Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (34): 7301-7309.doi: 10.12307/2025.889
Previous Articles Next Articles
Inhibition of hypertrophic scar in rats by beta-sitosterol-laden mesoporous silica nanoparticles
Zhang Fei1, Zuo Jun2
- 1Department of Plastic Surgery, First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha 410007, Hunan Province, China; 2Department of Plastic Surgery and Cosmetology, Affiliated Nanhua Hospital to University of South China, Hengyang 421000, Hunan Province, China
-
Received:2024-08-21Accepted:2024-10-11Online:2025-12-08Published:2025-01-17 -
Contact:Zuo Jun, MD, Associate chief physician, Department of Plastic Surgery and Cosmetology, Affiliated Nanhua Hospital to University of South China, Hengyang 421000, Hunan Province, China -
About author:Zhang Fei, MS, Attending physician, Department of Plastic Surgery, First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha 410007, Hunan Province, Chin -
Supported by:Hunan Natural Science Youth Fund Project, No. 2021JJ40487 (to ZJ)
CLC Number:
Cite this article
Zhang Fei, Zuo Jun. Inhibition of hypertrophic scar in rats by beta-sitosterol-laden mesoporous silica nanoparticles[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7301-7309.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
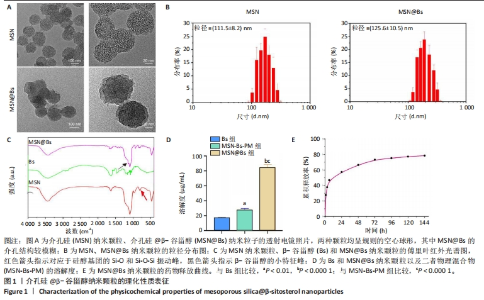
2.1 介孔硅@β-谷甾醇纳米颗粒的理化表征结果 透射电镜下可见两种纳米颗粒均呈规则的空心球形,其中介孔硅@β-谷甾醇纳米颗粒的平均粒径略有增大,并且介孔结构相比介孔硅纳米颗粒模糊,见图1A。介孔硅纳米颗粒和介孔硅@β-谷甾醇纳米颗粒的平均粒径分别为(111.5±8.2) nm和(125.6±10.5) nm,见图1B。 傅里叶红外光谱显示,介孔硅纳米颗粒在750 cm-1和1 000 cm-1处出现峰值,对应于硅醇基团的Si-O和Si-O-Si振动(红色箭头);而β-谷甾醇的振动峰在1 000-1 250 cm-1之间,当β-谷甾醇负载在介孔硅纳米粒中时其特征峰大部分消失,而在介孔硅@β-谷甾醇纳米颗粒中也可看到介孔硅纳米颗粒中没有的小特征峰(黑色箭头),但强度很低,见图1C,以上结果提示β-谷甾醇被不完全包封。 饱和水溶性实验结果显示,介孔硅@β-谷甾醇纳米颗粒的溶解度较游离β-谷甾醇增强近5倍[(17.2±0.7),(84.8±3.9) μg/mL,P < 0.000 1],也明显高于β-谷甾醇和介孔硅纳米颗粒的物理混合物[(27.4±2.3) μg/mL,P < 0.000 1],见图1D。介孔硅@β-谷甾醇纳米颗粒增加了β-谷甾醇的溶解速率,这可能与β-谷甾醇在介孔硅纳米颗粒孔隙内的无定形状态,以及纳米颗粒提供更高的表面积导致β-谷甾醇更快的扩散和释放有关。 介孔硅@β-谷甾醇纳米颗粒中β-谷甾醇在前6 h内呈线性释放趋势,累积释放量约为48.6%,之后缓慢释放长达6 d,见图1E。"

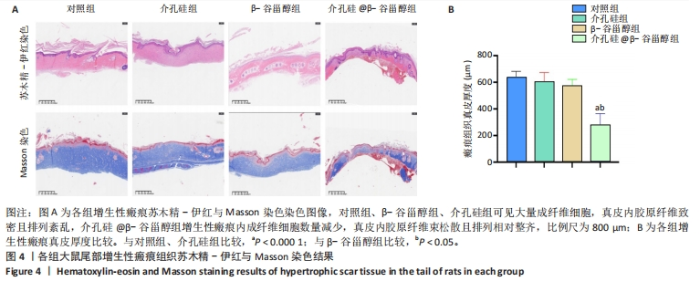
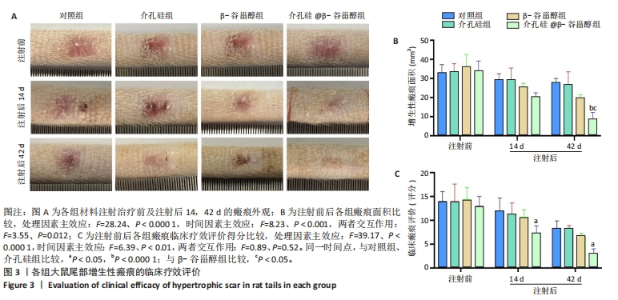
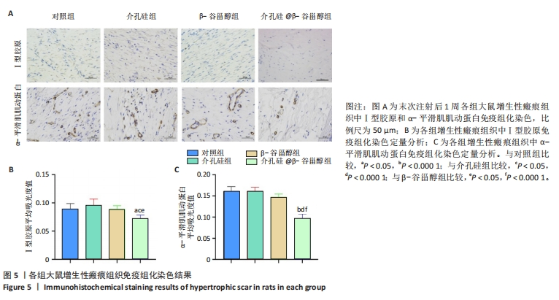
注射前,各组大鼠初始增生性瘢痕面积和临床评分比较差异均无显著性意义(P > 0.05)。注射后14 d,各组大鼠瘢痕面积比较差异无显著性意义(P > 0.05);注射后42 d,介孔硅@β-谷甾醇组大鼠瘢痕面积明显小于其他3组(P < 0.05,P < 0.000 1)。介孔硅@β-谷甾醇组注射后14,42 d的瘢痕评分低于对照组、介孔硅组(P > 0.05),其余组间瘢痕评分比较差异均无显著性意义(P > 0.05)。 2.2.4 各组大鼠瘢痕组织学观察结果 末次注射后1周,苏木精-伊红与Masson染色结果显示:对照组、β-谷甾醇组、介孔硅组增生性瘢痕内仍可见大量成纤维细胞,真皮厚度分别为(635.60±46.33),(574.10±47.65),(603.60±69.09) μm,真皮内胶原纤维致密,均呈多极、螺旋和结节状紊乱地排列;介孔硅@β-谷甾醇组增生性瘢痕内成纤维细胞数量减少,真皮厚度降低至(279.50±84.54) μm,胶原纤维束松散且存在间隙,排列相对整齐、方向规则,见图4。"

| [1] LYNAM EC, XIE Y, DAWSON R, et al. Severe hypoxia and malnutrition collectively contribute to scar fibroblast inhibition and cell apoptosis. Wound Repair Regen. 2015;23(5):664-671. [2] FENG Y, WU JJ, SUN ZL, et al. Targeted apoptosis of myofibroblasts by elesclomol inhibits hypertrophic scar formation. EBioMedicine. 2020;54:102715. [3] CHEN H, XU K, SUN C, et al. Inhibition of ANGPT2 activates autophagy during hypertrophic scar formation via PI3K/AKT/mTOR pathway. An Bras Dermatol. 2023;98(1):26-35. [4] ZHANG J, SONG F, WANG XQ. Role of autosis of fibroblasts in Hyper tropic Scar regression. J Shanghai Jiaotong Univ (Sci). 2022;42(1):44-50. [5] LEVINE B, KROEMER G. Biological Functions of Autophagy Genes:A Disease Perspective. Cell. 2019;176(1-2):11-42. [6] CHEN D, LI Q, ZHANG H, et al. Traditional Chinese medicine for hypertrophic scars-A review of the therapeutic methods and potential effects. Front Pharmacol. 2022;13:1025602. [7] 左俊,马少林.β-谷甾醇对增生性瘢痕成纤维细胞作用机制的网络药理学分析[J].中国组织工程研究,2024,28(2):216-223. [8] KHAN Z, NATH N, RAUF A, et al. Multifunctional roles and pharmacological potential of β-sitosterol: emerging evidence toward clinical applications. Chem Biol Interact. 2022;365:110117. [9] PANPIPAT W, CHAIJAN M, GUO Z. Oxidative stability of margarine enriched with different structures of β-sitosteryl esters during storage. Food Biosci. 2018;22:78-84. [10] JIA C, XIA X, WANG H, et al. Preparation of phytosteryl ornithine ester hydrochlo- ride and improvement of its bioaccessibility and cholesterol-reducing activity in vitro. Food Chem. 2020;331:127200. [11] MOHAMMADI M, JAFARI SM, HAMISHE- HKAR H, et al. Phytosterols as the core or stabilizing agent in different nanocarriers. Trends Food Sci Technol. 2020;101:73-88. [12] ZHU W, DONG Y, XU P, et al. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022;154:212-230. [13] KASIRZADEH S, GHAHREMANI MH, SETAYESH N, et al. β-sitosterol alters the inflammatory response in CLP rat model of sepsis by modulation of NFκB signaling. Biomed Res Int. 2021;2021:5535562. [14] HJELLESTAD M, STRAND LI, EIDE GE, et al. Clinimetric properties of a. translated and culturally adapted Norwegian version of the Patient and Observer Scar Assessment Scale for use in clinical practice and research. Burns. 2021;47(4):953-960. [15] MENASHE S, HELLER L. Keloid and Hypertrophic Scars Treatment. Aesthetic Plast Surg. 2024;48(13):2553-2560. [16] WANG H, WANG Z, ZHANG Z, et al. β-Sitosterol as a Promising Anticancer Agent for Chemoprevention. and Chemotherapy: Mechanisms of Action and Future Prospects. Adv Nutr. 2023;14(5): 1085-1110. [17] KHAN Z, NATH N, RAUF A, et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem Biol Interact. 2022;365:110117. [18] OKAGU OD, ABIOYE RO, UDENIGWE CC. Molecular Interaction of Pea Glutelin and Lipophilic Bioactive Compounds: Structure-Binding Relationship and Nano-/Microcomplexation. J Agric Food Chem. 2023;71(12):4957-4969. [19] SHISHIR MRI, XIE LH, SUN CD, et al. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci Technol. 2018;78: 34-60. [20] YUE X, CHEN X, LI H, et al. Nano Ag/Co3O4 Catalyzed Rapid Decomposition of Robinia pseudoacacia Bark for Production Biofuels and Biochemicals. Polymers (Basel). 2022;15(1):114. [21] LI D, SONG C, ZHANG J, et al.Targeted delivery and apoptosis induction activity of peptide-transferrin targeted mesoporous silica encapsulated resveratrol in MCF-7 cells. J Pharm Pharmacol. 2023;75(1):49-56. [22] LIN M, YAO W, XIAO Y, et al. Resveratrol-modified mesoporous silica nanoparticle for tumor-targeted therapy of gastric cancer. Bioengineered. 2021;12(1):6343-6353. [23] DONG J, CHEN F, YAO Y, et al. Bioactive mesoporous silica nanoparticle-functionalized titanium implants with controllable antimicrobial peptide release potentiate the regulation of inflammation and osseointegration. Biomaterials. 2024;305:122465. [24] ZHOU S, WANG W, ZHOU S, et al. A Novel Model for Cutaneous Wound Healing and Scarring in the Rat. Plast Reconstr Surg. 2019;143(2): 468-477. [25] LI M, WANG P, LI J, et al. NRP1 transduces mechanical stress inhibition via LATS1/YAP in hypertrophic scars. Cell Death Discov. 2023;9(1):341. [26] WANG P, PENG Z, YU L, et al. Verteporfin-Loaded Bioadhesive Nanoparticles for the Prevention of Hypertrophic Scar. Small Methods. 2024;8(8):e2301295. [27] RAMOS ML, GRAGNANI A, FERREIRA LM. Is there an ideal animal model to study hypertrophic scarring. J Burn Care Res. 2008;29:363-368. [28] BARONE N, SAFRAN T, VORSTENBOSCH J, et al. Current Advances in Hypertrophic Scar and Keloid Management. Semin Plast Surg. 2021;35(3):145-152. [29] LI Q, ZHANG B, LU J, et al. SNHG1 functions as a ceRNA in hypertrophic scar fibroblast proliferation and apoptosis through miR-320b/CTNNB1 axis. Arch Dermatol Res. 2023;315(6):1593-1601. [30] SHI J, XIAO H, LI J, et al. Wild-type p53-modulated autophagy and autophagic fibroblast apoptosis inhibit hypertrophic scar formation. Lab Invest. 2018;98(11):1423-1437. [31] YU Z, LI Y, FU R, et al. Platycodin D inhibits the proliferation and migration of hypertrophic scar-derived fibroblasts and promotes apoptosis through a caspase-dependent pathway. Arch Dermatol Res. 2023;315(5):1257-1267. [32] LU H, JIA C, WU D, et al. Fibroblast growth factor 21 alleviates senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the SIRT1-mTOR signaling pathway. Cell Death Dis. 2021;12(10):865. [33] DONG Y, CAO X, HUANG J, et al. Melatonin inhibits fibroblast cell functions and hypertrophic scar formation by enhancing autophagy through the MT2 receptor-inhibited PI3K/Akt /mTOR signaling. Biochim Biophys Acta Mol Basis Dis. 2024;1870(1):166887. [34] CHEN H, XU K, SUN C, et al. Inhibition of ANGPT2 activates autophagy during hypertrophic scar formation via PI3K/AKT/mTOR pathway. An Bras Dermatol. 2023;98(1):26-35. [35] ZHOU L, LIU Z, CHEN S, et al. Transcription factor EBmediated autophagy promotes dermal fibroblast differentiation and collagen production by regulating endoplasmic reticulum stress and autophagydependent secretion. Int J Mol Med. 2021;47(2):547-560. [36] DENG X, ZHAO F, ZHAO D, et al. Oxymatrine promotes hypertrophic scar repair through reduced human scar fibroblast viability, collagen and induced apoptosis via autophagy inhibition. Int Wound J. 2022; 19(5):1221-1231. [37] XU X, JIANG R, CHEN M, et al. Puerarin decreases collagen secretion in angII-induced atrial fibroblasts through inhibiting autophagy via the JNK–Akt–mTOR signaling pathway. J Cardiovasc Pharmacol. 2019; 73(6):373-382. [38] MATEI AE, CHEN CW, KIESEWETTER L, et al. Vascularised human skin equivalents as a novel in vitro model of skin fibrosis and platform for testing of antifibrotic drugs. Ann Rheum Dis. 2019;78(12):1686-1692. [39] LI Y, XIAO Y, HAN Y, et al. Blocking the MIR155HG/miR-155 axis reduces CTGF-induced inflammatory cytokine production and α-SMA expression via upregulating AZGP1 in hypertrophic scar fibroblasts. Cell Signal. 2024;120:111202. [40] DONG Y, CAO X, HUANG J, et al. Melatonin inhibits fibroblast cell functions and hypertrophic scar formation by enhancing autophagy through the MT2 receptor-inhibited PI3K/Akt /mTOR signaling. Biochim Biophys Acta Mol Basis Dis. 2024;1870(1):166887. [41] YAN M, XIE Y, YAO J, et al. The Dual-Mode Transition of Myofibroblasts Derived from Hepatic Stellate Cells in Liver Fibrosis. Int J Mol Sci. 2023;24(20):15460. [42] SAMPAIO LP, HILGERT GSL, SHIJU TM, et al. Topical Losartan and Corticosteroid Additively Inhibit Corneal Stromal Myofibroblast Generation and Scarring Fibrosis After Alkali Burn Injury. Transl Vis Sci Technol. 2022;11(7):9. [43] DING H, CHEN J, QIN J, et al. TGF-β-induced α-SMA expression is mediated by C/EBPβ acetylation in human alveolar epithelial cells. Mol Med. 2021;27(1):22. [44] LI M, SU Y, GAO X, et al. Transition of autophagy and apoptosis in fibroblasts depends on dominant expRession of HIF-1α or p53. J Zhejiang Univ Sci B. 2022;23(3):204-217. [45] ZUO J, MA S. Resveratrol-laden mesoporous silica nanoparticles regulate the autophagy and apoptosis via ROS-mediated p38-MAPK/HIF-1a /p53 signaling in hypertrophic scar fibroblasts. Heliyon. 2024; 10(4):e24985. |
| [1] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [2] | Jia Jinwen, Airefate·Ainiwaer, Zhang Juan. Effects of EP300 on autophagy and apoptosis related to allergic rhinitis in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1439-1449. |
| [3] | You Huijuan, Wu Shuzhen, Rong Rong, Chen Liyuan, Zhao Yuqing, Wang Qinglu, Ou Xiaowei, Yang Fengying. Macrophage autophagy in lung diseases: two-sided effects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1516-1526. |
| [4] | Liu Kexin, , Hao Kaimin, Zhuang Wenyue, , Li Zhengyi. Autophagy-related gene expression in pulmonary fibrosis models: bioinformatic analysis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1129-1138. |
| [5] | Hu Jing, Zhu Ling, Xie Juan, Kong Deying, Liu Doudou. Autophagy regulates early embryonic development in mice via affecting H3K4me3 modification [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1147-1155. |
| [6] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [7] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Advance in the mechanisms underlying miRNAs in steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1207-1214. |
| [8] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [9] | Bao Zhuoma, Hou Ziming, Jiang Lu, Li Weiyi, Zhang Zongxing, Liu Daozhong, Yuan Lin. Effect and mechanism by which Pterocarya hupehensis skan total flavonoids regulates the proliferation, migration and apoptosis of fibroblast-like synoviocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 816-823. |
| [10] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Mechanism by which vascular endothelial growth factor A targets regulation of angiogenesis in the treatment of steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 671-679. |
| [11] | Chen Ling, Mao Qiuhua, Xu Pu, Zhang Wenbo. Effect of water-soluble matrix of nano-pearl powder on proliferation, migration and apoptosis of mouse fibroblasts#br# [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 338-344. |
| [12] | Dong Chao, Zhao Mohan, Liu Yunan, Yang Zeli, Chen Leqin, Wang Lanfang. Effects of magnetic nano-drug carriers on exercise-induced muscle injury and inflammatory response in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 345-353. |
| [13] | Wang Yuhang, Zhang Han, Zhang Chaojing, Kou Xurong, Jing Tongtong, Lin Rimei, Liu Xinyu, Lou Shilei, Yan Hui, Sun Cong. Curcumin extraction and preparation and optimization of curcumin nanoparticles [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 362-374. |
| [14] | Xie Peisen, Guan Zhenpeng, Wei Xianjie, Zhang Keshi, Kang Qingyuan, Xiao Wentao, Guo Xiaoshuai. Cerium dioxide nanoparticles regulate expression of inflammatory factors in M1 macrophages and affect fibroblast co-culture system [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 375-383. |
| [15] | Li Congcong, Wufanbieke·Baheti, Zhao Li, Chen Xiaotao, Kong Chuifan, Yu Min. Physicochemical properties and biocompatibility of hydroxyapatite/graphene oxide/interleukin-4 composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 404-413. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||