Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (2): 362-374.doi: 10.12307/2025.497
Previous Articles Next Articles
Curcumin extraction and preparation and optimization of curcumin nanoparticles
Wang Yuhang1, Zhang Han1, Zhang Chaojing1, Kou Xurong1, Jing Tongtong1, Lin Rimei1, Liu Xinyu1, Lou Shilei2, Yan Hui2, Sun Cong2
- 1College of Pharmacy, 2College of Clinical Medicine, Changchun University of Chinese Medicine, Changchun 130117, Jilin Province, China
-
Received:2024-07-06Accepted:2024-09-05Online:2026-01-18Published:2025-06-17 -
Contact:Sun Cong, MD, Professor, Master’s supervisor, College of Clinical Medicine, Changchun University of Chinese Medicine, Changchun 130117, Jilin Province, China -
About author:Wang Yuhang, Master candidate, College of Pharmacy, Changchun University of Chinese Medicine, Changchun 130117, Jilin Province, China -
Supported by:Jilin Natural Science Foundation Project, No. YDZJ202401060ZYTS (to SC); Jilin Provincial Department of Education Scientific Research Project, No. JJKH20230949KJ (to YH)
CLC Number:
Cite this article
Wang Yuhang, Zhang Han, Zhang Chaojing, Kou Xurong, Jing Tongtong, Lin Rimei, Liu Xinyu, Lou Shilei, Yan Hui, Sun Cong. Curcumin extraction and preparation and optimization of curcumin nanoparticles[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 362-374.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
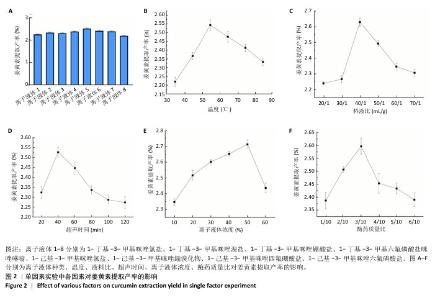
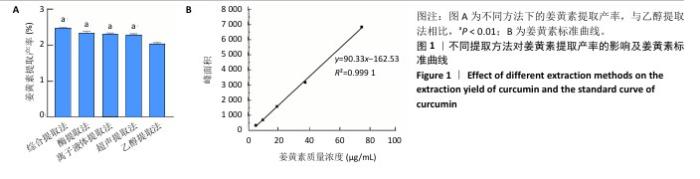
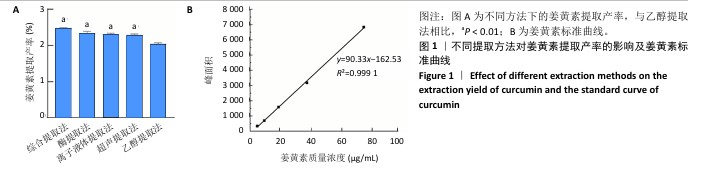
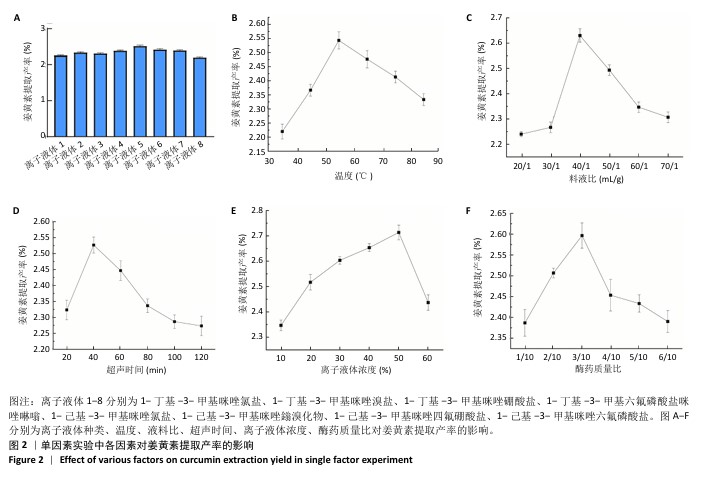
2.2 姜黄素提取工艺的单因素实验结果 如图2所示,随着各个因素值的增加,姜黄素提取产率均呈现先上升后下降的趋势;其中,在离子液体为1-己基-3-甲基咪唑氯盐、反应温度55 ℃、液料比为40 mL/g、超声时间为40 min、离子液体浓度50%、酶药质量比为3∶10时,姜黄素提取产率达最大。因此,确定最佳提取溶剂为离子液体5(1-己基-3-甲基咪唑氯盐)、最佳温度55 ℃、最佳液料比为40 mL/g进行后续实验,选择超声时间20,40,60 min,离子液体浓度40%,50%,60%,酶药质量比为2∶10,3∶10,4∶10进行后续响应面优化设计实验。 升高温度加速了姜黄素分子的热运动,使得姜黄素溶解度增大,但过高的温度可能会破坏姜黄素结构的稳定性。随着液料比的增加,在具有充足液体相的体系中更有利于姜黄素的传递,但液料比过大会因体系中存在过多液体而不利于姜黄素的提取。延长超声时间会使姜黄素提取更完全,但超声时间过长可能会使更多的姜黄素受热分解。增加离子液体浓度会加强提取溶剂提取姜黄素的能力,但过高的离子液体浓度会使体系内液体黏度增大,不利于溶质的传递。增加酶药质量比能使提取体系破坏细胞壁的能力增强,有助于溶质的流出,但酶药质量比的过度增加会导致体系内副产物增加[39]。 "
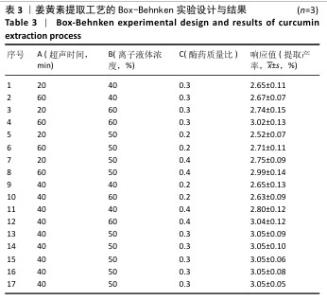
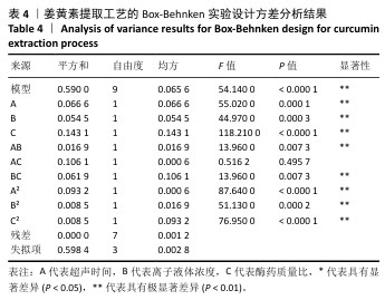
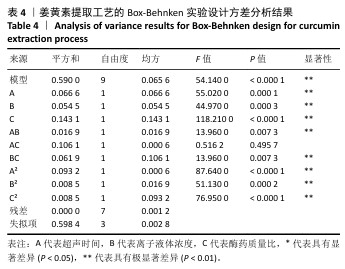
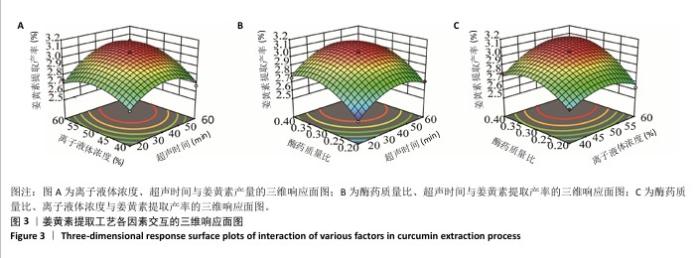
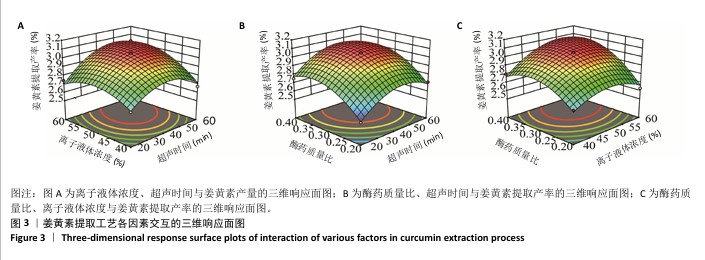
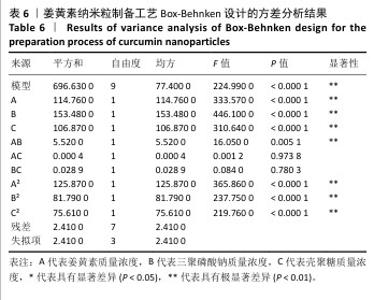
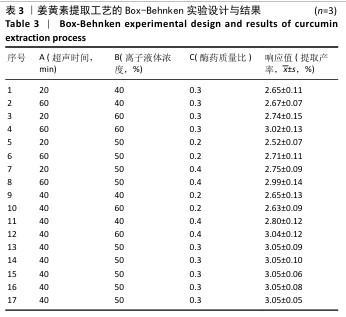
2.3 姜黄素提取工艺的Box-Behnken响应面实验结果 分析表3实验数据可知,拟合的回归模型拟合效果较好,可用于分析实验结果,回归方程为:Y(%)=3.05+0.091 2A+0.082 5B+0.133 8C+0.065 0AB+0.012 5AC+0.065 0BC-0.158 7A2-0.121 2B2-0.148 7C2。 对表3数据进行多项拟合回归分析,A(超声时间)、B(离子液体5浓度)、C(酶药质量比)3个因素对响应值(Y)的交互作用关系较好(表4,图3)。经分析可知,离子液体联合超声辅助酶法提取姜黄素的最优提取参数为超声时间57 min、离子液体5浓度57%、酶药质量比3.5∶10,最优提取工艺响应值为3.107 8%。"
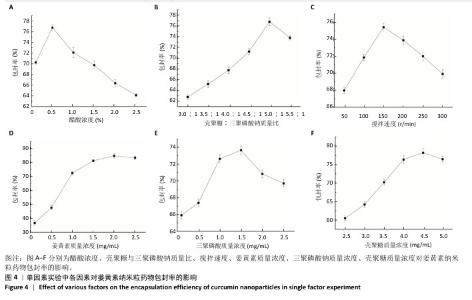
2.4 姜黄素提取最佳工艺参数验证结果 通过响应面实验且结合实际,校正最优提取参数为超声时间57 min、离子液体5(1-己基-3-甲基咪唑氯盐)浓度57%、酶药质量比为3.5∶10,3次验证平行实验得出姜黄素提取产率平均值为(3.10±0.01)%,与预测值偏差极小,无显著差异,说明经响应面优化的制备工艺有效可靠。 2.5 姜黄素纳米粒制备工艺的单因素实验结果 由图4可见,随着各个因素值的增加,姜黄素纳米粒药物包封率均呈先上升后下降趋势。在冰醋酸浓度0.5%、壳聚糖与三聚磷酸钠质量比为5.0∶1、搅拌速度为150 r/min、姜黄素质量浓度为2 mg/mL、三聚磷酸钠质量浓度为1.5 mg/mL、壳聚糖质量浓度为4.5 mg/mL时,姜黄素纳米粒的药物包封率达到最大值。因此,确定冰醋酸浓度为0.5%、壳聚糖与三聚磷酸钠质量比5.0∶1、搅拌速度为150 r/min进行后续实验,选择姜黄素质量浓度为1.5,2.0,2.5 mg/mL、三聚磷酸钠质量浓度1.0,1.5,2.0 mg/mL、壳聚糖质量浓度4.0,4.5,5.0 mg/mL进行后续响应面优化设计实验。 在姜黄素纳米粒合成过程中,壳聚糖与三聚磷酸钠质量比是影响纳米粒尺寸的关键因素[40]。当壳聚糖与三聚磷酸钠质量比较小时,其能提供姜黄素进入的空间有限,以致一部分姜黄素会游离或者吸附聚集在壳聚糖-三聚磷酸钠表面[32];而随着壳聚糖与三聚磷酸钠质量比的增加,能够提供足够的空间用来包埋姜黄素,进而提高药物包封率,但壳聚糖与三聚磷酸钠质量比的过度增加会导致其能够承载的药量减少,并且影响纳米粒的稳定性[41]。随着搅拌速度的增大,姜黄素能够充分地包埋进壳聚糖-三聚磷酸钠中,但当搅拌速度过大时,较强的机械作用力会破坏纳米粒。在制备姜黄素纳米粒过程中,姜黄素动态地进出壳聚糖-三聚磷酸钠[40],当姜黄素进出壳聚糖-三聚磷酸钠达到上限后,继续增加姜黄素质量浓度,姜黄素无法完全包埋到壳聚糖-三聚磷酸钠中。当药物包封率达到最大值后,在壳聚糖质量浓度不变的情况下,随着三聚磷酸钠质量浓度的增加,过多的三聚磷酸钠磷酸基团丢失结合对象,使药物包封率略有下降;在三聚磷酸钠质量浓度不变的情况下,随着壳聚糖质量浓度的增加,过多的壳聚糖自由氨基丢失结合对象,使药物包封率略有下降。"
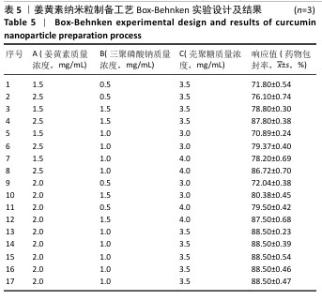
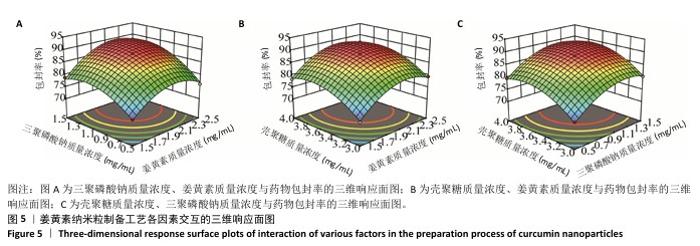
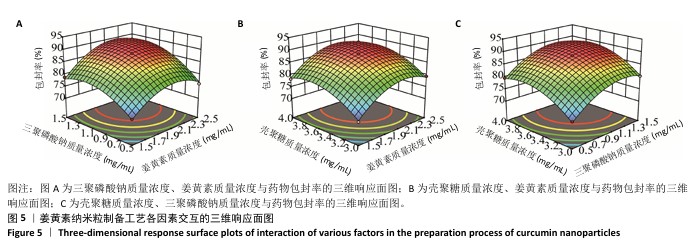
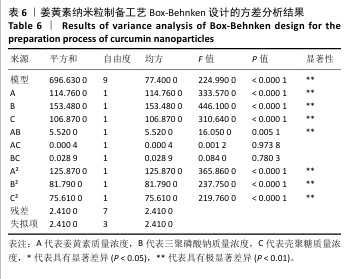
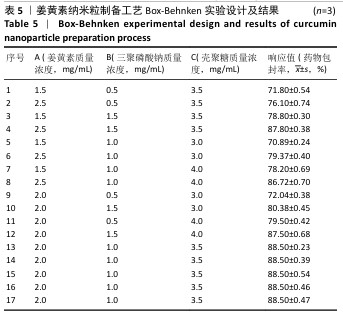
2.6 姜黄素纳米粒制备工艺的Box-Behnken响应面实验结果 分析表5实验结果分析可得模型具有显著差异(P < 0.01),3个因素对响应值(Y)的影响交互作用关系较好(图5)。对结果进行多元二次回归方程拟合及方差分析,见表6。响应值对各因素的多元二次回归模型方程为:Y(药物包封率)%=88.50+3.79A+4.38B+3.65C+1.17AB-0.010 0AC+0.008 5BC-5.47A2-4.41B2-4.24C2。经响应面实验优化后的工艺处方为姜黄素质量浓度2.229 97 mg/mL、三聚磷酸钠质量浓度1.446 32 mg/mL、壳聚糖质量浓度3.632 43 mg/mL,优化后提取工艺的响应值为90.617 6%。 2.7 姜黄素纳米粒制备最佳工艺参数验证结果 根据响应面实验结果并结合实际,将最佳制备工艺参数优化为姜黄素质量浓度2.23 mg/mL、三聚磷酸钠质量浓度1.45 mg/mL、壳聚糖质量浓度3.63 mg/mL,以此工艺制备姜黄素纳米粒,进行3次平行实验,测得平均药物包封率为(90.61±0.01)%,与预测值偏差极小,无显著差异,表明模型预测性良好,优化工艺有效可靠。"
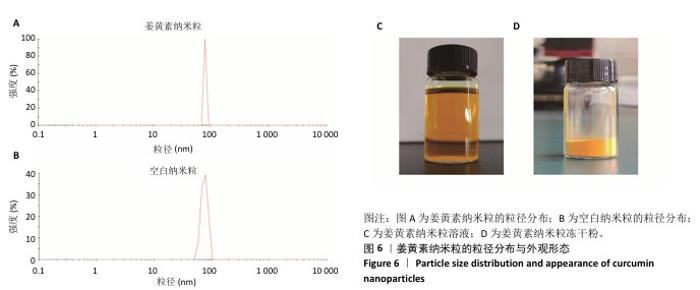
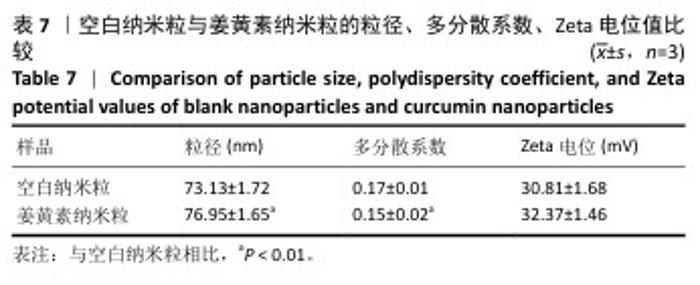
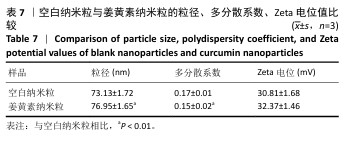
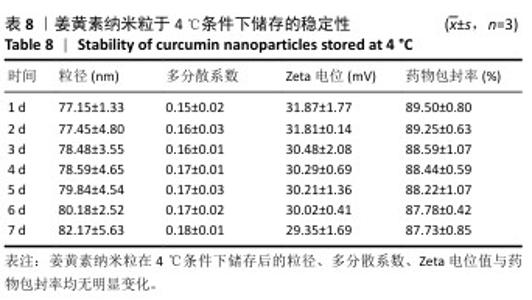
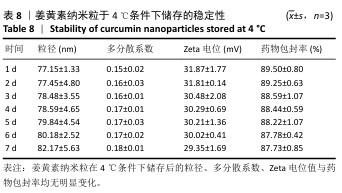
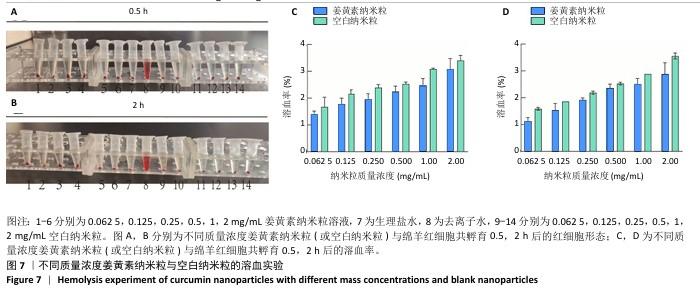
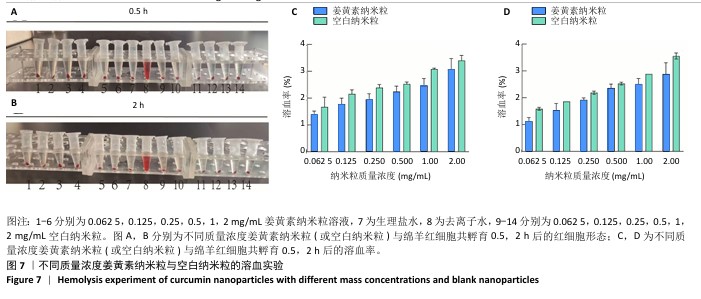
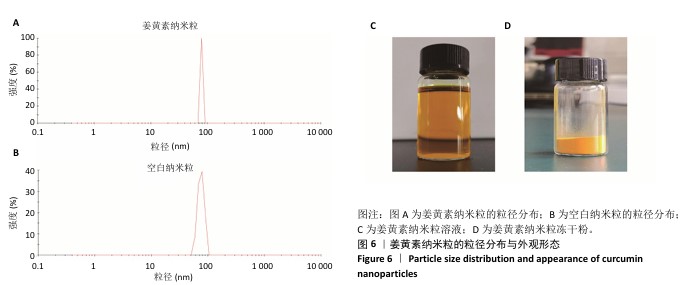
2.8 姜黄素纳米粒的制备和表征 优化前的姜黄素纳米粒载药量为(11.247±0.28)%,优化后姜黄素纳米粒的载药量为(14.494±0.23)%,数据表明经优化后姜黄素纳米粒的载药量显著高于优化前(P < 0.01)。 空白纳米粒与姜黄素纳米粒的粒径分布见图6A,B。姜黄素纳米粒的粒径大于空白纳米粒(P < 0.01),多分散系数小于空白纳米粒(P < 0.01),二者Zeta电位值比较差异无显著性意义(P > 0.05),见表7。姜黄素纳米粒外观形态呈微黄色乳光的溶液(图6C),其冻干粉为均匀细腻的黄色粉末(图6D)。姜黄素纳米粒在4 ℃条件下储存后的粒径、多分散系数、Zeta电位值与药物包封率均无明显变化,见表8,并且未见沉淀或浑浊现象,表明姜黄素纳米粒粒径稳定,纳米颗粒均匀,具有良好的分散性,不易聚集[42]。 姜黄素纳米粒和空白纳米粒分别与稀释绵羊红细胞匀浆孵育0.5,2 h后,红细胞均无明显破损,形态均保持完好(图7A,B),姜黄素纳米粒和空白纳米粒导致的溶血率在0.998%-3.635%之间(图7C,D),远低于国家标准GB/T16886.4-2022,表明姜黄素纳米粒和空白纳米粒对红细胞具有良好的血液相容性。 "
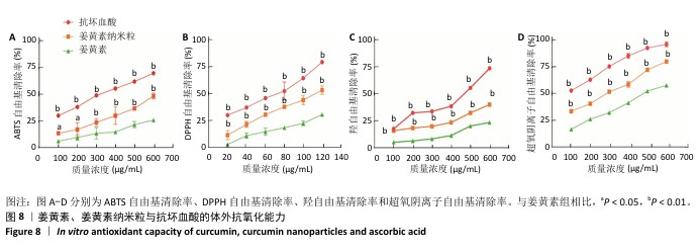
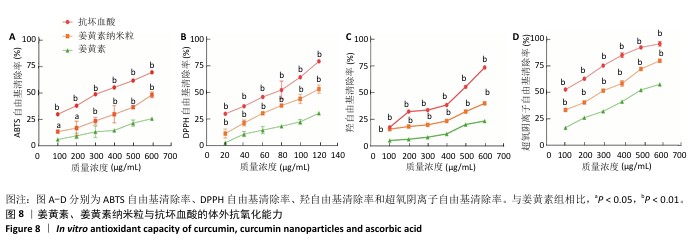
2.9 姜黄素纳米粒体外抗氧化实验结果 姜黄素、姜黄素纳米粒、抗坏血酸对DPPH自由基、ABTS自由基、羟自由基和超氧阴离子自由基的清除率与其对应质量浓度均呈现正相关,并且同质量浓度姜黄素纳米粒的自由基清除率高于姜黄素(图8)。由此可知,将姜黄素制成姜黄素纳米粒可增强其体外抗氧化能力。 姜黄素是一种供氢体,可以提供活泼的氢离子而阻止自由基发生连锁反应[43]。DPPH和ABTS是用来检测化合物抗氧化活性的天然的稳定自由基[44]。羟自由基是一种会对机体产生损害且十分活泼的自由基,抗氧化剂可以抑制其在活细胞中与生物大分子发生的自由基连锁反应[45],从而发挥抗氧化作用。超氧阴离子是一种活性氧自由基,过量的超氧阴离子会导致细胞的异常凋亡,甚至导致疾病[46]。抗氧化剂可以通过中和超氧阴离子自由基达到抗氧化作用,因此,通常将测定超氧阴离子自由基清除能力作为检测化合物抗氧化活性的一种重要手段。"
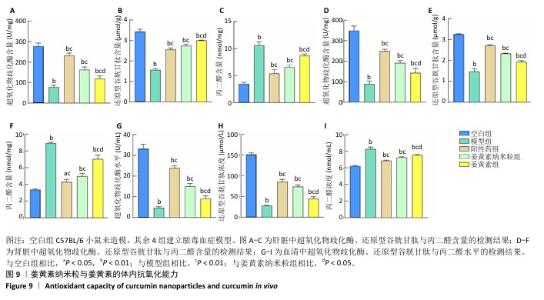
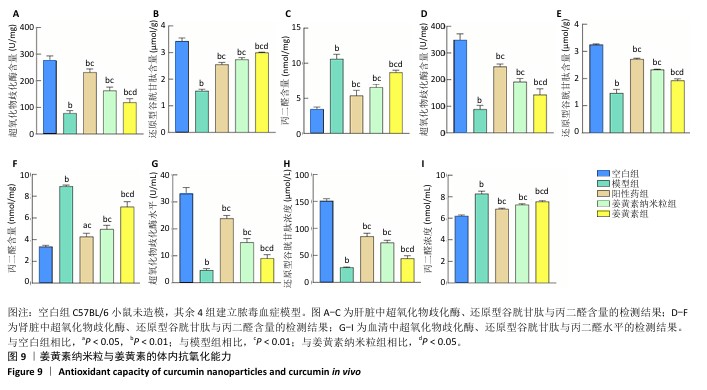
2.10 姜黄素纳米粒体内抗氧化实验结果 造模后12 h无实验动物死亡,60只小鼠全部进入结果分析。 空白组小鼠状态无异常;模型组、阳性药组、姜黄素纳米粒组和姜黄素组造模12 h后小鼠均出现毛发直立、行为异常、眼角出现分泌物、对外界刺激反应迟钝、呼吸缓慢等异常状态,说明脓毒血症模型造模成功。空白组、模型组、阳性药组、姜黄素纳米粒组和姜黄素组小鼠脓毒血症评分分别为0,25.32±0.24,20.24±0.40,20.56±0.19,24.21±0.17,模型组小鼠脓毒血症评分高于空白组(P<0.01),阳性药组、姜黄素纳米粒组和姜黄素组小鼠脓毒血症评分低于模型组(P < 0.01),姜黄素纳米粒组小鼠脓毒血症评分低于姜黄素组(P < 0.05)。 超氧化物歧化酶、还原型谷胱甘肽和丙二醛等对体系活性氧的稳定性具有重要作用[47-48]。与正常动物相比,在脓毒血症模型疾病微环境中,丙二醛含量显著上升,超氧化物歧化酶、还原型谷胱甘肽含量显著下降[49]。实验结果显示,与空白组相比,模型组小鼠血清、肝脏和肾脏中的超氧化物歧化酶、还原型谷胱甘肽含量均显著下降,丙二醛含量显著上升(P均< 0.01);与模型组相比,阳性药组、姜黄素纳米粒组、姜黄素组小鼠血清、肝脏和肾脏中的超氧化物歧化酶、还原型谷胱甘肽含量显著上升(P均< 0.01),丙二醛含量显著下降(P均< 0.01);姜黄素纳米粒组小鼠血清、肝脏和肾脏中的超氧化物歧化酶、还原型谷胱甘肽、丙二醛含量与姜黄素组比较差异均有显著性意义(P均< 0.05),见图9。说明姜黄素纳米粒和姜黄素均有一定的体内抗氧化能力,并且姜黄素纳米粒的体内抗氧化能力强于姜黄素。 "
| [1] 国家药典委员会.中华人民共和国药典(一部)[M].北京:中国医药科技出版社,2020. [2] DOS SANTOS PDF, FRANCISCO CRL, COQUEIRO A, et al. The nanoencapsulation of curcuminoids extracted from Curcuma longa L. and an evaluation of their cytotoxic, enzymatic, antioxidant and anti-inflammatory activities. Food Funct. 2019;10(2):573-582. [3] ZHOU H, FENG X, YAN Y, et al. Optimization of an ultrasonic-assisted aqueous two-phase extraction method for four flavonoids from Lysionotus pauciflorus. Prep Biochem Biotechnol. 2022;52(7): 770-782. [4] XU T, ZHANG H, WANG S, et al. A review on the advances in the extraction methods and structure elucidation of Poria cocos polysaccharide and its pharmacological activities and drug carrier applications. Int J Biol Macromol. 2022;217:536-551. [5] 苏适,于德涵,柴宝丽,等.响应面法优化超声辅助离子液体提取黑豆花青素工艺研究[J].中国调味品,2019,44(7):154-159. [6] 周龙珠,王朋,董双,等.可溶性膳食纤维的制备及其在畜牧生产中的应用研究进展[J].中国畜牧杂志,2022,58(4):11-15. [7] 张帅,郭晓雪,任丽琨,等.酶法改性影响膳食纤维的构成及生物作用效果的研究进展[J].食品安全质量检测学报,2022,13(4): 1089-1098. [8] GONG S, SUN M, LEE Y, et al. Bulk‐like Pt (100)‐oriented Ultrathin Surface: Combining the Merits of Single Crystals and Nanoparticles to Boost Oxygen Reduction Reaction. Angew Chem Int Ed Engl. 2023; 135(4):e202214516. [9] ARDEAN C, DAVIDESCU CM, NEMEŞ NS, et al. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int J Mol Sci. 2021;22(14):7449. [10] HEJJAJI EM, SMITH AM, MORRIS GA. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS: TPP) ratios. Int J Biol Macromol. 2018;120:1610-1617. [11] 杨天硕,肖小兰,阮文权,等.响应面法优化负压原位碱度蒸汽汽提脱氨处理兰炭废水的研究[J].煤炭转化,2024,47(4):79-88. [12] 许珂,缪秉陶,曹洪杰,等.鮟鱇鱼皮活性肽的分离纯化及其对HK-2细胞损伤保护的作用[J].广东海洋大学学报,2024,44(4):1-10. [13] 李慧卿,曹叶霞,黄坊娇.DPPH·和ABTS+·比较研究毛建草多酚、黄酮和多糖的抗氧化协同作用[J].山西农业大学学报(自然科学版),2022,42(3):98-105. [14] HOU D, LIAO H, HAO S, et al. Curcumin simultaneously improves mitochondrial dynamics and myocardial cell bioenergy after sepsis via the SIRT1-DRP1/PGC-1α pathway. Heliyon. 2024;10(7):e28501. [15] JIANG C, SHI Q, YANG J, et al. Ceria nanozyme coordination with curcumin for treatment of sepsis-induced cardiac injury by inhibiting ferroptosis and inflammation. J Adv Res. 2024;63:159-170. [16] XIAO Z, YU X, ZHANG S, et al. The Expression Levels and Significance of GSH, MDA, SOD, and 8‐OHdG in Osteochondral Defects of Rabbit Knee Joints. Biomed Res Int. 2022;2022(1):6916179. [17] 王月光,穆晓红,蒋昇源,等.急性脊髓损伤患者早期血清标志物与AISA分级的相关性[J].中国组织工程研究,2024,28(34): 5494-5499. [18] WU J, SU C, JIANG J, et al. The potential role of serum lipoprotein in children with sepsis. Medicine (Baltimore). 2023;102(48):e36311. [19] TAO H, SHEN L. Research progress of curcumin in the treatment of sepsis. Shock. 2024;61(6):805-816. [20] SULZBACHER MM, SULZBACHER LM, PASSOS FR, et al.Adapted murine sepsis score: improving the research in experimental sepsis mouse model. Biomed Res Int. 2022;2022(1):5700853. [21] 胡钰,傅纤雯,阙珊奇,等.干燥和提取方式对栀子花成分及抑菌活性的影响[J].食品科技,2024,49(5):200-207. [22] MOHAMMADPOUR H, SADRAMELI SM, ESLAMI F, et al. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind Crops Prod. 2019;131:106-116. [23] LIANG H, WANG W, XU J, et al. Optimization of ionic liquid-based microwave-assisted extraction technique for curcuminoids from Curcuma longa L. Food Bioprod Process. 2017;104:57-65. [24] ALEXANDRE EM, SILVA S, SANTOS SA, et al. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res Int. 2019;115:167-176. [25] KIM DW, YOUSAF AM, LI DX, et al.Development of RP-HPLC method for simultaneous determination of docetaxel and curcumin in rat plasma: Validation and stability. Asian J Pharm Sci. 2017;12(1): 105-113. [26] ZHENG S, ZHU Y, JIAO C, et al. Extraction and analysis of gigantol from Dendrobium officinale with response surface methodology. Molecules. 2018;23(4):818. [27] 郑晓庆,王德达,慕鸿雁.装载鱼油的壳聚糖-三聚磷酸盐复合体系工艺研究[J].食品科技,2022,47(4):267-274. [28] INDA A, TETTAMANTI CS, MARTINEZ SM, et al. New RP-HPLC method for the simultaneous determination of process yield and percentage of encapsulation of Gallein in albumin nanoparticles. J Chromatogr B Analyt Technol Biomed Life Sci. 2024;1240:124161. [29] 吴瑛,李阳,李凤琴,等.黄芩苷壳聚糖纳米粒的制备及表征[J].中国实验方剂学杂志,2014,20(15):15-19. [30] 李宛蓉,刘佩,余静怡,等.乳清分离蛋白与单宁酸相互作用提高稻米油Pickering乳液的稳定性[J].食品科学,2020,41(18):1-7. [31] HASSAN S, BILAL N, KHAN TJ, et al. Bioinspired chitosan based functionalization of biomedical implant surfaces for enhanced hemocompatibility, antioxidation and anticoagulation potential: an in silico and in vitro study. RSC Adv. 2024;14(29):20691-20713. [32] GUO X, LI W, WANG H, et al. Preparation, characterization, release and antioxidant activity of curcumin-loaded amorphous calcium phosphate nanoparticles. J Non Cryst Solids. 2018;500:317-325. [33] 赵彩云,高文静,吴斌,等.杏干多酚的提取工艺及体外抗氧化活性研究[J].保鲜与加工,2024,24(5):61-68. [34] 余青松,马欣龙,陈玉霞,等.茯苓菌固态发酵香菇柄基质的酶活力、营养成分及抗氧化活性变化[J].华中农业大学学报,2024,43(4): 230-238. [35] 刘燕,马雪萍,史玉柱,等.滨蒿提取物对脂多糖诱导小鼠急性肺损伤的保护作用及抗氧化活性[J].中药药理与临床,2024,40(4): 78-82. [36] WANG G, MA X, HUANG W, et al. Macrophage biomimetic nanoparticle-targeted functional extracellular vesicle micro-RNAs revealed via multiomics analysis alleviate sepsis-induced acute lung injury. J Nanobiotechnology. 2024;22(1):362. [37] WANG J, WEI L, LIU C, et al. Taurine treatment alleviates intestinal mucositis induced by 5-fluorouracil in mice. Plant Foods Hum Nutr. 2022;77(3):399-404. [38] AIMAIER S, TAO Y, LEI F, et al. Protective effects of the Terminalia bellirica tannin-induced Nrf2/HO-1 signaling pathway in rats with high-altitude pulmonary hypertension. BMC Complement Med Ther. 2023;23(1):150. [39] ZHANG L, FAN G, KHAN MA, et al. Ultrasonic-assisted enzymatic extraction and identification of anthocyanin components from mulberry wine residues. Food Chem. 2020;323:126714. [40] TSAI WH, YU KH, HUANG YC, et al. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int J Nanomedicine. 2018;13:903-916. [41] DU Z, LIU J, ZHANG T, et al. A study on the preparation of chitosan-tripolyphosphate nanoparticles and its entrapment mechanism for egg white derived peptides. Food Chem. 2019;286:530-536. [42] 管庆霞,林泽榆,夏昭睿,等.马钱子碱聚乳酸-羟基乙酸共聚物纳米粒表征及体内药动学评价[J].吉林中医药,2023,43(10): 1193-1198. [43] WEI ZZ, ZHOU TQ, XIA ZM, et al. Four organosulfur compounds from the seeds of Capsella bursa-pastoris and their anti-inflammatory activities. Nat Prod Res. 2023;37(16):2688-2696. [44] HCINI K, BAHI A, ZARROUG MB, et al. Polyphenolic profile of tunisian thyme (Thymbra capitata L.) post-distilled residues: evaluation of total phenolic content and phenolic compounds and their contribution to antioxidant activity. Molecules. 2022;27(24):8791. [45] KAŹMIERCZAK-BARAŃSKA J, BOGUSZEWSKA K, ADAMUS-GRABICKA A, et al. Two faces of vitamin C—antioxidative and pro-oxidative agent. Nutrients. 2020;12(5):1501. [46] JIE Z, LIU J, SHU M, et al. Detection strategies for superoxide anion: A review. Talanta. 2022;236: 122892. [47] SANDHU S K, RAUT J, KUMAR S, et al. Nanocurcumin and viable Lactobacillus plantarum based sponge dressing for skin wound healing. Int J Pharm. 2023;643:123187. [48] LI Q, DING J, XIA B, et al. L-theanine alleviates myocardial ischemia/reperfusion injury by suppressing oxidative stress and apoptosis through activation of the JAK2/STAT3 pathway in mice. Mol Med. 2024;30(1):98. [49] ABDELNASER M, ALAAELDIN R, ATTYA ME, et al. Modulating Nrf-2/HO-1, apoptosis and oxidative stress signaling pathways by gabapentin ameliorates sepsis-induced acute kidney injury. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(2):947-958. [50] 赵蝴蝶,易帆,马越,等.天然姜黄色素的应用及性能改善研究进展[J].印染助剂,2023,40(11):1-9. [51] 郭玉,任迪峰,郭子烟,等.姜黄素增溶技术研究进展[J].食品与发酵工业,2024,50(14):342-348. [52] 张欣,孙敬蒙,贾珍珍,等.叶黄素复合纳米颗粒的制备工艺优化及其稳定性和抗氧化活性分析[J].食品工业科技,2024,45(16): 102-113. [53] FAN Y, YI J, ZHANG Y, et al. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018;239: 1210-1218. [54] 陈煜淳,林勇,刘勇,等.姜黄素的应用、制备及改性研究进展[J].现代食品科技,2024,40(6):327-335. [55] KHAN A, WANG C, SUN X, et al. Physicochemical and microstructural properties of polymerized whey protein encapsulated 3, 3′-Diindolylmethane nanoparticles. Molecules. 2019;24(4):702. [56] YEDGAR S, BARSHTEIN G, GURAL A. Hemolytic activity of nanoparticles as a marker of their hemocompatibility. Micromachines (Basel). 2022; 13(12):2091. [57] NAMGOONG JW, KIM HM, KIM SH, et al. Synthesis and characterization of metal phthalocyanine bearing carboxylic acid anchoring groups for nanoparticle dispersion and their application to color filters. Dyes Pigm. 2021;184:108737. [58] TIAN Z, MAI Y, MENG T, et al. Nanocrystals for improving oral bioavailability of drugs: intestinal transport mechanisms and influencing factors. AAPS PharmSciTech. 2021;22(5):179. [59] 向晓燕,余忠姝,谢佳希,等.壳聚糖杂化吴茱萸碱复合纳米粒的药动学研究[J].医药导报,2021,40(8):1022-1025. [60] 杨晓宇,罗寒,盛剑勇,等.配体修饰的口服纳米载药系统的研究进展[J].医药导报,2020,39(9):1234-1239. |
| [1] | Qi Junlong, Liu Junyi, He Yuzhou, Qiang Wei, Zhang Shiying, Liu Qiao, Zhu Hongda. Functionalized self-assembled micelles enhance effect of tranexamic acid in treatment of cutaneous hyperpigmentation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6061-6069. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||