Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (22): 3561-3568.doi: 10.12307/2024.490
Previous Articles Next Articles
Biological and physicochemical properties of bioactive ion modified brushite cements
Zeng Cheng1, Yu Huanhuan1, Gong Yukang1, Wang Chenhao2, Zhang Yinen1, Gao Wenshan1
- 1Department of Orthopedics, Affiliated Hospital of Hebei University, Baoding 071000, Hebei Province, China; 2School of Basic Medicine, Hebei University, Baoding 071000, Hebei Province, China
-
Received:2023-08-21Accepted:2023-10-07Online:2024-08-08Published:2024-01-20 -
Contact:Gao Wenshan, Chief physician, Professor, Doctoral supervisor, Department of Orthopedics, Affiliated Hospital of Hebei University, Baoding 071000, Hebei Province, China -
About author:Zeng Cheng, Master candidate, Department of Orthopedics, Affiliated Hospital of Hebei University, Baoding 071000, Hebei Province, China
CLC Number:
Cite this article
Zeng Cheng, Yu Huanhuan, Gong Yukang, Wang Chenhao, Zhang Yinen, Gao Wenshan. Biological and physicochemical properties of bioactive ion modified brushite cements[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3561-3568.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
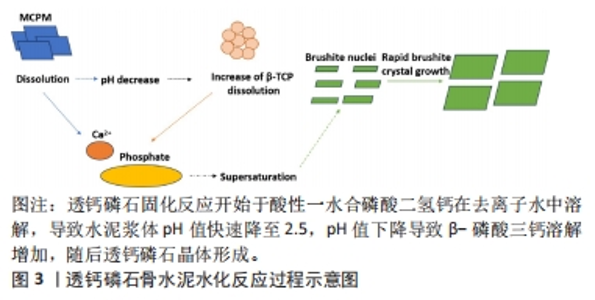
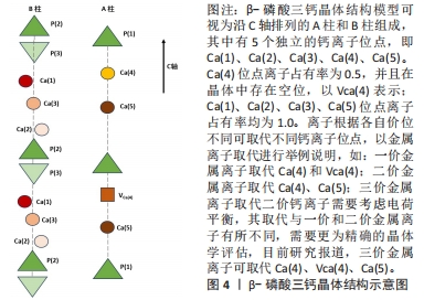
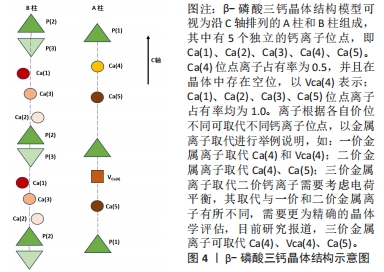
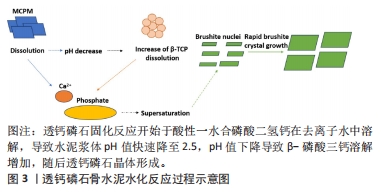
2.1 透钙磷石骨水泥概述 透钙磷石骨水泥是1989年首次由Mirtchi和Lemaitre将酸性磷酸钙[一水合磷酸二氢钙(Ca(H2PO4)2?H2O,MCPM)]与碱性磷酸钙[β-磷酸三钙(Ca3(PO4)2,β-TCP)]组成粉末与水混合形成可模制的糊状物,并在放热反应中逐渐固化形成主要由二水磷酸氢钙(CaHPO4?2H2O)组成的硬质材料,其矿物名称为“透钙磷石”;反应公式如下:β-Ca3(PO4)2+Ca(H2PO4)2?H2O+7 H2O → 4 CaHPO4?2H2O[9],见图3。透钙磷石骨水泥的获得均是酸碱反应的过程,目前已提出的制备透钙磷石骨水泥的配方:β-TCP+MCPM 、β-TCP+H3PO4、TetCP(磷酸四钙)+MCPM+CaO,其中β-TCP+MCPM 是研究及应用最为广泛的[10-11]。"
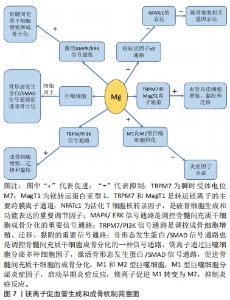
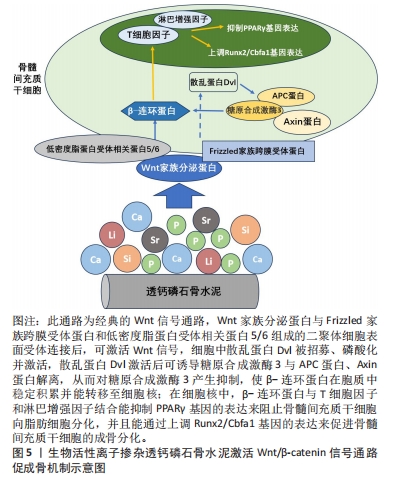
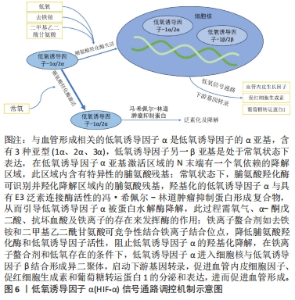
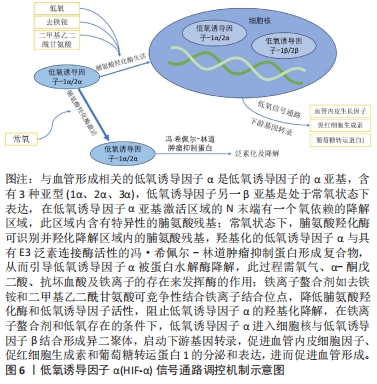
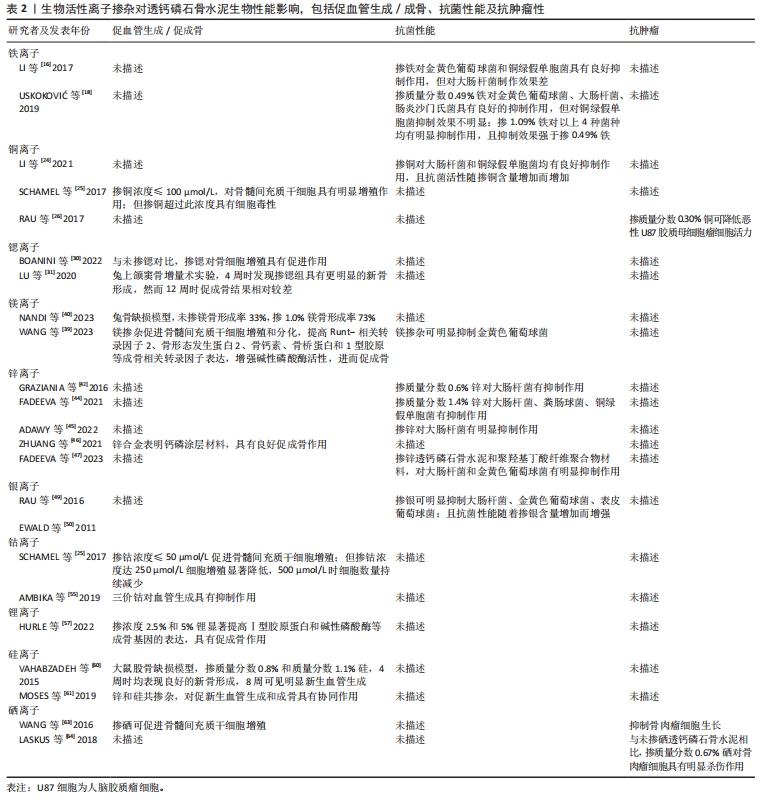
对理化性能影响的研究显示,与未掺铁透钙磷石骨水泥相比,1%和5%铁掺杂可显著延长凝固时间,分别达20 min和123 min[16]。 VAHABZADEH等[17]的研究也表明掺不同浓度铁(质量分数0.25%,0.5%,1.00%)的透钙磷石骨水泥,其凝固时间均明显延长,且存在剂量依赖;该研究还发现,与未掺铁透钙磷石骨水泥抗压强度(7.05±1.60) MPa相比,质量分数0.25%和0.5%铁掺杂对抗压强度无明显影响,但质量分数1.00%铁会导致抗压强度明显降低至(3.12±1.06) MPa,说明铁离子作为掺杂剂影响透钙磷石骨水泥晶体的相纯度和稳定性,进而改变其力学性能,可见铁改性作用效果与其浓度密切相关。 针对生物性能方面的研究,发现掺铁的透钙磷石骨水泥对金黄色葡萄球菌和铜绿假单胞菌具有良好的抑制作用,但对大肠杆菌敏感性相对较差;尽管大肠杆菌和铜绿假单胞菌同属革兰阴性菌,其细胞壁上肽聚糖层次少,但铁掺杂对铜绿假单胞菌抑制作用强于大肠杆菌,这可能是由于掺铁透钙磷石骨水泥产生了特殊的微环境,减弱了对大肠杆菌的毒性作用,具体机制需进一步研究[16]。USKOKOVI?等[18]将不同浓度铁(质量分数0.49%和1.09%)掺入透钙磷石骨水泥,评估其抗菌性能(金黄色葡萄球菌、大肠杆菌、铜绿假单胞菌和肠炎沙门氏菌),结果显示低浓度(质量分数0.49%)铁掺入骨水泥组对铜绿假单胞菌的抗菌效果不明显,但对其他3种菌株有明显的抑制作用;质量分数1.09%掺铁骨水泥对以上4种菌株均有明显的抑制作用,且抗菌效果较低浓度组更好。 以上研究表明,铁掺杂能显著提高透钙磷石骨水泥的力学、机械及生物性能,其作用效果与铁浓度密切相关,且具有一定的抗菌性,在骨修复领域具有良好的应用前景。掺入铁最适宜的浓度是未来需要重点关注的方向之一,以达到最佳改性效果。 2.3.2 铜 铜离子可促进成骨细胞分化,抑制破骨细胞分化,并通过缺氧激活低氧诱导因子α通路,上调血管内皮细胞生长因子的表达,促进内皮细胞增殖、迁移和血管化,发挥促血管生成的作用,进而促进成骨[19-20]。铜离子具备良好的生物相容性,还表现出抗菌性,能有效抑制大肠杆菌、金黄色葡萄球菌和耐甲氧西林金黄色葡萄球菌等[21-23]。 铜离子依赖其良好的血管生成活性、成骨活性、生物相容性和抗菌性,在骨科领域具有良好的应用前景。有学者进行了铜改性透钙磷石骨水泥的研究。对理化性能方面的研究显示,与未掺铜透钙磷石骨水泥凝固时间12 min相比,5%铜掺杂显著延长了凝固时间,达到了50 min;未掺铜透钙磷石骨水泥抗压强度为24 MPa,1%铜掺杂可显著提高抗压强度达27 MPa,而3%和5%铜掺杂透钙磷石骨水泥抗压强度分别为25 MPa和24 MPa;但无论是否掺铜对注射性能无明显影响,其注射系数均约为88%[24]。 针对生物性能方面,有研究认为铜离子的作用效果与其浓度相关。LI等[24]发现掺铜的透钙磷石骨水泥的抗菌活性随掺铜量的增加而增加。SCHAMEL等[25]研究了不同浓度的铜离子对骨髓间充质干细胞增殖的影响,结果显示当掺入铜离子浓度≤ 100 μmol/L时,随着培养时间的增加,骨水泥对细胞增殖具有明显的促进作用,但掺入铜离子浓度更高时,可产生细胞毒性,观察到细胞数量减少甚至死亡。有趣的是,RAU等[26]研究发现质量分数0.30%含铜透钙磷石骨水泥可降低恶性U87胶质母细胞瘤细胞活力,表明铜具有一定的抗肿瘤潜力。 以上研究表明,铜离子掺杂可增强透钙磷石骨水泥的使透钙磷石骨水泥具备良好的促进血管生成、成骨和抗感染甚至抗肿瘤能力,在需要预防感染以及肿瘤引起的骨缺损治疗具有良好的应用前景。 2.3.3 锶 锶离子能同时促进骨形成并抑制骨吸收,对人体骨代谢具有重要作用[27]。基于锶离子双重作用的特性,临床上将锶制剂雷奈酸锶应用于抗骨质疏松的治疗[28]。近年来有学者将锶掺入透钙磷石骨水泥,以期能应用于局部骨组织的修复中,尤其是骨质疏松性骨再生的应用。 对理化性能影响的研究显示,质量分数2.4%和6.6%锶掺杂的透钙磷石骨水泥最初凝固时间分别延长为12 min和16 min,最终凝固时间分别延长为21 min和25 min,注射系数分别增加为66%和69%;但进一步增加锶含量(质量分数7.4%和8.9%),凝固时间和可注射性则显著降低[29]。质量分数6.6%锶掺杂的透钙磷石骨水泥抗压强度由1.32 MPa增加到34 MPa[29]。 对生物性能影响的研究显示,BOANINI等[30]的研究发现掺锶的透钙磷石骨水泥在刺激胶原蛋白生成和抑制破骨细胞活性方面具有显著效果,证实了锶掺杂在促进骨骼生长和抑制过度骨吸收方面的有益作用。但也有研究持不同观点,LU等[31]将锶掺杂的透钙磷石骨水泥应用于兔上颌窦骨增量术中,发现在4周时表现出最佳的骨传导性和新骨形成能力;然而随着时间的推移,在12周时表现出相对较差的最终骨再生和植入物移除扭矩,这主要是由于空间保持能力差和吸收过快。 以上研究表明,合适浓度的锶掺杂可改善透钙磷石骨水泥机械性能,也可促进骨形成、减少骨吸收,但需注意其降解速度快及空间保持能力差的局限性。未来的研究需进一步寻找最适宜的锶掺杂浓度,以发挥其最大优势。 2.3.4 镁 镁离子是维持骨骼健康的重要因子,一方面,镁离子通过激活MAPK/ERK信号通路,直接促进骨髓间充质干细胞的增殖和成骨分化;另一方面,镁离子可刺激巨噬细胞分泌细胞因子,并通过骨形态发生蛋白/SMAD信号通路促进成骨分化;同时镁还通过TRPM/PI3K信号通路促进成骨细胞增殖、迁移和黏附;也可抑制核转录因子κB通路,直接抑制NFATc1的表达,抑制破骨细胞相关基因的表达,从而抑制破骨细胞形成及骨吸收活性[32-33]。 此外,大量研究证实镁通过TRPM7和MagT1离子通道可增强血管内皮细胞增殖、黏附和迁移;且可促进M1向M2型巨噬细胞转化,抑制炎症因子分泌,表明了镁具有促血管生成和调节免疫反应的功能[34-36],见图7。因此,镁在骨再生的研究应用中有着巨大的潜力和吸引力,有学者利用镁改性透钙磷石骨水泥,取得一定的进展。"
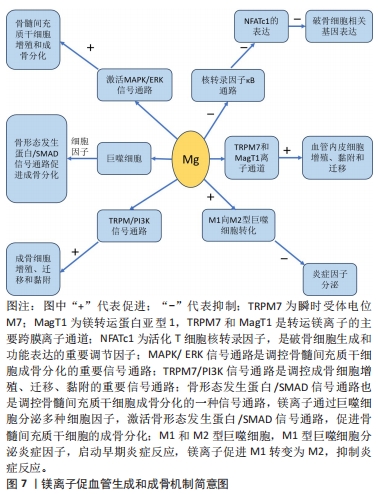
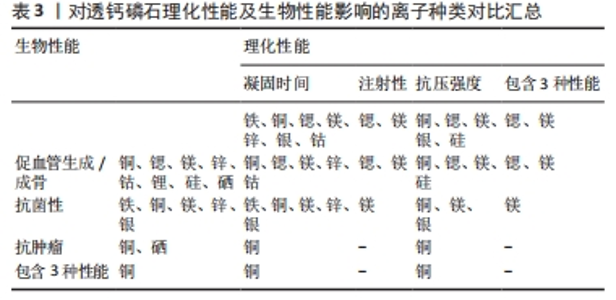
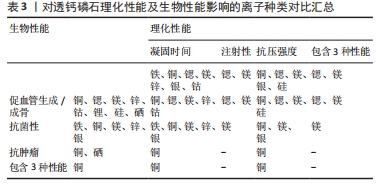
对理化性能影响的研究显示,随着掺杂镁含量的增加,初始和最终凝固时间随之增加,与未掺杂镁最终凝固时间4 min相比,当透钙磷石骨水泥晶体结构中镁掺杂浓度为镁离子/(镁离子+钙离子)摩尔比=0.1时,其最终凝固时间可达到33 min,可注射性由未掺杂镁的10%提高到77%,抗压强度也得到提高[37];ALKHRAISAT等[38]的研究也发现掺杂13.3%的镁也显著延长了最终凝固时间。WANG等[39]通过镁基有机金属框架的添加将镁离子引入透钙磷石骨水泥体系,发现添加比例达0.5%时,透钙磷石骨水泥抗压强度由未掺镁的27 MPa提高到32 MPa。 针对生物性能,NANDI等[40]利用兔骨缺损模型,发现与纯透钙磷石骨水泥骨形成率33%相比,掺质量分数1.0%镁的透钙磷石骨水泥可达73%。WANG等[39]也发现镁掺杂促进了骨髓间充质干细胞增殖和成骨分化,提高了Runt-相关转录因子2、骨形态发生蛋白2、骨钙素、骨桥蛋白和Ⅰ型胶原等成骨相关转录因子的表达,并增强了碱性磷酸酶活性。此外,该研究还采用脂多糖诱导的巨噬细胞模型评估了复合水泥的抗炎特性,发现镁掺杂可调节炎症因子并促进了M1巨噬细胞向M2巨噬细胞的复极化;在抗菌性方面,镁掺杂透钙磷石复合水泥可明显抑制金黄色葡萄球菌(存活率< 10%)[39]。 以上研究表明,镁掺杂透钙磷石骨水泥可明显提高凝固时间、可注射性和抗压强度,并在成骨、抗炎和抗菌等生物性能改善方面发挥重要作用。 2.3.5 锌 锌离子不仅是骨组织的组成成分,还促进骨胶原蛋白合成、成骨细胞新骨生成和矿化,并参与调节OPG/RANK/RANKL信号通路,利于骨骼重塑[41]。在理化性能影响方面,未掺杂锌、质量分数0.6%和1.2%锌掺杂的透钙磷石骨水泥凝固时间分别为7,13和19 min[42]。质量分数0.25%锌掺杂可延长透钙磷石骨水泥最初和最终凝固时间,但抗压强度降低[43]。与之相反,有研究发现质量分数1.4%锌显著改善了骨水泥的抗压强度,达到(17.5±1.6) MPa,这可能是由于该浓度锌掺杂使透钙磷石骨水泥孔隙率更低,结构密度更大[44]。在生物性能影响方面,质量分数1.4%锌掺杂提升了骨水泥的生物相容性[44],质量分数0.25%锌联合0.5%硅可促进骨再生[43]。质量分数0.6%锌掺杂的透钙磷石骨水泥可抑制大肠杆菌[42];质量分数1.4%锌掺杂可抑制大肠杆菌,粪肠球菌和铜绿假单胞菌[44];ADAWY等[45]的研究也证实锌掺杂具有较好的抗菌性。 锌合金因具有适宜降解速率和良好的生物相容性,吸引了研究者们将锌离子以合金的形式应用于骨缺损修复的研究中,如ZHUANG等[46]制备了一种表面钙磷涂层改性的新型多孔锌合金支架,结果表明,支架材料具有良好的生物相容性、促进成骨细胞分化和新骨形成的特性。还有研究将锌以复合材料的形式应用于骨缺损修复的研究中, FADEEVA等[47]将掺锌透钙磷石骨水泥涂覆在聚羟基丁酸纤维聚合物上,该复合材料对大肠杆菌和金黄色葡萄球菌均有明显的抑制作用,同时表现出较好的生物相容性,有望用于合并细菌感染的骨缺损修复治疗中。 以上研究表明,锌离子可显著改善透钙磷石骨水泥的理化性能和生物相容性,并具有抗菌特性;以锌合金或复合材料的形式应用于骨组织工程中具有一定的潜力。 2.3.6 其他金属离子 银离子具有良好的抗菌特性,可作为掺杂剂掺入骨水泥应用于骨髓炎的治疗中[48]。关于理化性能的影响,RAU等[49]发现随着掺入银含量的增加(质量分数0%,0.6%和1.0%),凝固时间逐渐增加(分别为3,5和7 min),而抗压强度逐渐降低。但有研究却发现掺杂1%银能使透钙磷石骨水泥的抗压强度提高30%以上[50]。两项研究显示,银掺杂对透钙磷石骨水泥抗压强度产生了完全相反的影响,这可能是由于掺入质量分数0.6%和1.0%银的透钙磷石骨水泥终产物中形成了二价多磷酸钙银。针对生物性能的研究,多项研究均证明,掺杂银可显著提高透钙磷石骨水泥的抗菌性能,抑制大肠杆菌、金黄色葡萄球菌和表皮葡萄球菌,且随银含量的增加其抗菌性逐渐增强[49-50]。SAYAHI等[51]的研究显示,与纯透钙磷石骨水泥相比,银掺杂使其对脂肪间充质干细胞具有更小的毒性作用,且增强了碱性磷酸酶表达。 钴离子在缺氧微环境下通过激活低氧诱导因子1α信号通路,上调血管内皮生长因子的表达,从而诱导血管生成[52]。有研究报道较低浓度(0.25%)钴掺杂可提高骨替代材料的成骨和新生血管生成[53]。在理化性能影响方面,与未掺钴透钙磷石骨水泥相比,质量分数1.2%钴掺杂可使透钙磷石骨水泥初始凝固时间达(6.80±0.08) min,且最终凝固时间增加2.2-2.7倍;但质量分数0.3%和0.5%钴掺杂却有所减少;不同浓度钴掺杂对凝固时间产生了相反影响,质量分数1.2%钴掺杂延长凝固时间可能是由于钴离子比钙离子更小,较高浓度钴使得透钙磷石骨水泥晶体结构稳定性更高,导致溶解度降低,而质量分数0.3%和0.5%钴掺杂减少凝固时间可能归因于较低浓度钴改性过程中形成了高溶解度的磷酸一氢钴[54]。同时抗压强度也产生了剂量依赖性的负面影响,其中质量分数1.2%钴掺杂透钙磷石骨水泥抗压强度由(3.17±1.04) MPa降低至(1.09±0.21) MPa[54]。由此可见,掺钴的浓度是一个关键因素,不同浓度的钴会对透钙磷石骨水泥的物相组成产生不同影响。对于生物性能影响方面,当掺钴浓度≤ 50 μmol/L时透钙磷石骨水泥促进骨髓间充质干细胞增殖,但浓度达250 μmol/L时细胞增殖显著降低,500 μmol/L则导致细胞数量持续下降[25]。高浓度钴掺杂可能具有细胞毒性,反而抑制细胞增殖,进而影响成骨和新生血管生成。值得注意的是,有研究报道三价钴对血管生成具有抑制作用[55]。故对于钴掺杂的研究应用中,除了探索其最适浓度外,还应注意严格控制钴离子价位,避免对骨修复产生负面影响。 锂离子可通过激活Wnt/β-catenin信号通路,增加血管内皮生长因子、转化生长因子β和胰岛素样生长因子1的表达,进而促进新生血管形成[56]。有研究报道,2.5%和5%锂掺杂的透钙磷石骨水泥均表现出较低的细胞毒性,且显著提高了Ⅰ型胶原蛋白和碱性磷酸酶等成骨基因的表达,然而该研究中凝固时间和抗压强度却随着锂含量的增加而降低,可能是由于锂掺杂导致透钙磷石骨水泥晶体结构、稳定性及溶解度的改变[57]。 2.4 非金属离子 目前关于非金属离子改性透钙磷石骨水泥的研究较少,主要集中在硅和硒离子,在理化和生物性能改善方面也展现出一定优势。 2.4.1 硅 硅是骨组织发育必需的微量元素,可刺激成骨细胞分化和矿化,促进体内骨矿物质密度增加[58-59]。理化性能研究显示,与未掺杂硅的透钙磷石骨水泥相比,质量分数1.1%硅掺杂使其抗压强度由(4.78±0.21) MPa提升至(5.53±0.53) MPa[60]。生物性能研究发现,质量分数0.8%和1.1%硅掺杂的透钙磷石骨水泥,4周时表现出良好的新骨形成,8周时出现明显的新生血管生成[60]。此外,MOSES等[61]分别将锌、硅、锌+硅掺杂透钙磷石骨水泥,发现锌和硅具有协同效应,锌和硅共同掺杂进一步增强了碱性磷酸酶表达,促进新骨形成和新生血管生成。 2.4.2 硒 硒在促进软骨前体细胞增殖和分化中有重要作用,硒缺乏与克山病、大骨节病、类风湿关节炎及骨质疏松密切相关[62]。中国张胜民教授团队开发出了硒掺杂羟基磷灰石生物材料,发现其促进骨髓间质干细胞增殖,抑制骨肉瘤细胞生长,还可显著改善健康组织功能,在骨恶性肿瘤切除术后骨修复领域具有良好前景[63]。LASKUS等[64]研究发现,与未掺硒透钙磷石骨水泥相比,体积分数0.67%硒掺杂对骨肉瘤细胞具有明显杀伤作用。 表1归纳总结了生物活性离子对透钙磷石骨水泥凝固时间、注射性、抗压强度的影响,这些离子对透钙磷石骨水泥促血管生成/成骨、抗菌性、抗肿瘤的影响见表2。表3汇总了能改善透钙磷石骨水泥凝固时间、注射性、抗压强度及促血管生成/成骨、抗菌性、抗肿瘤性能的离子种类。 由表1可见,铁、铜、锶、镁、锌、银、钴可延长透钙磷石骨水泥的凝固时间,锂可减少凝固时间,其中需要注意的是铁、钴和锂的掺杂浓度:质量分数0.5%和1%铁掺杂可延长凝固时间,但0.25%铁对凝固时间无影响;质量分数1.2%钴掺杂延长凝固时间,但0.3%和0.5%钴减少凝固时间;2.5%和5%锂掺杂减少凝固时间,且随锂离子含量增加而减少。锶、镁可提高透钙磷石骨水泥注射性,其中质量分数2.4%和6.6%锶提高注射性,但7.4%和8.9%锶掺杂却降低注射性。铜、锶、镁、银、硅掺杂可提高透钙磷石骨水泥抗压强度,而钴、锂掺杂降低抗压强度,特别强调的是两项关于锌掺杂的研究显示出不同的结果,VAHABZADEH等[43]发现掺质量分数0.25%锌抗压强度下降,FADEEVA等[44]发现掺质量分数1.4%锌显著提高抗压强度,可达(17.5±1.6) MPa,由此可见,锌离子对抗压强度的影响与掺杂浓度密切相关。"
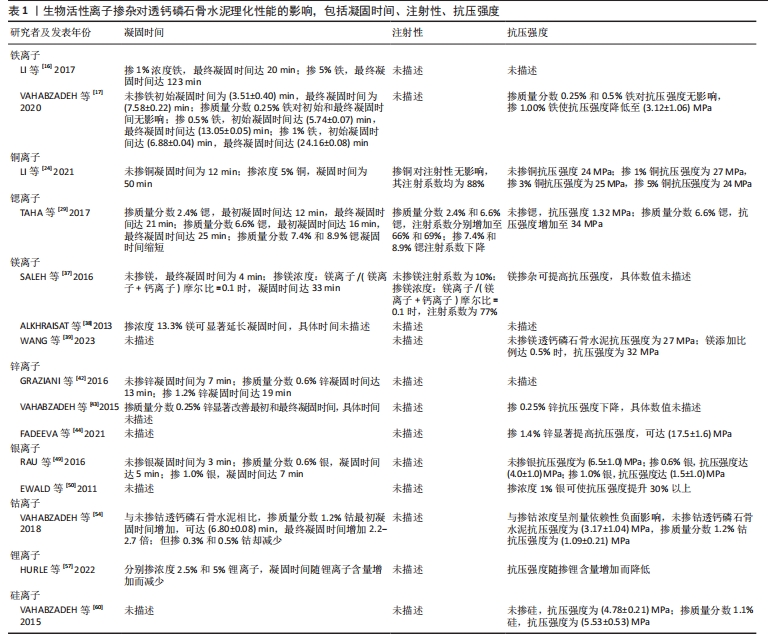

由表2可见,铜、锶、镁、锌、钴、锂、硅、硒掺杂透钙磷石骨水泥具有促血管生成/成骨作用,值得注意的是铜和钴掺杂浓度,掺铜浓度100 μmol/L以内时显著促进骨髓间充质干细胞增殖,但超过此浓度便具有细胞毒性,此外,掺钴浓度50 μmol/L以内时显著促进骨髓间充质干细胞增殖,但钴掺杂浓度大于250 μmol/L时则出现细胞增殖明显降低,且三价钴对血管生成具有抑制作用。铁、铜、镁、锌、银掺杂透钙磷石骨水泥具有抗菌性能。铜和硒掺杂透钙磷石骨水泥具有抗肿瘤特性。需要特别指出的是,目前关于以上生物活性离子改性,只有镁离子的抗炎性有研究进行报道,故此处未将抗炎性纳入总结标准。 由表3可见,生物活性离子掺杂可提升透钙磷石骨水泥的理化性能及生物性能,对于能改善其各种性能的离子种类总结如下:延长凝固时间:铁、铜、锶、镁、锌、银、钴;提高注射性:锶、镁;增强抗压强度:铜、锶、镁、银、硅;改善凝固时间+注射性+抗压强度:锶、镁;促血管生成/成骨:铜、锶、镁、锌、钴、锂、硅、硒;抗菌性:铁、铜、镁、锌、银;抗肿瘤:铜、硒;具有促血管生成/成骨+抗菌性+抗肿瘤:铜;改善凝固时间+注射性+抗压强及具有促血管生成/成骨+抗菌性:镁。"

| [1] SI L, WINZENBERG TM, JIANG Q, et al. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos Int. 2015;26(7):1929-1937. [2] LODOSO-TORRECILLA I, VAN DEN BEUCKEN JJJP, JANSEN JA. Calcium phosphate cements: optimization toward biodegradability. Acta Biomater. 2021;119:1-12. [3] NASROLLAHI N, DEHKORDI AN, JAMSHIDIZAD A, et al. Preparation of brushite cements with improved properties by adding graphene oxide. Int J Nanomedicine. 2019;14:3785-3797. [4] SHEIKH Z, ZHANG YL, GROVER L, et al. In vitro degradation and in vivo resorption of dicalcium phosphate cement based grafts. Acta Biomater. 2015;26:338-346. [5] 彭磊,丁秀明,陈克伟,等.羟基磷灰石体系透钙磷灰石骨水泥的理化性能[J].中国组织工程研究,2018,22(6):821-826. [6] DING L, WANG H, LI J, et al. Preparation and characterizations of an injectable and biodegradable high-strength iron-bearing brushite cement for bone repair and vertebral augmentation applications. Biomater Sci. 2023;11:96-107. [7] LU TL, WANG JC, YUAN XY, et al. Zinc-doped calcium silicate additive accelerates early angiogenesis and bone regeneration of calcium phosphate cement by double bioactive ions stimulation and immunoregulation. Biomater Adv. 2022;141:213120. [8] BOSE S, FIELDING G, TARAFDER S, et al. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013;31(10):594-605. [9] MIRTCHI AA, LEMAITRE J, TERAO N. Calcium phosphate cements: study of the beta-tricalcium phosphate--monocalcium phosphate system. Biomaterials. 1989;10(7):475-480. [10] BOHNER M. Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements.Injury. 2000;31 Suppl 4:37-47. [11] HURLE K, OLIVEIRA JM, REIS RL, et al. Ion-doped brushite cements for bone regeneration. Acta Biomater. 2021;123:51-71. [12] KANNAN S, GOETZ-NEUNHOEFFER F, NEUBAUER J, et al. Rietveld structure and in vitro analysis on the influence of magnesium in biphasic (hydroxyapatite and beta-tricalcium phosphate) mixtures. J Biomed Mater Res B Appl Biomater. 2009;90B(1):404-411. [13] LAURA T, VAQUERO M. Chronic iron deficiency as an emerging risk factor for osteoporosis: a hypothesis. Nutrients. 2015;7(4):2324-2344. [14] BALOGH E, PARAGH G, JENEY V. Influence of iron on bone homeostasis. Pharmaceuticals (Basel). 2018;11(4):107. [15] 贾鹏,邓廉夫.低氧诱导因子-α信号通路与骨形成[J].中华骨科杂志,2015,35(6):676-680. [16] LI G, NAN Z, ZHAO S, et al. Fe-doped brushite bone cements with antibacterial property. Mater Lett. 2017;215:27-30. [17] VAHABZADEH S, FLECK S, MARBLE J, et al. Role of iron on physical and mechanical properties of brushite cements, and interaction with human dental pulp stem cells. Ceram Int. 2020;46(8 Pt B):11905-11912. [18] USKOKOVIĆ V, GRAZIANI V, WU V, et al. Gold is for the mistress, silver for the maid: enhanced mechanical properties, osteoinduction and antibacterial activity due to iron doping of tricalcium phosphate bone cements. Mater Sci Eng C Mater Biol Appl. 2019;94:798-810. [19] SAGHIRI MA, ASATOURIAN A, ORANGI J, et al. Functional role of inorganic trace elements in angiogenesis-Part II: Cr, Si, Zn, Cu, and S. Crit Rev Oncol Hematol. 2015;96(1):143-155. [20] 郭苏童,郭宇,王凌,等.铜离子在骨组织工程中的应用:生物相容性,抗菌性,血管生成活性及成骨活性[J].中国组织工程研究,2022,26(21):3410-3414. [21] RYAN EJ, RYAN AJ, GONZÁLEZ-VÁZQUEZ A, et al. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo.Biomaterials. 2019;197:405-416. [22] YAN JL, XIA DD, ZHOU WH, et al. pH-responsive silk fibroin-based CuO/Ag micro/nano coating endows polyetheretherketone with synergistic antibacterial ability, osteogenesis, and angiogenesis. Acta Biomater. 2020;115:220-234. [23] DU WL, NIU SS, XU YL, et al. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym. 2009;75(3):385-389. [24] LI XY, LI GD, ZHANG K, et al. Cu-loaded brushite bone cements with good antibacterial activity and operability. J Biomed Mater Res B Appl Biomater. 2021;109(6):877-889. [25] SCHAMEL M, BERNHARDT A, QUADE M, et al. Cu(2+), Co(2+) and Cr(3+) doping of a calcium phosphate cement influences materials properties and response of human mesenchymal stromal cells. Mater Sci Eng C Mater Biol Appl. 2017;73:99-110. [26] RAU J, WU VW, GRAZIANI V, et al. The bone building blues: self-hardening copper-doped calcium phosphate cement and its in vitro assessment against mammalian cells and bacteria. Mater Sci Eng C Mater Biol Appl. 2017;79:270-279. [27] XU HL, ZHU L, TIAN F, et al. In vitro and in vivo evaluation of injectable strontium-modified calcium phosphate cement for bone defect repair in rats. Int J Mol Sci. 2022;24(1):568. [28] KOŁODZIEJSKA B, STĘPIEŃ N, KOLMAS J. The influence of strontium on bone tissue metabolism and its application in osteoporosis treatment. Int J Mol Sci. 2021;22(12):6564. [29] TAHA A, AKRAM M, JAWAD Z, et al. Strontium doped injectable bone cement for potential drug delivery applications. Mater Sci Eng C Mater Biol Appl. 2017;80:93-101. [30] BOANINI E, PAGANI S, TSCHON M, et al. Monetite vs. brushite: different influences on bone cell response modulated by strontium functionalization. J Funct Biomater. 2022;13(2):65. [31] LU DZ, ZHANG YB, DONG W, et al. Effectiveness of strontium-doped brushite, bovine-derived hydroxyapatite and synthetic hydroxyapatite in rabbit sinus augmentation with simultaneous implant installation. J Biomed Mater Res B Appl Biomater. 2020;108(8):3402-3412. [32] ZHENG LZ, WANG JL, XU JK, et al. Magnesium and vitamin C supplementation attenuates steroid-associated osteonecrosis in a rat model. Biomaterials. 2020;238:119828. [33] KANG Y, XU C, MENG L, et al. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact Mater. 2022;18:26-41. [34] ZHANG XT, HUANG PZ, JIANG GW, et al. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater Sci Eng C Mater Biol Appl. 2021;121:111868. [35] MA LM, CHENG S, JI XF, et al. Immobilizing magnesium ions on 3D printed porous tantalum scaffolds with polydopamine for improved vascularization and osteogenesis. Mater Sci Eng C Mater Biol Appl. 2020;117:111303. [36] CERQUEIRA A, ROMERO-GAVILÁN F, GARCÍA-ARNÁEZ I, et al. Characterization of magnesium doped sol-gel biomaterial for bone tissue regeneration:The effect of Mg ion in protein adsorption. Mater Sci Eng C Mater Biol Appl. 2021;125:112114. [37] SALEH A, LING LS, HUSSAIN R. Injectable magnesium-doped brushite cement for controlled drug release application. J Mater Sci. 2016;51(16):7427-7439. [38] ALKHRAISAT MH, CABREJOS-AZAMA J, RODRÍGUEZ CR, et ai. Magnesium substitution in brushite cements. Mater Sci Eng C Mater Biol Appl. 2013;33(1):475-481. [39] WANG B, CHEN H, PENG S, et al. Multifunctional magnesium-organic framework doped biodegradable bone cement for antibacterial growth, inflammatory regulation and osteogenic differentiation. J Mater Chem B. 2023;11(13):2872-2885. [40] NANDI SK, ROY M, BANDYOPADHYAY A, et al. In vivo biocompatibility of SrO and MgO doped brushite cements. J Biomed Mater Res B Appl Biomater. 2023;111(3):599-609. [41] MOLENDA M, KOLMAS J. The role of zinc in bone tissue health and regeneration-a review. Biol Trace Elem Res. 2023. doi:10.1007/s12011-023-03631-1 [42] GRAZIANI AV, FOSCA AM, EGOROV AA, et al. Zinc-releasing calcium phosphate cements for bone substitute materials. Ceramics Int. 2016;42(15):17310-17316. [43] VAHABZADEH S, BANDYOPADHYAY A, BOSE S, et al. IGF-loaded silicon and zinc doped brushite cement:physico-mechanical characterization and in vivo osteogenesis evaluation. Integr Biol (Camb). 2015;7(12):1561-1573. [44] FADEEVA IV, GOLDBERG MA, PREOBRAZHENSKY II, et al. Improved cytocompatibility and antibacterial properties of zinc-substituted brushite bone cement based on beta-tricalcium phosphate. J Mater Sci Mater Med. 2021;32(9):99. [45] ADAWY A, DIAZ R. Probing the structure, cytocompatibility, and antimicrobial efficacy of silver-, strontium-, and zinc-doped monetite. ACS Appl Bio Mater. 2022;5(4):1648-1657. [46] ZHUANG Y, LIU QC, JIA GZ, et al. A Biomimetic zinc alloy scaffold coated with brushite for enhanced cranial bone regeneration. ACS Biomater Sci Eng. 2021;7(3):893-903. [47] FADEEVA IV, DEYNEKO DV, KNOTKO AV, et al. Antibacterial composite material based on polyhydroxybutyrate and zn-doped brushite cement. Polymers (Basel). 2023;15(9):2106. [48] CHOI YS, KIM YH, AN HM, et al. Efficacy of silver nanoparticles-loaded bone cement against an mrsa induced-osteomyelitis in a rat model. Medicina (Kaunas). 2023;59(4):811. [49] RAU JV, FOSCA M, GRAZIAN V, et al. Silver-doped calcium phosphate bone cements with antibacterial properties. J Funct Biomater. 2016;7(2):10. [50] EWALD A, HÖSEL D, PATEL S, et al. Silver-doped calcium phosphate cements with antimicrobial activity. Acta biomaterialia. 2011;7(11):4064-4070. [51] SAYAHI M, SANTOS C, EL-FEKI H, et al. Brushite (Ca,M)HPO4, 2H2O doping with bioactive ions (M=Mg2+, Sr2+, Zn2+, Cu2+, and Ag+): a new path to functional biomaterials? Mater Today Chem. 2020;16:100230. [52] RANA NK, SINGH P, KOCH B. CoCl(2) simulated hypoxia induce cell proliferation and alter the expression pattern of hypoxia associated genes involved in angiogenesis and apoptosis. Biol Res. 2019;52(1):12. [53] LI JG, ZHAO CQ, LIU C, et al. Cobalt-doped bioceramic scaffolds fabricated by 3D printing show enhanced osteogenic and angiogenic properties for bone repair. Biomed Eng Online. 2021;20(1):70. [54] VAHABZADEH S, FLECK S, DUVVURU M, et al. Effects of cobalt on physical and mechanical properties and in vitro degradation behavior of brushite cement. JOM. 2018;71:315-320. [55] AMBIKA S, MANOJKUMAR Y, ARUNACHALAM S, et al. Biomolecular interaction, anti-cancer and anti-angiogenic properties of cobalt (III) schiff base complexes. Sci Rep. 2019;9(1):2721. [56] LIU L, LIU Y, FENG C, et al. Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials. 2019;192:523-536. [57] HURLE K, MAIA FR, RIBEIRO VP, et al. Osteogenic lithium-doped brushite cements for bone regeneration. Bioact Mater. 2022;16:403-417. [58] BECK GR JR, HA SW, CAMALIER CE, et al. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomedicine. 2012;8(6):793-803. [59] APARICIO JL, RUEDA C, MANCHÓN A, et al. Effect of physicochemical properties of a cement based on silicocarnotite/calcium silicate on in vitro cell adhesion and in vivo cement degradation. Biomed Mater. 2016;11(4):045005. [60] VAHABZADEH R, ROY M, BOSE S. Effects of silicon on osteoclast cell mediated degradation, in vivo osteogenesis and vasculogenesis of brushite cement. J Mater Chem B. 2015;3(46):8973-8982. [61] MOSES M, DEY M, DEVI KB, et al. Synergistic effects of silicon/zinc doped brushite and silk scaffolding in augmenting the osteogenic and angiogenic potential of composite biomimetic bone grafts. ACS Biomater Sci Eng. 2019;5(3):1462-1475. [62] DENG H, LIU HB, YANG Z, et al. Progress of selenium deficiency in the pathogenesis of arthropathies and selenium supplement for their treatment. biological trace element research. 2022;200(10):4238-4249. [63] WANG YF, WANG JL, HAO H, et al. In vitro and in vivo mechanism of bone tumor inhibition by selenium-doped bone mineral nanoparticles. ACS Nano. 2016;10(11):9927-9937. [64] LASKUS A, ZGADZAJ A, KOLMAS J. Selenium-enriched brushite: a novel biomaterial for potential use in bone tissue engineering. Int J Mol Sci. 2018;19(12):4042. |
| [1] | Zhou Shijie, Li Muzhe, Yun Li, Zhang Tianchi, Niu Yuanyuan, Zhu Yihua, Zhou Qinfeng, Guo Yang, Ma Yong, Wang Lining. Effect of Wenshen Tongluo Zhitong formula on mouse H-type bone microvascular endothelial cell/bone marrow mesenchymal stem cell co-culture system [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 8-15. |
| [2] | Wang Chenglong , Yang Zhilie , Chang Junli , Zhao Yongjian , Zhao Dongfeng , Dai Weiwei , Wu Hongjin , Zhang Jie , Wang Libo , Xie Ying , Tang Dezhi , Wang Yongjun , Yang Yanping. Restoration of osteogenic differentiation of bone marrow mesenchymal stem cells in mice inhibited by cyclophosphamide with psoralen [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 16-23. |
| [3] | Fu Jiaqi, Chen Xiubao, Cui Xing, Chen Zetao . Mechanism by which Angelica sinensis polysaccharide regulates bone marrow hematopoietic microenvironment for aplastic anemia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 44-51. |
| [4] | Wang Zhikun, Bai Shaoxuan, Zhao Wei, Wang Chenyu. Exercise preconditioning combined with bone marrow mesenchymal stem cell transplantation for myocardial infarction in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 65-73. |
| [5] | Zheng Qian, Liu Pingping, Gu Yujie, Xie Lei. Effect of ursolic acid on osteogenic differentiation of human periodontal ligament stem cells#br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 80-86. |
| [6] | Yan Ru, Wang Kairu, Zhang Feiyan, Jia Shaobin, Cong Guangzhi. Endothelial cell-specific bone morphogenetic protein 2 affects angiogenesis: bioinformatics analysis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 103-110. |
| [7] | Xie Qinglin, Zhang Xiaodong. Effect of bone marrow fat on bone metastasis and quantitative evaluation by magnetic resonance imaging [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 128-135. |
| [8] | Qin Hao, Kang Teng, Liu Gang. Long non-coding RNA directly or indirectly affects osteoporosis through p38MAPK signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 175-184. |
| [9] | Zhang Mianyu, Han Jie, Zeng Hao, Chen Xiangshan, Gao Zhengang. Regulating ferroptosis of osteoblasts by traditional Chinese medicine in treatment of steroid-induced avascular necrosis of femoral head [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 185-192. |
| [10] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [11] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [12] | Wang Menghan, Qi Han, Zhang Yuan, Chen Yanzhi. Three kinds of 3D printed models assisted in treatment of Robinson type II B2 clavicle fracture [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1403-1408. |
| [13] | Yang Cekai, Cai Zhuoyan, Chen Ming, Liu Hao, Weng Rui, Cui Jianchao, Zhang Shuncong, Yao Zhensong. Relationship between degeneration of paraspinal muscle and refractures in postmenopausal women treated by percutaneous vertebroplasty [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1414-1419. |
| [14] | Zhuang Xinyi, Peng Yuanhao, Yu Ting, Lyu Dongmei, Wen Xiujie, Cheng Qian. Cone-beam CT evaluation of bone mass in the external oblique line of the mandible in adolescents with different cervical vertebral bone ages [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1253-1258. |
| [15] | Xiaheida·Yilaerjiang, Nijiati·Tuerxun, Reyila·Kuerban, Baibujiafu·Yelisi, Chen Xin. Three-dimensional finite element analysis of the distribution pattern of stress in bone tissues with different characteristics [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1277-1282. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||