enhances Bone Regeneration In vitro and In vivo. Int J Bioprint. 2022;8(3):591. [54] SEVERSON AR, INGRAM RT, FITZPATRICK LA. Matrix proteins associated with bone calcification are present in human vascular smooth muscle cells grown in vitro. In Vitro Cell Dev Biol Anim. 1995;31(11):853-857. [55] SHANAHAN CM, PROUDFOOT D, TYSON KL, et al. Expression of mineralisation-regulating proteins in association with human vascular calcification. Z Kardiol. 2000;89 Suppl 2:63-68. [56] OLESEN P, NGUYEN K, WOGENSEN L, et al. Calcification of human vascular smooth muscle cells: associations with osteoprotegerin expression and acceleration by high-dose insulin. Am J Physiol Heart Circ Physiol. 2007;292(2):H1058-1064. [57] AYARI H, BRICCA G. Microarray analysis reveals overexpression of IBSP in human carotid plaques. Adv Med Sci. 2012;57(2):334-340. [58] FARROKHI E, SAMANI KG, CHALESHTORI MH. Oxidized low-density lipoprotein increases bone sialoprotein expression in vascular smooth muscle cells via runt-related transcription factor 2. Am J Med Sci. 2015;349(3):240-243. [59] BUETTMANN EG, YONEDA S, HU P, et al. Postnatal Osterix but not DMP1 lineage cells significantly contribute to intramembranous ossification in three preclinical models of bone injury. Front Physiol. 2023;13:1083301. [60] CHELLAN B, ROJAS E, ZHANG C, et al. Editorial Expression of Concern: Enzyme-modified non-oxidized LDL (ELDL) induces human coronary artery smooth muscle cell transformation to a migratory and osteoblast-like phenotype. Sci Rep. 2020;10(1):11050. [61] GONZÁLEZ-SALVATIERRA S, GARCÍA-FONTANA C, LACAL J, et al. Cardioprotective function of sclerostin by reducing calcium deposition, proliferation, and apoptosis in human vascular smooth muscle cells. Cardiovasc Diabetol. 2023;22(1):301. [62] DONMEZ BO, KARAGUR ER, DONMEZ AC, et al. Calcium‑dependent activation of PHEX, MEPE and DMP1 in osteocytes. Mol Med Rep. 2022;26(6):359. [63] CHUANG SF, CHEN YH, MA PX, et al. Dentin Sialoprotein/Phosphophoryn (DSP/PP) as Bio-Inductive Materials for Direct Pulp Capping. Polymers (Basel). 2022;14(17):3656. [64] YOUNESI FS, MILLER AE, BARKER TH, et al. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat Rev Mol Cell Biol. 2024 Apr 8. doi: 10.1038/s41580-024-00716-0. [65] KUSCHMAN HP, PALCZEWSKI MB, THOMAS DD. Nitric oxide and hydrogen sulfide: Sibling rivalry in the family of epigenetic regulators. Free Radic Biol Med. 2021;170:34-43. [66] LIU Z, WANG Q, ZHANG J, et al. The Mechanotransduction Signaling Pathways in the Regulation of Osteogenesis. Int J Mol Sci. 2023;24(18): 14326. [67] KOMORI T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int J Mol Sci. 2019;20(7):1694. [68] SILVER SV, POPOVICS P. The Multifaceted Role of Osteopontin in Prostate Pathologies. Biomedicines. 2023;11(11):2895. [69] VIIAYKUMAR A, DYRKACZ P, VIDOVIC-ZDRILIC I, et al. Expression of BSP-GFPtpz Transgene during Osteogenesis and Reparative Dentinogenesis. J Dent Res. 2020;99(1):89-97. [70] LIU Y, ZHONG Y, ZHENG B, et al. Extracellular vesicles derived from M1 macrophages enhance rat midpalatal suture expansion by promoting initial bone turnover and inflammation. Prog Orthod. 2023;24(1):34. [71] AKKELLE BS, VOLKAN B, TUTAR E, et al. Characteristics of Siblings With Celiac Disease Diagnosed by Family Screening. Indian Pediatr. 2022;59(11):867-870. [72] PHADWAL K, TAN X, KOO E, et al. Metformin ameliorates valve interstitial cell calcification by promoting autophagic flux. Sci Rep. 2023;13(1):21435. [73] GROUND M, WAQANIVAVALAGI S, PARK YE, et al. Fibroblast growth factor 2 inhibits myofibroblastic activation of valvular interstitial cells. PLoS One. 2022;17(6):e0270227. [74] WEI J, MARISETTY A, SCHRAND B, et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019;129(1):137-149. [75] ZHANG X, SHU Q, LIU Z, et al. Recombinant osteopontin provides protection for cerebral infarction by inhibiting the NLRP3 inflammasome in microglia. Brain Res. 2021;1751:147170. [76] SHI B, LI H, HE X. Advancing lifelong precision medicine for cardiovascular diseases through gut microbiota modulation. Gut Microbes. 2024;16(1):2323237. |
| [1] | Ding Yu, Chen Jingwen, Chen Xiuyan, Shi Huimin, Yang Yudie, Zhou Meiqi, Cui Shuai, . Circulating inflammatory proteins and myocardial hypertrophy: large sample analysis of European populations from GWAS Catalog and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1047-1057. |
| [2] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei, Zhou Jiatan. Based on Mendelian randomization, the causal relationship between 1400 metabolites and sarcopenia and the correlation analysis of cardiovascular disease were investigated [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-11. |
| [3] | E Zhengkang, Xin Hongwei, Yu Qingbo, Zhang Yunshuai. miR-192-5p targets CKIP-1 to promote osteogenic differentiation of bone marrow mesenchymal stem cells in osteoporosis patients [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2641-2647. |
| [4] | Li Xiupeng, Su Yuying, Wang Yuetong, Peng Liang, Wang Yida, Jing Wen. Effect of low-volume high-intensity interval training on cardiovascular risk factor in obese or overweight populations: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(12): 2590-2604. |
| [5] | Li Xiafei, Zhao Lingling, Liang Feng, Zhang Xuewei, Zhang Jinjin, Lin Fei, Yang Tuo, Zhao Liang. Preparation of heparinized acellular vascular scaffold and hemocompatibility evaluation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2631-2636. |
| [6] | Han Weiyu, Chen Yuanxing, Huang Youyang, Liu Weiwei, Zhao Yongchao, Zhao Ranzun. Regulation of cardiovascular diseases by histone deacetylation modification [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5707-5713. |
| [7] | Zheng Yi, Wei Zhoudan, Guo Dong, Shi Ziqing, Niu Weiran, Xu Zhuoyu, Li Pengcui, Li Wenjin. Changes in periapical tissue of type 2 diabetic mice during tooth occlusal elongation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5196-5202. |
| [8] | Wang Nanfeng, Shen Shengmei, Zhang Peisheng, Teng Wei. Effects of hydrogels loaded with hepatocyte growth factor on myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4802-4808. |
| [9] | Peng Fengli, Li Chaofu, Shi Bei. Application of the nanovesicle delivery system in cardiovascular diseases [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4862-4868. |
| [10] | Jiang Honghui, Kong Yuanyuan, Liu Jing, Wang Zhihong. Preparation and applications of decellularized extracellular matrix bioink in cardiovascular fields [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4904-4911. |
| [11] | Wei Qin, Amanguli·Ruze, Chen Bingxin, Zhao Ling, Zhao Banghao, Jiang Tao, Zhang Chun, Li Zhiqiang, Gao Xiaoming, Duan Mingjun. Relaxin protects myocardial microvascular endothelial cells from hypoxia-reoxygenation injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(28): 4519-4524. |
| [12] | Luo Wenhao, Feng Le, Huang Haixia, Liu Min, Wang Pin. Effect of glycogen synthase kinase-3 beta inhibitor Tideglusib on proliferation and osteogenic differentiation of rat bone marrow mesenchymal stem cells under high glucose microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(19): 2968-2974. |
| [13] | Guo Tingting, Cui Chaoqiang, Liu Baokun, Gu Hao, Zhou Dong. Application of endovascular steerable catheter system in interventional surgery [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(16): 2609-2615. |
| [14] | Feng Zhanpeng, Jiang Yan, Feng Xuesong. Establishment of the equation for calculating peak oxygen pulse rate of young people [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(29): 4693-4698. |
| [15] | Liao Jun, Xu Pu. Effect of nanonized freshwater pearl powder on the expression of osteogenic related genes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4325-4329. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||