Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (28): 6070-6082.doi: 10.12307/2025.481
Previous Articles Next Articles
Oriented electrostatically spun polycaprolactone/silk fibroin scaffold loaded with calcium titanate promotes peripheral nerve regeneration
Liu Xiaojun1, 2, Shang Yuqing3, Guan Wenchao3, Xu Linlin1, 2, Li Guicai3
- 1Henan Institute for Drug and Medical Device Inspection, Zhengzhou 450018, Henan Province, China; 2Key Laboratory for Quality Evaluation of Medical Protective and Implant Devices of National Medical Products Administration, Zhengzhou 450018, Henan Province, China; 3Key Laboratory of Neuroregeneration of Ministry of Education, Nantong University, Nantong 226001, Jiangsu Province, China
-
Received:2024-06-01Accepted:2024-07-30Online:2025-10-08Published:2024-12-07 -
Contact:Li Guicai, PhD, Professor, Key Laboratory of Neuroregeneration of Ministry of Education, Nantong University, Nantong 226001, Jiangsu Province, China -
About author:Liu Xiaojun, MS, Engineer, Henan Institute for Drug and Medical Device Inspection, Zhengzhou 450018, Henan Province, China; Key Laboratory for Quality Evaluation of Medical Protective and Implant Devices of National Medical Products Administration, Zhengzhou 450018, Henan Province, China -
Supported by:National Natural Science Foundation of China, No. 32171352 (to LGC); Key Laboratory of Medical Biological Protection and Implant Device Quality Evaluation Open Project of National Medical Products Administration, No. 2023-02 (to LGC)
CLC Number:
Cite this article
Liu Xiaojun, Shang Yuqing, Guan Wenchao, Xu Linlin, Li Guicai. Oriented electrostatically spun polycaprolactone/silk fibroin scaffold loaded with calcium titanate promotes peripheral nerve regeneration[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6070-6082.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

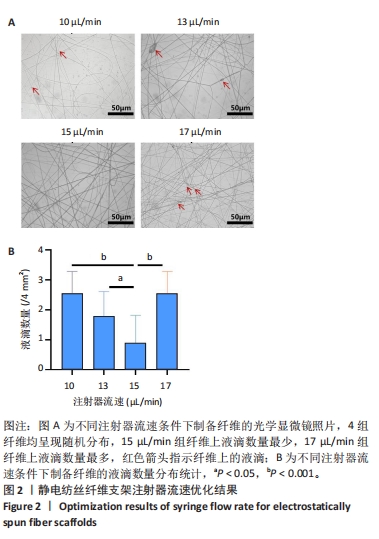
2.1 静电纺丝纤维支架参数优化结果 2.1.1 流速优化结果 采用金属板作为接收装置,通过改变注射器流速(10,13,15,17 μL/min)制备了纺丝时间为30 s的4组静电纺丝纳米纤维,采用光学显微镜记录纺丝纤维的形貌(图2A),4组纤维均呈现随机分布,10,13,15 μL/min组表现出随着注射器流速的增加,视野下纤维上液滴的数目逐渐降低,15 μL/min组纤维液滴最少,大部分纤维呈连续性分布;17 μL/min组纤维液滴数目反而更多,这可能是由于注射器流速进一步增大,纤维在牵引拉伸至接收装置时被吹散,影响了纤维的连续性,最终表现为纤维表面存在较多液滴。随后对4组纤维的液滴数量进行定量分析,结果显示15 μL/min组纳米纤维液滴数量低于其他3组(P < 0.05,P < 0.001),见图2B。因此,后续实验选择15 μL/min作为制备静电纺丝纤维的流速。"
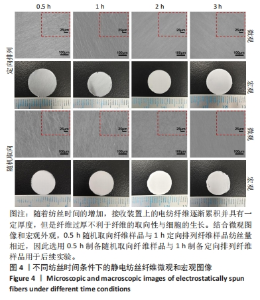
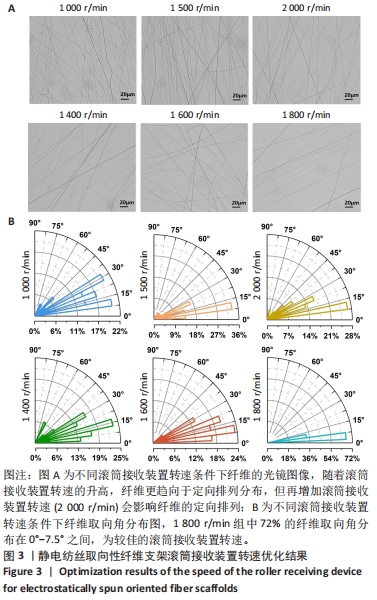
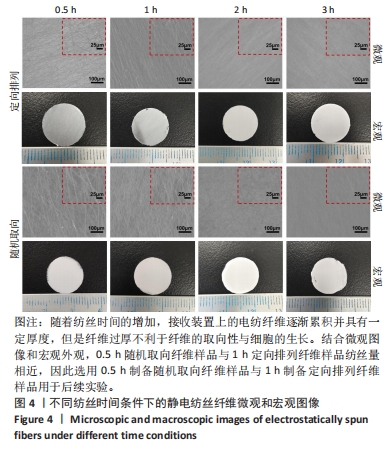
为了进一步确定滚筒接收装置转速,进而设置了1 400,1 600,1 800 r/min组进行滚筒接收装置转速筛选,由图3可得,3组静电纺丝支架大多纤维取向角都分布在0°-30°之间,1 400 r/min组中14%纤维取向角分布在0°-7.5°,1 600 r/min组中17%纤维取向角分布在0°-7.5°,但1 800 r/min组中72%的纤维取向角分布在0°-7.5°之间,比例远大于其他两组。因此,后续实验选择1 800 r/min为滚筒接收装置转速进行静电纺丝。 2.1.3 纺丝时间优化结果 不同纺丝时间条件下制备的静电纺丝纤维微观图像和宏观图像如图4所示,随着纺丝时间的增加,接收装置上的电纺纤维逐渐累积并具有一定厚度,这会导致接收装置在牵引拉伸纤维时静电力下降,进而影响纤维的取向性。此外,玻片纤维的覆盖率可能会影响后续细胞的生长,过低的纤维覆盖率会导致细胞培养过程中与小圆玻片直接接触,进而影响支架发挥作用及细胞生长。因此,制备的纺丝支架需要较大程度覆盖住小圆玻片又不宜太厚,选择1 h定向排列纤维样品用于后续实验。另外,结合微观图像和宏观外观,0.5 h随机取向纤维样品与1 h定向排列纤维样品纺丝量相近,因此选用0.5 h制备随机取向纤维样品与1 h制备定向排列纤维样品用于后续实验。"
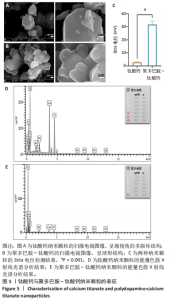
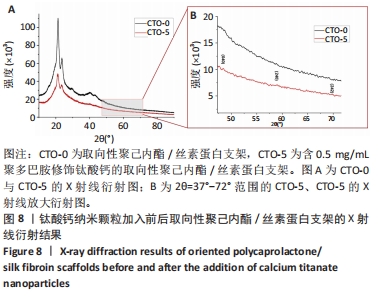
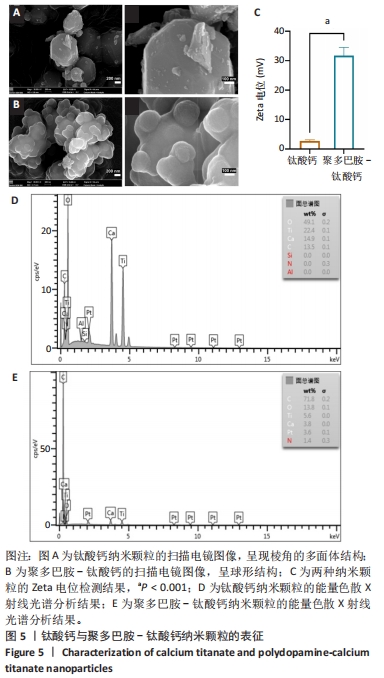
2.2 钛酸钙纳米颗粒表征结果 通过扫描电镜观察到钛酸钙、聚多巴胺-钛酸钙纳米颗粒的形貌,钛酸钙呈现棱角的多面体结构,见图5A,当钛酸钙被聚多巴胺功能化时,多面体钛酸钙纳米颗粒表面被聚多巴胺覆盖,成为一个球形结构,见图5B,这种结构应该是微小的钛酸钙纳米结构聚集的结果。此外,在聚多巴胺聚合过程中,一些小的钛酸钙颗粒团聚形成层状结构,见图5B,说明在多巴胺的氧化聚合作用下,钛酸钙被聚多巴胺功能化。如图5C所示,聚多巴胺-钛酸钙纳米颗粒的Zeta电位显著高于钛酸钙纳米颗粒(P < 0.001),表明聚多巴胺-钛酸钙纳米颗粒在水溶液体系的稳定性提高。能量色散X射线光谱检测结果显示,钛酸钙中碳元素比例为13.5%,不含氮元素,并且氧元素含量占比最高,见图5D;聚多巴胺-钛酸钙的碳元素比例升高至71.6%,并且检测到氮元素含量为1.4%,这说明聚多巴胺成功修饰在钛酸表面,并且聚多巴胺-钛酸钙中碳元素比例显著高于钛酸钙,见图5E。"
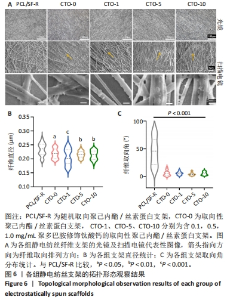
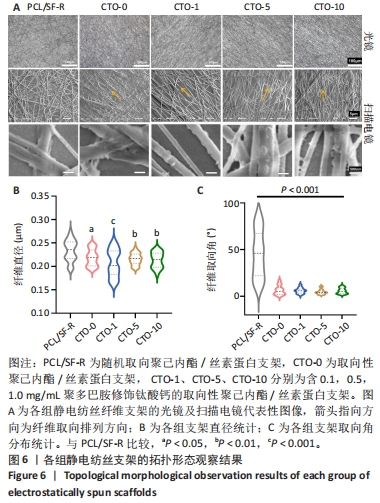
2.3 各组静电纺丝支架拓扑形貌观察结果 5组静电纺丝支架在光镜和扫描电镜下的纤维形态如图6A所示。PCL/SF-R组静电纺丝支架的光镜和电镜观察结果一致,纤维分布较为杂乱,没有明显取向性,而其余4组静电纺丝支架中大部分纤维排列方向较为一致,呈现取向排列;随着钛酸钙纳米颗粒质量浓度的增加,显微镜视野下黏附在纤维上的钛酸钙颗粒数量逐渐增加且呈均匀分布。扫描电镜观察结果显示, CTO-1组、CTO-5组、CTO-10组纤维支架都成功掺入了钛酸钙纳米颗粒并均匀分散到纤维丝上。通过对各组支架的直径进行定量统计,如图6B所示,PCL/SF-R组纤维直径大于其他4组(P < 0.05,P < 0.01,P < 0.001),而后4组间纤维直径比较无差异(P > 0.05),说明加钛酸钙颗粒不会影响纤维的直径,而纺丝方式会影响纤维直径:与采用滚筒接收装置制备的纤维相比,采用金属网作为接收装置制备的静电纺丝纳米纤维具有更大的直径。通过对各组纤维取向角分布进行统计,如图6C所示,PCL/SF-R组取向角分布范围很广,在0°-100°范围内都有分布,而其他几组取向角分布比较集中,大多位于0°-15°之间,由此进一步证明在金属板上制备的静电纺丝纳米纤维呈随机方向分布,并且加入钛酸钙纳米颗粒不会影响纤维在滚筒上制备纤维的取向分布。"
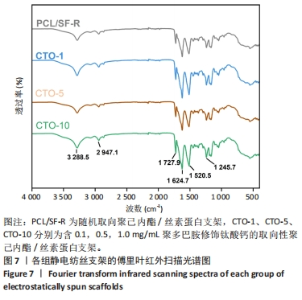
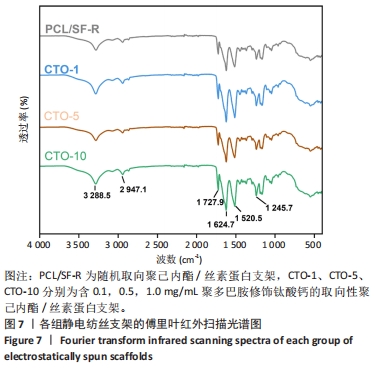
2.4 各组静电纺丝支架的物理表征结果 2.4.1 傅里叶红外扫描结果 使用傅里叶变换红外光谱仪对PCL/SF-R、CTO-1、CTO-5、CTO-10进行红外谱图分析(图7),结果显示4组样品都出现了丝素蛋白的酰胺Ⅰ带(C=O拉伸)、酰胺Ⅱ带(N=H弯曲)和酰胺Ⅲ带(C=N 弯曲振动)[33],3个酰胺带的主要吸收峰分别位于1 624.7,1 520.5,1 245.7 cm-1,这说明经过乙醇交联后丝素蛋白的空间构象发生改变,主要为β折叠结构;此外,可观察到位于2 947 cm-1(不对称-CH2拉伸)、1 727.9 cm-1(C-O振动)、3 288 cm-1(-OH伸缩振动),这与相关研究一致[34],表明了4组纤维膜中都有聚己内酯的存在。根据查阅资料显示,聚多巴胺的特征峰处于1 600-1 450 cm-1之间,这是芳香族化合物的苯环中的C=C键振动引起的[35],但此次实验傅里叶变换红外光谱结果中并不明显,可能是由于CTO-1、CTO-5、CTO-10中聚多巴胺修饰的钛酸钙纳米颗粒质量浓度太低,并且与丝素蛋白的特征峰波数重叠,导致特征峰不明显。"
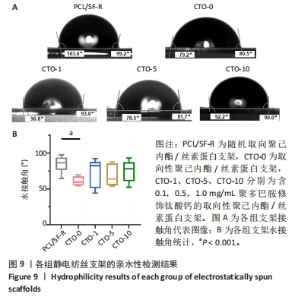
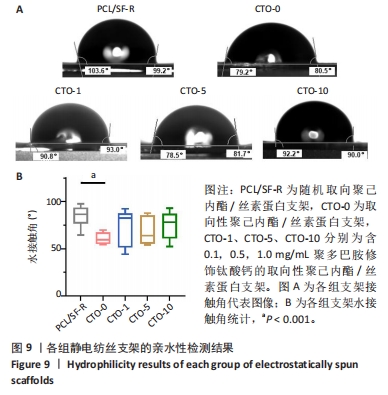
2.4.3 亲水性检测 为了评估静电纺丝纤维的排列结构及钛酸钙纳米颗粒的加入是否会影响膜的亲水性,通过测量支架表面的水接触角并对其进行量化分析,各组的接触角代表图像结果如图9A所示,统计结果如图9B所示。PCL/SF-R组、CTO-0组、CTO-1组、CTO-5组、CTO-10组纤维水接触角分别为(93.78±4.05)°,(84.20±2.35)°,(89.40±7.30)°,(86.95±5.62)°,(90.00±5.50)°。PCL/SF-R组纤维水接触角大于CTO-0组,并且最高水接触角为99°;CTO-1组水接触角高于CTO-0组,这表明向支架中加入低质量浓度钛酸钙纳米颗粒后材料亲水性降低;但当再增加钛酸钙纳米颗粒质量浓度时,CTO-5组支架的水接触角会减小,意味着膜亲水性的改善,这归因于聚多巴胺修饰后的钛酸钙纳米颗粒表面存在亲水羟基和氨基;然而,将钛酸钙纳米颗粒质量浓度增加到1 mg/mL并不会导致水接触角的进一步减小,这可能是与支架的表面粗糙度有关,支架的表面粗糙度随着钛酸钙纳米颗粒质量浓度的增加而增加,这可归因于纳米颗粒的团聚。纳米颗粒由于其高表面能而在较高质量浓度下团聚,这导致它们在较高浓度的聚合物溶液中分散很少,团聚会增加膜的表面粗糙度,从而导致支架亲水性降低。"
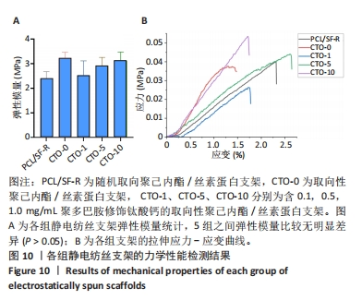
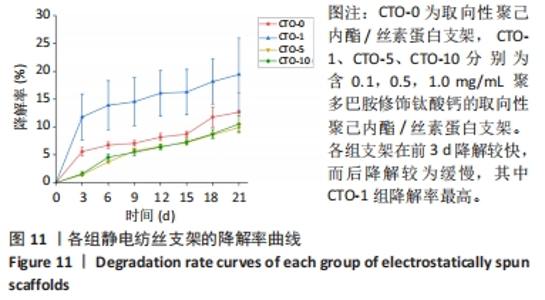
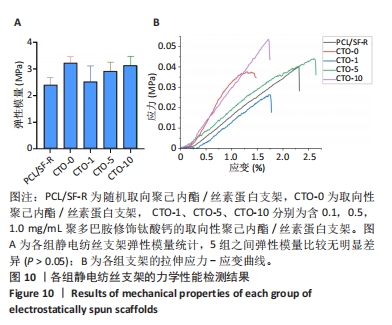
2.4.4 力学性能检测 各组静电纺丝支架的力学性能检测结果,如图10所示。图10A显示了各组支架弹性模量统计结果,PCL/SF-R组、CTO-0组、CTO-1组、CTO-5组、CTO-10组弹性模量分别为(2.40±0.28),(3.22±0.24),(2.53±0.59),(2.90±0.34),(3.13±0.35) MPa。图10B是各组静电纺丝支架的代表性应力-应变曲线。各组弹性模量经统计后没有显著差异,但PCL/SF-R组弹性模量略低于CTO-0支架,这表明纤维的取向排列方式会影响纤维的力学性能,随机取向纤维的力学性能要低于定向排列的纤维支架;并且掺杂聚多巴胺修饰的钛酸钙颗粒后,CTO-1组弹性模量要略低于未加入纳米颗粒的CTO-0组,然而随着钛酸钙纳米颗粒负载质量浓度的增加,支架的弹性模量呈上升趋势,但彼此间没有显著差异。"
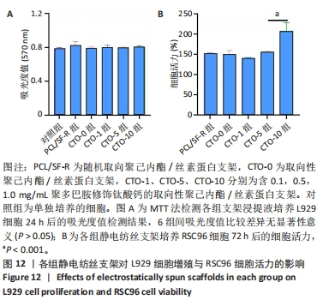
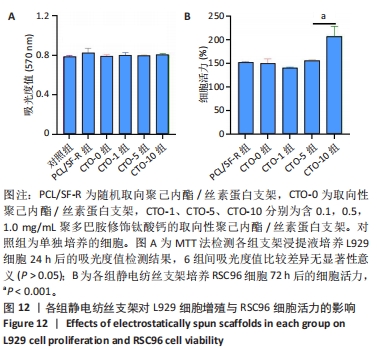
2.5 各组静电纺丝支架的细胞毒性检测结果 MTT检测结果显示,各组支架上的细胞吸光度值与对照组相比较差异无显著性意义(P > 0.05),见图12A所示。以上结果表明实验制备的5组支架材料无明显的细胞毒性,适用于后续的RSC96细胞培养。 2.6 各组静电纺丝支架对RSC96细胞活力的影响 通过CCK-8检测结果计算各组RSC96细胞活力,结果见图12B所示。结果显示,PCL/SF-R、CTO-0、CTO-1、CTO-5组之间的RSC96细胞活力无显著差异(P > 0.05),随着钛酸钙纳米颗粒质量浓度的进一步增加,支架促进细胞活力的效果更加显著,CTO-10组细胞活力显著高于其他4组(P < 0.001)。"
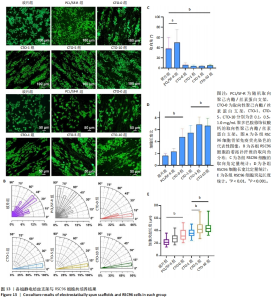
2.7 各组静电纺丝支架对RSC96细胞形态指数的影响 为了观察RSC96细胞在破片与各组静电纺丝支架上的排列和生长情况,对细胞骨架进行了免疫荧光染色和荧光显微镜观察,结果如图13A所示。在低倍率荧光显微镜下,CTO-0组、CTO-1组、CTO-5组、CTO-10组RSC96细胞呈定向排列,细胞呈长梭形,伪足沿着纤维方向延伸;而在玻璃片上(对照组)和PCL/SF-R上培养的RSC96细胞呈圆形,突起较短,分布与排列的方向不规则。 为进一步比较各组RSC96细胞的定向排列情况,对RSC96细胞取向角进行了统计,结果见图13B,C所示。对照组、PCL/SF-R组RSC96细胞取向角分布在0°-90°之间,而CTO-0组、CTO-1组、CTO-5组、CTO-10组RSC96细胞取向角集中分布在0°-15°之间,表明取向纤维支架上培养的RSC96细胞取向角显著低于PCL/SF-R组和对照组。 另外,CTO-0组RSC96细胞的长宽比和细胞突起长度显著高于PCL/SF-R组和对照组(P < 0.001),CTO-5组RSC96细胞的长宽比和细胞突起长度著高于CTO-0组(P < 0.001),见图13D,E。"
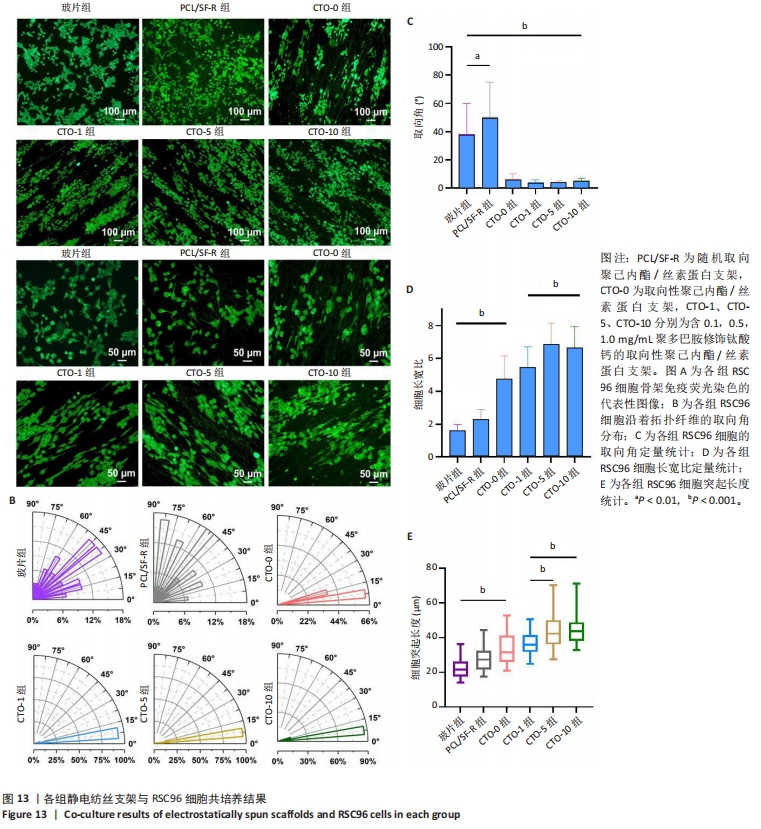
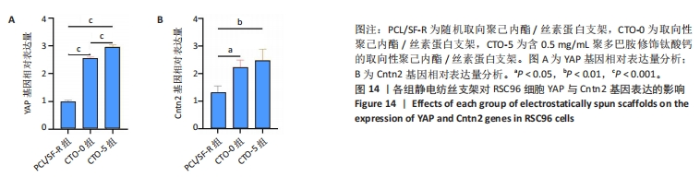
以上结果都表明,取向静电纺丝纤维支架对施万细胞的定向生长具有引导作用,并且负载钛酸钙纳米颗粒后会进一步促进导向作用。 2.8 各组静电纺丝支架对RSC96细胞相关基因表达的影响 采用qPCR技术对各组细胞骨架相关基因YAP和细胞黏附相关基因Cntn2相对表达进行了分析,结果如图14所示。结果显示,CTO-0组和CTO-5组YAP mRNA表达高于PCL/SF-R组(P < 0.001),CTO-5组YAP mRNA表达高于CTO-0组(P < 0.001),明钛酸钙纳米颗粒和拓扑支架协同诱导RSC96细胞骨架发生变化,从而引导细胞沿着拓扑纤维延伸;CTO-0组和CTO-5组Cntn2 mRNA表达高于PCL/SF-R组(P < 0.05,P < 0.01),表明该CTO-0、CTO-5能促进RSC96细胞黏附。"
| [1] SOLOMEVICH SO, ORANGES CM, KALBERMATTEN DF, et al. Natural polysaccharides and their derivatives as potential medical materials and drug delivery systems for the treatment of peripheral nerve injuries. Carbohydr Polym. 2023;315:120934. [2] JUCKETT L, SAFFARI TM, ORMSETH B, et al. The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury. Biomolecules. 2022;12(12):1856. [3] ADIDHARMA W, WANG Y, KOTSIS SV, et al. Utilization Trends of Nerve Autograft Alternatives for the Reconstruction of Peripheral Nerve Defects. Plast Reconstr Surg. 2024;153(4):863-872. [4] BITTNER GD, BUSHMAN JS, GHERGHEREHCHI CL, et al. Typical and atypical properties of peripheral nerve allografts enable novel strategies to repair segmental-loss injuries. J Neuroinflammation. 2022;19(1):60. [5] BU Y, WANG X, LI L, et al. Lithium Loaded Octa-Poly(Ethylene Glycol) Based Adhesive Facilitates Axon Regeneration and Reconnection of Transected Peripheral Nerves. Adv Healthc Mater. 2020;9(13):e2000268. [6] THIBODEAU A, GALBRAITH T, FAUVEL CM, et al. Repair of peripheral nerve injuries using a prevascularized cell-based tissue-engineered nerve conduit. Biomaterials. 2022;280:121269. [7] SAMADIAN H, MALEKI H, FATHOLLAHI A, et al. Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration. Int J Biol Macromol. 2020;154:795-817. [8] HUANG X, AN Y, YUAN S, et al. Silk fibroin carriers with sustained release capacity for treating neurological diseases. Front Pharmacol. 2023;14: 1117542. [9] SEMMLER L, NAGHILOU A, MILLESI F, et al. Silk-in-Silk Nerve Guidance Conduits Enhance Regeneration in a Rat Sciatic Nerve Injury Model. Adv Healthc Mater. 2023;12(11):e2203237. [10] YAO D Y, WANG T, ZHANG X L, et al. High Concentration Crystalline Silk Fibroin Solution for Silk-Based Materials. Materials (Basel). 2022; 15(19):6930. [11] XUAN H, TANG X, ZHU Y, et al. Freestanding Hyaluronic Acid/Silk-Based Self-healing Coating toward Tissue Repair with Antibacterial Surface. ACS Appl Bio Mater. 2020;3(3):1628-1635. [12] GU X, CHEN X, TANG X, et al. Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration. Nanotechnol Rev. 2021;10(1):10-19. [13] ZHANG H, ZHANG H, WANG H, et al. Natural proteins-derived asymmetric porous conduit for peripheral nerve regeneration. Appl Mater Today. 2022; 27:101431. [14] CHEN X, TANG X, WANG Y, et al. Silk-inspired fiber implant with multi-cues enhanced bionic microenvironment for promoting peripheral nerve repair. Mater Sci Eng C Mater Biol Appl. 2022;135:112674. [15] AZNAR-CERVANTES SD, VICENTE-CERVANTES D, MESEGUER-OLMO L, et al. Influence of the protocol used for fibroin extraction on the mechanical properties and fiber sizes of electrospun silk mats. Mater Sci Eng C Mater Biol Appl. 2013;33(4):1945-1950. [16] DíAZ E, SANDONIS I, VALLE MB. In Vitro Degradation of Poly(caprolactone)/nHA Composites. J Nanomater. 2014;2014:8. [17] MAO X, LI T, CHENG J, et al. Nerve ECM and PLA-PCL based electrospun bilayer nerve conduit for nerve regeneration. Front Bioeng Biotechnol. 2023;11:1103435. [18] XU H, GAO Z, WANG Z, et al. Electrospun PCL Nerve Conduit Filled with GelMA Gel for CNTF and IGF-1 Delivery in Promoting Sciatic Nerve Regeneration in Rat. ACS Biomater Sci Eng. 2023;9(11):6309-6321. [19] HAJIALI F, TAJBAKHSH S, SHOJAEI A. Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review. Polym Rev. 2018;58(1):164-207. [20] CHEN HW, LIN MF. Characterization, biocompatibility, and optimization of electrospun SF/PCL/CS composite nanofibers. Polymers. 2020;12(7):1439. [21] WANG X, PENG Y, WU Y, et al. Chitosan/silk fibroin composite bilayer PCL nanofibrous mats for bone regeneration with enhanced antibacterial properties and improved osteogenic potential. Int J Biol Macromol. 2023; 230:123265. [22] JIA X, ZHOU J, NING J, et al. The polycaprolactone/silk fibroin/carbonate hydroxyapatite electrospun scaffold promotes bone reconstruction by regulating the polarization of macrophages. Regen Biomater. 2022;9: rbac035. [23] GUAN Y, REN Z, YANG B, et al. Dual-bionic regenerative microenvironment for peripheral nerve repair. Bioact Mater. 2023;26:370-386. [24] LI G, XIAO Q, MCNAUGHTON R, et al. Nanoengineered porous chitosan/CaTiO(3) hybrid scaffolds for accelerating Schwann cells growth in peripheral nerve regeneration. Colloids Surf B Biointerfaces. 2017;158:57-67. [25] VIEIRA FP, GONINI JÚNIOR A, PIVA E, et al. Experimental resin-based dual-cured calcium aluminate and calcium titanate materials for vital pulp therapy. Braz Oral Res. 2022;36:e037. [26] MERT T, GUNES Y, GUVEN M, et al. Effects of calcium and magnesium on peripheral nerve conduction. Pol J Pharmacol. 2003;55(1):25-30. [27] THUY BA LINH N, MONDAL D, LEE BT. In vitro study of CaTiO3-hydroxyapatite composites for bone tissue engineering. ASAIO J. 2014;60(6):722-729. [28] WU LL, ZHENG TT, GUAN WC, et al. Anisotropic chitosan micropatterning containing metformin functionalized calcium titanate (CaTiO3) nanoparticles for regulating dorsal root ganglion behavior. Surf Interfaces. 2022;35:102414. [29] JANG SR, KIM JI, PARK CH, et al. The controlled design of electrospun PCL/silk/quercetin fibrous tubular scaffold using a modified wound coil collector and L-shaped ground design for neural repair. Mater Sci Eng C Mater Biol Appl. 2020;111:110776. [30] XUE W, SHI W, KONG Y, et al. Anisotropic scaffolds for peripheral nerve and spinal cord regeneration. Bioact Mater. 2021;6(11):4141-4160. [31] LI L, LI H, QIAN Y, et al. Electrospun poly (ɛ-caprolactone)/silk fibroin core-sheath nanofibers and their potential applications in tissue engineering and drug release. Int J Biol Macromol. 2011;49(2):223-232. [32] 武郭敏.改良丝素蛋白/聚己内酯纳米纤维膜的制备及其皮肤再生或骨再生性能的研究[D].武汉:武汉大学,2020. [33] RAMAPPA VK, SINGH V, SRIVASTAVA D, et al. Fabrication of mulberry leaf extract (MLE)- and tasar pupal oil (TPO)-loaded silk fibroin (SF) hydrogels and their antimicrobial properties. 3 Biotech. 2023;13(2):37. [34] AFZAL A, JALALAH M, NOOR A, et al. Development and Characterization of Drug Loaded PVA/PCL Fibres for Wound Dressing Applications. Polymers (Basel). 2023;15(6):1355. [35] XU Y, DENG Z, CHEN Y, et al. Preparation and characterization of mussel-inspired hydrogels based on methacrylated catechol-chitosan and dopamine methacrylamide. Int J Biol Macromol. 2023;229:443-451. [36] YUAN L, CHEN Q, RIVIERE JE, et al. Pharmacokinetics and tumor delivery of nanoparticles. J Drug Deliv Sci Technol. 2023;83:104404. [37] HU SY, WU JH, CUI ZX, et al. Study on the mechanical and thermal properties of polylactic acid/hydroxyapatite@polydopamine composite nanofibers for tissue engineering. J Appl Polym Sci. 2020;137(36):e49077. [38] ZHANG JL, WU QL, ZHANG XQ, et al. Mechanical and water resistance performance of physically cross-linked poly(vinyl alcohol) composite with poly(dopamine) modified cellulose nanocrystals. Mater Today Commun. 2022;33:104413. [39] ZHANG JC, XU YW, ZHANG YQ, et al. Facile construction of calcium titanate-loaded silk fibroin scaffolds hybrid frameworks for accelerating neuronal cell growth in peripheral nerve regeneration. Heliyon. 2023;9(4):e15074. [40] HOFFMAN-KIM D, MITCHEL JA, BELLAMKONDA RV. Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203-231. [41] JEANETTE H, MARZIALI LN, BHATIA U, et al. YAP and TAZ regulate Schwann cell proliferation and differentiation during peripheral nerve regeneration. Glia. 2021;69(4):1061-1074. [42] ZOU D, ZHOU X, LIU J, et al. MiR-34a regulates Schwann cell proliferation and migration by targeting CNTN2. Neuroreport. 2020;31(17):1180-1188. [43] ZHENG TT, WU LL, XU JW, et al. YR/DFO@DCNT functionalized anisotropic micro/nano composite topography scaffolds for accelerating long-distance peripheral nerve regeneration. Compos B Eng. 2022;246:110242. [44] GUAN W, GAO H, SUN S, et al. Multi-scale, multi-level anisotropic silk fibroin/metformin scaffolds for repair of peripheral nerve injury. Int J Biol Macromol. 2023;246:125518. |
| [1] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [2] | Gang Fangli, Dang Zhongxiu, Li Ruiyun, Li Xiao, Sun Xiaoyang. Techniques and performance of biominerals for fabricating bone tissue engineering scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 5957-5967. |
| [3] | An Jiangru, Zhang Jinyi, Wang Qiuhua, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Mesenchymal stem cells combined with polycaprolactone-hyaluronic acid electrospinning membrane in repair of endometrial injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3369-3379. |
| [4] | Tian Jiayu, Li Duohua, Zhang Feng, Feng Hu, Sun Wei. Construction and performance evaluation of polycaprolactone/nanodiamond-phospholipid composite materials [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3380-3387. |
| [5] | Li Yonghang, Li Wenming, Yan Caiping, Wang Xingkuan, Xiang Chao, Zhang Yuan, Jiang Ke, Chen Lu. Critical bone defect repaired with anti-fibrosis and “H”-type core-shell bionic scaffold [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3420-3431. |
| [6] | Shen Ziqing, Xia Tian, Shan Yibo, Zhu Ruijun, Wan Haoxin, Ding Hao, Pan Shu, Zhao Jun. Vascularized tracheal substitutes constructed by exosome-load hydrogel-modified 3D printed scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 697-705. |
| [7] | Gu Mingxi, Wang Changcheng, Tian Fengde, An Ning, Hao Ruihu, Guo Lin. Preparation and in vitro evaluation of a three-dimensional porous cartilage scaffold made of silk fibroin/gelatin/chitosan [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 366-372. |
| [8] | Guo Qingxia, Wang Yue, Wu Tong. Silk fibroin methacryloyl hydrogel loaded with Scriptaid regulates polarization of microglia cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4628-4633. |
| [9] | Wang Lu, Xu Jie, Xia Yijing, Zhang Xinsong, Zhao Bin. Preparation and characterization of silk fibroin/bioactive glass composite fiber membrane [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3457-3463. |
| [10] | Liu Ziyang, Lao An, Xu Chenci, AiRi Shin, Wu Jiaqing, Liu Jiaqiang. Polycaprolactone-polydopamine-AOPDM1 scaffold promotes bone formation in a high-glucose environment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2667-2674. |
| [11] | Li Shuo, Hu Haolei, Yang Jie, Xu Tao, Yin Gang, Li Yi. Repair of chronic tympanic membrane perforation by bone marrow mesenchymal stem cells-loaded high-porosity polycaprolactone-collagen nanofiber membrane scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2371-2377. |
| [12] | Li Xiaoyin, Yang Xiaoqing, Chen Shulian, Li Zhengchao, Wang Ziqi, Song Zhen, Zhu Daren, Chen Xuyi. Collagen/silk fibroin scaffold combined with neural stem cells in the treatment of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 890-896. |
| [13] | Wang Ziqi, Li Xiaoyin, Jiang Jipeng, Song Zhen, Li Zhengchao, Chen Shulian, Chen Xuyi. Collagen/silk fibroin scaffold combined with human umbilical cord-derived mesenchymal stem cells in the treatment of traumatic brain injury in dogs [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5483-5490. |
| [14] | Huang Jinsheng, Zhang Geyi, Li Senrui, Li Jiangnan, Lu Laijin, Zhou Nan. Lymphatic endothelial cells-derived exosomes promote axonal regeneration following peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5314-5319. |
| [15] | Liu Jichao, Zhao Jinlong, Yu Yang. Silk fibroin collagen composite scaffold combined with platelet-rich plasma for repairing skin injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3971-3976. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||