Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (34): 7310-7317.doi: 10.12307/2025.492
Previous Articles Next Articles
Inhibitory effect of ferroptosis inhibitor toxicity induced by cobalt nanoparticles through reactive oxygen species
Wang Chen1, Zhang Weinan2, Shen Jining2, Liu Fan3, Yuan Jishan1, Liu Yake3
- 1Affiliated People’s Hospital of Jiangsu University, Zhenjiang 212000, Jiangsu Province, China; 2Nantong University, Nantong 226000, Jiangsu Province, China; 3Affiliated Hospital of Nantong University, Nantong 226000, Jiangsu Province, China
-
Received:2024-08-24Accepted:2024-09-20Online:2025-12-08Published:2025-01-17 -
Contact:Liu Yake, MD, Master’s supervisor, Associate chief physician, Affiliated Hospital of Nantong University, Nantong 226000, Jiangsu Province, China -
About author:Wang Chen, Resident physician, Affiliated People’s Hospital of Jiangsu University, Zhenjiang 212000, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 82172519 (to LF); National Natural Science Youth Science Foundation of China, 82002282 (to LYK)
CLC Number:
Cite this article
Wang Chen, Zhang Weinan, Shen Jining, Liu Fan, Yuan Jishan, Liu Yake. Inhibitory effect of ferroptosis inhibitor toxicity induced by cobalt nanoparticles through reactive oxygen species[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7310-7317.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
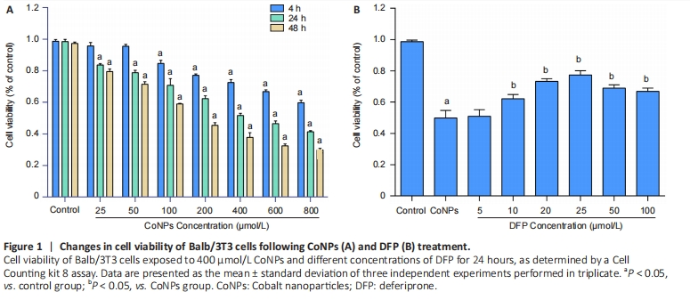
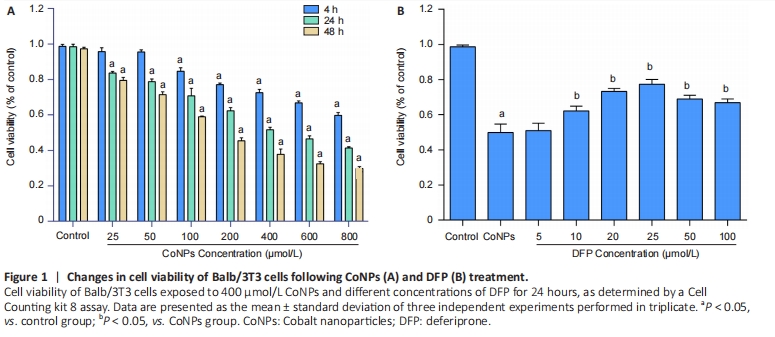
Ferroptosis inhibitor protected against cytotoxicity induced by CoNPs in Balb/3T3 cells Cells were treated with different concentrations of CoNPs for 4, 24, or 48 hours and the effect of CoNPs on Balb/3T3 cell survival was determined by CCK-8 assay. The percentage of survived cells strikingly decreased with the increase of CoNPs concentration (Figure 1A). The IC50 value was 400 μmol/L 24 hours later, respectively. Cells cultured in the medium with varying concentrations of DFP showed a dose-dependent survival rate. When the concentration of DFP increased, the viability of Balb/3T3 cells increased. When cells were cultured in 400 μmol/L CoNPs and different concentrations of DFP, the viability of cells was significantly higher than the cells treated with CoNPs (Figure 1B)."
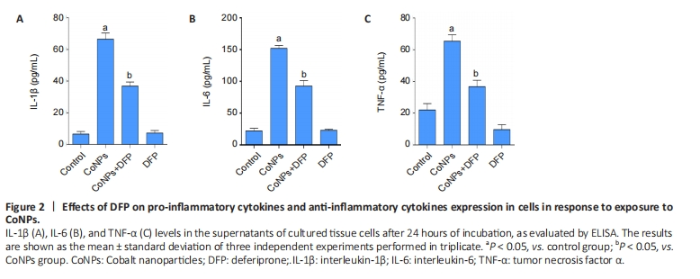
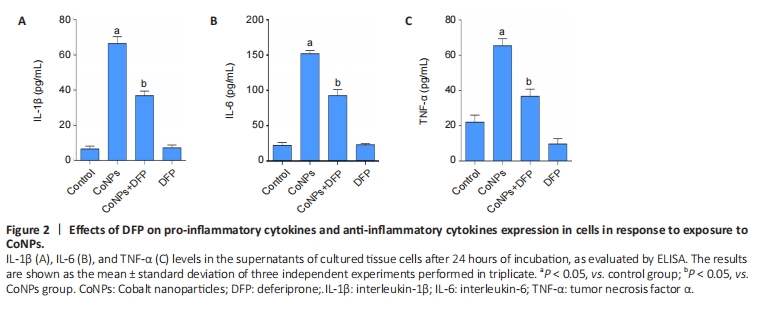
Ferroptosis inhibitor reduced inflammatory cytokines in Balb/3T3 cells The results are shown in Figure 2. The levels of TNF-α, IL-1β, and IL-6 in the CoNPs group were noticeably higher than those in the control group. Compared with the CoNPs group, DFP treatment had a significant protective effect and significantly reduced TNF-α (P < 0.05), IL-1β (P < 0.05), and IL-6 (P < 0.05) induced by cobalt nanoparticles expression. However, there was no significant difference between the single DFP group and the CoNPs group."
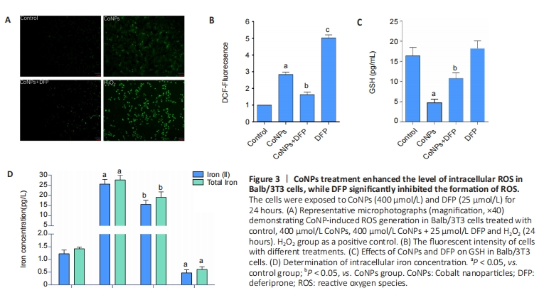
The oxidative damage induced by ROS was considered an important cause of the toxicity of cobalt nanoparticles. ROS was also the main incentive of ferroptosis. In this study, as shown in Figure 3, after 400 μmol/L CoNPs were treated for 24 hours, the ROS level of Balb/3T3 cells increased significantly (P < 0.05), while DFP (25 μmol/L) could significantly inhibit this increase (P < 0.05), and the difference was statistically significant (P < 0.05) (Figure 3A, B). GSH is the key cellular endogenous antioxidant that can scavenge toxic free radicals, and depletion of GSH appears to promote intracellular ROS accumulation, leading to apoptosis. Altered GSH levels represent the increased cellular response to oxidative stress. The results suggested that CoNPs treatment decreased GSH levels (P < 0.05), and DFP inhibited this decrease (P < 0.05) as measured by the GSH assay kit (Figure 3C). ROS promotes lipid peroxidation, leading to damage to the cell membrane and eventual cell death. When the concentration of iron ions in the cell increases, the likelihood of ferroptosis is significantly heightened. Conversely, reducing the intracellular free iron content or using iron chelators can effectively inhibit or slow down the process of ferroptosis[25-26]. Our results showed that CoNPs could lead to significant increases in Fe2+ ion and total iron concentration in Balb/3T3 cells compared with the control group. However, the increase was inhibited when cells were exposed to DFP (Figure 3D). These results indicate that CoNPs can induce the occurrence of ferroptosis in Balb/3T3 cells by stimulating oxidative stress."
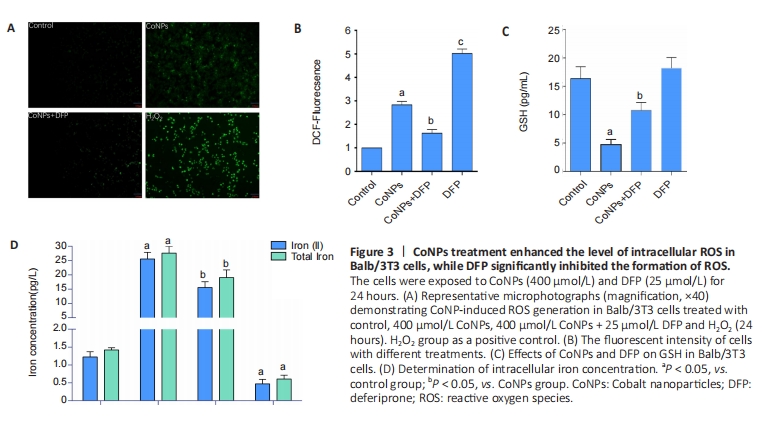
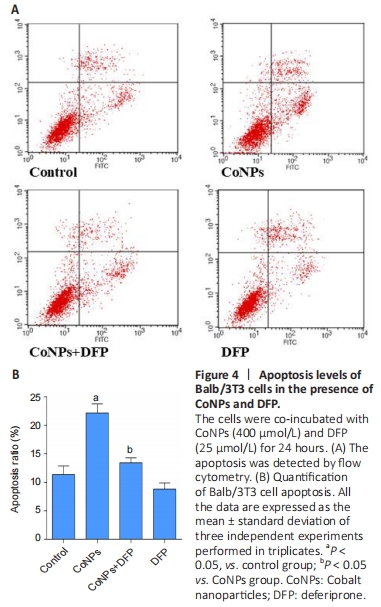
Apoptosis assay in Balb/3T3 cells To investigate the apoptosis rate of cells treated with 400 μmol/L CoNPs and 25 μmol/L DFP, the AnnexinV-FITC assay kit was used. Incubation with 400 μmol/L CoNPs for 24 hours mildly increased the cell apoptosis rate compared with the control group. When DFP was added as a treatment before exposure to CoNPs, the apoptosis was obviously alleviated (Figure 4A, B)."
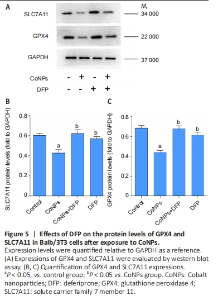
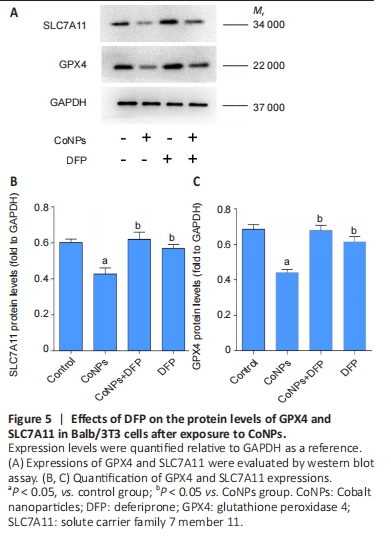
Ferroptosis inhibitor exerted the positive regulation on GPX4 in Balb/3T3 cells GPX4 is the central regulator of ferroptosis, and the decline of GPX4 is often used as a marker of ferroptosis[27-28]. SLC7A11 is a unit of the glutamate-cystine antiporter Xc-, resulting in enhanced lipid oxidation and ferroptosis. Western blot assay showed that CoNPs noticeably decreased the protein amount of GPX4 and SLC7A11. However, the decrease in GPX4 and SLC7A11 levels was inhibited when cells were treated with 25 μmol/L DFP and 400 μmol/L CoNPs for 24 hours. These results suggest that CoNPs might induce ferroptosis and the ferroptosis inhibitor DFP exerts therapeutic action against CoNPs (Figure 5)."
| [1] SINGH JA, SLOAN JA. Health-related quality of life in total hip and total knee arthroplasty. Rheumatology (Oxford). 2008;47(12):1826-1831. [2] JACOBS J, HALLAB N, SKIPOR A, et al. Metal degradation products: a cause for concern in metal-metal bearings? Clin Orthop Relat Res. 2003;(417):139-147. [3] LIN X, CHEN C, CHEN J, et al. Long Noncoding RNA NR_030777 alleviates cobalt nanoparticles-induced neurodegenerative damage by promoting autophagosome-lysosome fusion. ACS Nano. 2024; 18(36):24872-24897. [4] LIU Y, ZHU W, NI D, et al. Alpha lipoic acid antagonizes cytotoxicity of cobalt nanoparticles by inhibiting ferroptosis-like cell death. J Nanobiotechnology. 2020;18(1):141. [5] SABBIONI E, FORTANER S, FARINA M, et al. Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: an in vitro model. Nanotoxicology. 2014;8(4):455-464. [6] PAPAGEORGIOU I, BROWN C, SCHINS R, et al. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials. 2007;28(19):2946-2958. [7] WAN R, MO Y, ZHANG Z, et al. Cobalt nanoparticles induce lung injury, DNA damage and mutations in mice. Part Fibre Toxicol. 2017;14(1):38. [8] MONTEILLER C, TRAN L, MACNEE W, et al. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64(9):609-615. [9] COLOGNATO R, BONELLI A, PONTI J, et al. Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro. Mutagenesis. 2008;23(5):377-382. [10] RAJIV S, JEROBIN J, SARANYA V, et al. Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Hum Exp Toxicol. 2016;35(2):170-183. [11] HOREV-AZARIA L, KIRKPATRICK CJ, KORENSTEIN R, et al. Predictive toxicology of cobalt nanoparticles and ions: comparative in vitro study of different cellular models using methods of knowledge discovery from data. Toxicol Sci. 2011;122(2):489-501. [12] Martin O, VLADIMIR G, STEN O, et al. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12(5):913-922. [13] MASUI T, SAKANO S, HASEGAWA Y, et al. Expression of inflammatory cytokines, RANKL and OPG induced by titanium, cobalt-chromium and polyethylene particles. Biomaterials. 2005;26(14):1695-1702. [14] GOODMAN SB, HUIE P, SONG Y, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br. 1998;80(3):531-539. [15] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-1072. [16] HAN C, LIU Y, DAI R, et al. Ferroptosis and its potential role in human diseases. Front Pharmacol. 2020;11:239. [17] XIE Y, HOU W, SONG X, et al. Ferroptosis: process and function. 2016; 23(3):369-379. [18] YANG WS, SRIRAMARATNAM R, WELSCH ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317-331. [19] JIANG L, KON N, LI T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57-62. [20] ZOU Y, SCHREIBER SL. Progress in understanding ferroptosis and challenges in its targeting for therapeutic benefit. Cell Chem Biol. 2020;27(4):463-471. [21] IMAI H, MATSUOKA M, KUMAGAI T, et al. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143-170. [22] ZHANG Y, SUN C, ZHAO C, et al. Ferroptosis inhibitor SRS 16-86 attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury. Brain Res. 2019;1706:48-57. [23] LIU Y, HONG H, LU X, et al. L-ascorbic acid protected against extrinsic and intrinsic apoptosis induced by cobalt nanoparticles through ROS attenuation. Biol Trace Elem Res. 2017;175(2):428-439. [24] JIANG H, LIU F, YANG H, et al. Effects of cobalt nanoparticles on human T cells in vitro. Biol Trace Elem Res. 2012;146(1):23-29. [25] LI J, CAO F, YIN HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. [26] PANG Q, TANG Z, LUO L. The crosstalk between oncogenic signaling and ferroptosis in cancer. Crit Rev Oncol Hematol. 2024;197:104349. [27] YANG W, SRIRAMARATNAM R, WELSCH M, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317-331. [28] FRIEDMANN ANGELI J, SCHNEIDER M, PRONETH B, et al. Inactivation of the ferroptosis regulator GPX4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180-1191. [29] WANG S, WANG C, ZHANG W, et al. Bioactive nano-selenium antagonizes cobalt nanoparticle-mediated oxidative stress via the Keap1-Nrf2-ARE signaling pathway. J Nanopart Res. 2022;24(1):1-12. [30] SABBIONI E, FORTANER S, FARINA M, et al. Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: an in vitro model. Nanotoxicology. 2014;8(4):455-464. [31] YAN X, LIU Y, XIE T, et al. alpha-Tocopherol protected against cobalt nanoparticles and cocl2 induced cytotoxicity and inflammation in Balb/3T3 cells. Immunopharmacol Immunotoxicol. 2018;40(2):179-185. [32] LU LQ, TIAN J, LUO XJ, et al. Targeting the pathways of regulated necrosis: a potential strategy for alleviation of cardio-cerebrovascular injury. Cell Mol Life Sci. 2021;78(1):63-78. [33] THOMAS V, HALLORAN B, AMBALAVANAN N, et al. In vitro studies on the effect of particle size on macrophage responses to nanodiamond wear debris. Acta Biomater. 2012;8(5):1939-1947. [34] HALLAB NJS. Biologic responses to orthopedic implants: innate and adaptive immune responses to implant debris. Spine. 2016;41 Suppl 7:S30-S31. [35] KAUFMAN A, ALABRE C, RUBASH H, et al. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. J Biomed Mater Res A. 2008;84(2):464-474. [36] DIXON SJ, PRATT DA. Ferroptosis: a flexible constellation of related biochemical mechanisms. Mol Cell. 2023;83(7):1030-1042. [37] IMAI H, MATSUOKA M, KUMAGAI T, et al. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143-170. [38] CHEN D, FAN Z, RAUH M, et al. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36(40):5593-5608. [39] ZHANG W, WANG C, ZHU W, et al. Ferrostatin-1 alleviates cytotoxicity of cobalt nanoparticles by inhibiting ferroptosis. Bioengineered. 2022; 13(3):6163-6172. [40] ZHU W, ZHANG R, LIU S, et al. The effect of nanoparticles of cobalt-chromium on human aortic endothelial cells in vitro. J Appl Toxicol. 2021;41(12):1966-1979. |
| [1] | Liu Hongjie, Mu Qiuju, Shen Yuxue, Liang Fei, Zhu Lili. Metal organic framework/carboxymethyl chitosan-oxidized sodium alginate/platelet-rich plasma hydrogel promotes healing of diabetic infected wounds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1929-1939. |
| [2] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [3] | Chen Haojie, Wang Dai, Shen Shan. Immune inflammatory microenvironment mechanisms in peri-implantitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2054-2062. |
| [4] | Liu Dawei, Cui Yingying, Wang Fanghui, Wang Zixuan, Chen Yuhan, Li Yourui, Zhang Ronghe. Epigallocatechin gallate-mediated bidirectional regulation of reactive oxygen species and its application in nanomaterials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2101-2112. |
| [5] | Zhu Kuicheng, Du Chunyan, Zhang Jintao. Mechanism by which hairless gene mutation promotes white adipose tissue browning in hairless mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1424-1430. |
| [6] | Li Hao, Tao Hongcheng, Zeng Ping, Liu Jinfu, Ding Qiang, Niu Chicheng, Huang Kai, Kang Hongyu. Mitogen-activated protein kinase signaling pathway regulates the development of osteoarthritis: guiding targeted therapy with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1476-1485. |
| [7] | Chen Ju, Zheng Jinchang, Liang Zhen, Huang Chengshuo, Lin Hao, Zeng Li. Effect and mechanism of beta-caryophyllene in mice with osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1341-1347. |
| [8] | Wen Guangwei, Zhen Yinghao, Zheng Taikeng, Zhou Shuyi, Mo Guoye, Zhou Tengpeng, Li Haishan, Lai Yiyi. Effects and mechanisms of isoginkgetin on osteoclastogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1348-1358. |
| [9] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [10] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [11] | Peng Tuanhui, Song Hongming, Yang Ling, Ding Xiaoge, Meng Pengjun. Effects of long-term endurance exercise on kl/FGF23 axis and calcium-phosphorus metabolism in naturally aging mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1089-1095. |
| [12] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [13] | Hu Jing, Zhu Ling, Xie Juan, Kong Deying, Liu Doudou. Autophagy regulates early embryonic development in mice via affecting H3K4me3 modification [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1147-1155. |
| [14] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| [15] | Bao Zhuoma, Hou Ziming, Jiang Lu, Li Weiyi, Zhang Zongxing, Liu Daozhong, Yuan Lin. Effect and mechanism by which Pterocarya hupehensis skan total flavonoids regulates the proliferation, migration and apoptosis of fibroblast-like synoviocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 816-823. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||