Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (12): 1956-1961.doi: 10.12307/2024.015
Previous Articles Next Articles
Decompression and fusion for degenerative lumbar spondylolisthesis affect sagittal disequilibrium of the spine
Shi Haoran, Guan Haishan, Wang Yueyong, Liu Tao
- 山西医科大学第二医院骨科,山西省太原市 030001
-
Received:2023-02-20Accepted:2023-03-24Online:2024-04-28Published:2023-08-23 -
Contact:Guan Haishan, Chief physician, Department of Orthopedics, Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Shi Haoran, Master candidate, Department of Orthopedics, Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
CLC Number:
Cite this article
Shi Haoran, Guan Haishan, Wang Yueyong, Liu Tao. Decompression and fusion for degenerative lumbar spondylolisthesis affect sagittal disequilibrium of the spine[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(12): 1956-1961.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

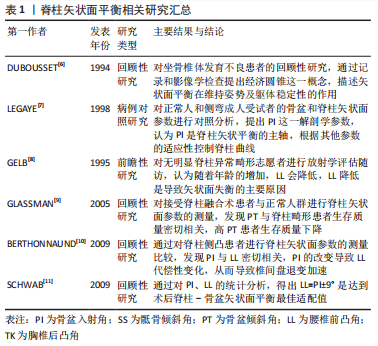
2.1 脊柱矢状面平衡发展概述 人类的脊柱与生物力学密切相关:正常脊柱在矢状面上有多个生理曲度,颈椎、胸椎、腰椎、骶椎以及骨盆的有序排列,通过调整脚之间的重心,最大限度地减少关节、肌肉和韧带的影响,从而提高效率[2]。矢状垂直轴是用来评估整体脊柱对齐最经典简易的指标,C7中点铅垂线落在S1椎体终板后上角前方为脊柱矢状面正平衡,反之为负平衡。骨盆倾斜角的出现为脊柱矢状面失衡建立了良好的补偿机制[3],较大的骨盆倾斜角可以影响脊柱矢状面的几何结构。近几年随着对脊柱矢状面参数的不断评估,已经逐渐认识到骨盆入射角和骨盆形态显著影响脊柱矢状面平衡,特别是腰椎前凸角与骨盆入射角的适配性[4]。除此以外,较大的骨盆入射角、较小的骶骨倾斜角以及较小的腰椎前凸角也被认为与腰椎退行性疾病有关[5]。因此,对于脊柱-骨盆矢状面平衡的研究对手术方式的改良及患者的预后均有重要意义。 文章对脊柱矢状面平衡相关研究作一总结[6-11],见表1。"
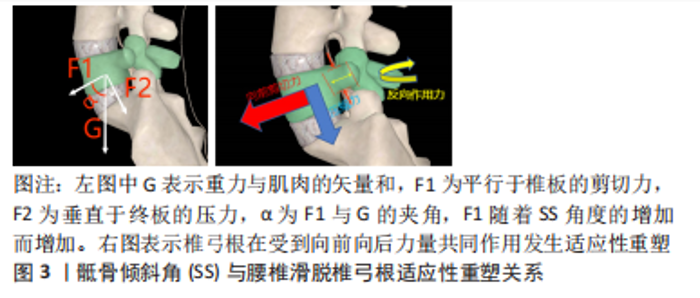
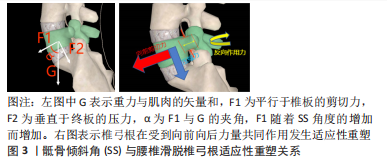
2.2 脊柱矢状面平衡概念 脊柱矢状面平衡是人体以最小能耗维持脊柱整体、局部生理曲度和力线结构的状态,可以减轻背部肌肉的压力及负荷,并改善腰椎手术的效果[12]。退变、炎症、创伤、感染、肿瘤、先天或医源性因素等导致椎体序列以及力线的改变引起的脊柱矢状面失衡是患者发生脊柱疼痛和活动受限的主要原因。由于腰椎滑脱常与其他畸形相结合通常会导致脊柱整体矢状不平衡。脊柱-骨盆矢状位失衡是造成腰椎退行性滑脱的一个重要因素[13],有研究指出较大的骶骨倾斜角更容易导致滑脱的进展[14],而腰椎融合术在于复位滑脱的椎体及牢靠地固定,而导致滑脱的较大的骶骨倾斜角未进行矫正,可能会导致融合节段或者邻近节段进展为腰椎滑脱。节段性前凸的保留或恢复与邻近节段退行性变、整体矢状面平衡和术后不良预后密切相关。由于腰椎矢状面平衡影响因素较多,暂无统一的诊疗标准来改善矢状面平衡。 2.3 矢状面平衡相关参数 2.3.1 骨盆入射角(pelvic incidence,PI) 是指经骶骨上终板中点与骶骨上终板相垂直的直线,与骶骨上终板中点和股骨头中点连线所形成夹角。骨盆入射角是一个解剖学参数,不受体位变化而变化。从4-18岁逐渐增加,成年后几乎没有固定的变化,在矢状面平衡以及腰椎退化过程中起到重要作用,对于任何脊柱畸形或者腰椎超过2个节段的融合手术,需要测量骨盆入射角。骨盆入射角的标准值为53°±9°,中国人报道为45°,以此为界分为小骨盆入射角和大骨盆入射角,大骨盆入射角增加了腰椎退行性变的风险,因为相应的补偿机制需要平衡增加的剪切力[15],骨盆入射角越小也代表骨盆代偿能力越弱。因此,术前评估患者骨盆入射角对术式的选择及术中的操作具有重要意义。 2.3.2 腰椎前凸角(lumber lordosis,LL) 是指L1上终板与S1上终板间夹角。腰椎前凸角对于矢状面平衡以及腰椎退化也起重要作用,腰椎前凸的生理意义在于抵抗纵向压力;腰椎退变会导致腰椎生理曲度变直,重力线前移导致腰椎前中柱抵抗纵向压力减小,后柱的负荷增加。有研究证实退行性腰椎滑脱的患者生物力学改变是因为矢状面平衡的前移导致腰椎前凸角的丧失,强调了恢复腰椎前凸角来调整腰骶部的不平衡是腰椎手术的重要目标之一[16]。 腰椎前凸角与骨盆入射角有着密切的联系,腰椎前凸角的大小取决于骨盆入射角的值。理想公式为LL=PI±9°,如果两个参数不匹配,容易造成矢状面失衡[17]。Senteler等[18]研究证明,PI-LL失配患者相邻节段的剪切应力较高,发生邻近节段退行性改变的风险也越高。按照Roussouly分型,骨盆入射角越小,腰前凸节段数越少(3-5个),腰前凸顶点越靠近尾端(L4、L5),腰椎前凸角恢复更加严格。最近的几项研究不仅证实了退变性腰椎滑脱患者的主要生物力学特征是矢状面平衡的前向平移和腰椎前凸角随骨盆倾斜角增加而丧失[16],但也证明,术后恢复矢状面平衡可以改善长期临床结果,并降低矢状面失衡的风险。术前积极评估腰椎前凸角以及骨盆入射角,术中尽可能改善其适配性是手术操作的一个重点,也是影响患者预后的一个关键因素。 2.3.3 骶骨倾斜角(sacral slope,SS) 是指S1上终板与水平线的夹角。骶骨倾斜角作为骨盆矢状面的姿势参数用以描述骨盆空间位置,随着人体姿势的变化而改变,用以判断骨盆后倾状态;骶骨倾斜角减小骨盆后倾,骶骨倾斜角增大骨盆前倾。有学者发现骶骨倾斜角与L4椎体滑移距离百分比呈正相关[14];另一部分学者发现退变性滑脱患者由于滑移水平剪切力的作用,导致对应椎弓根发生适应性重塑,即椎弓根会变得更细,内倾更小[19],见图3。骶骨倾斜角的测量以及椎弓根形态的评估有助于评估脊柱骨盆矢状位平衡,并对椎弓根螺钉的放置起到指导作用。"

骶骨倾斜角与骨盆入射角有密切联系,PI=SS+PT[7]。PT为骨盆倾斜角,同样作为骨盆矢状面的姿势参数,这3个参数是近些年学者研究的重点,因为脊柱矢状面的失衡需要骨盆参数的代偿和调节。对于涉及骶椎的融合固定,需要充分考虑骶骨倾斜角的变化;对于术前骶骨倾斜角较大的患者,也应尽可能降低其角度,否则融合固定后容易出现拔钉、断钉等风险。 2.3.4 脊柱矢状轴(sagittal vertical axis,SVA) 是指S1终板后上角与C7铅垂线之间的垂直距离,被认为是脊柱矢状位平衡的生命线;正常值小于5 cm,当脊柱矢状轴绝对值大于5 cm时称为脊柱矢状位的失衡。一项回顾性研究也表明脊柱矢状轴> 5 cm的患者术后矢状面排列不良导致随访3年结果显著恶化[20]。除此以外,骨盆入射角与脊柱矢状轴之间也有一定关联,KOBAYASHI等[21]也发现大的骨盆入射角是脊柱矢状轴恶化的危险因素之一。长节段的融合固定对脊柱矢状造成影响,导致预后差,因此行超过3个节段的脊柱融合固定时应测量评估脊柱矢状轴,关于矢状面参数及其各自标准值和范围的描述见表2,矢状面参数测量方法见图4。"

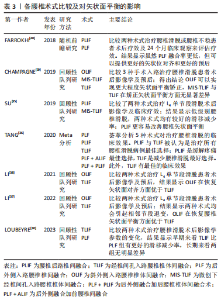
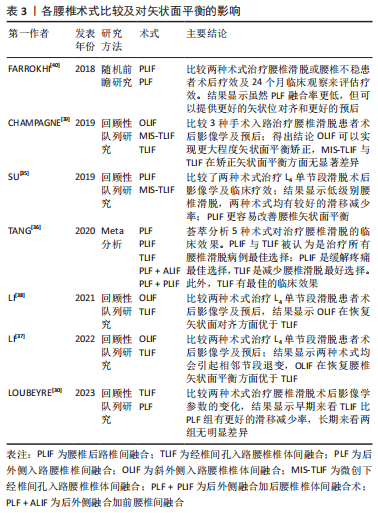
2.4 脊柱矢状面失衡的危险因素 2.4.1 术式选择 退变性腰椎滑脱的特点是上位椎体相对下位椎体的滑移进而导致中央椎管狭窄或不稳定。手术干预可以通过复位滑脱椎体来缓解椎管狭窄,同时缓解神经根及硬膜囊的压迫[22]。手术的目的主要是改善神经系统症状、彻底减压以及牢靠的内固定以避免再次失稳[23]。腰椎减压融合术是治疗腰椎滑脱的首选方式,虽然单纯减压可以用来治疗某些退变性腰椎滑脱,但仅减压不进行融合可能会进一步破坏脊椎的稳定性,因此更常采用融合减压术[24]。经椎间孔腰椎椎体融合术(transforaminal lumbar interbody fusion,TLIF)和后路腰椎间融合术(posterior lumbar interbody fusion,PLIF)均被用来治疗退变性腰椎滑脱[25]。 PLIF术作为退变性腰椎滑脱的一种经典有效的手术方法,具有高融合率和有效的神经根减压的特点[26],一直是骨科医生用来处理退变性腰椎滑脱的一种手术方法。PLIF术通过椎弓根螺钉的固定及融合器的牵引来显著改善矢状面的平衡,该术式可以更好地将矢状位对齐。也有学者提出观点[27],一期后入路融合被证明是一种安全有效的治疗严重腰椎滑脱的技术,这项技术可以恢复局部和整体对齐,无永久性神经并发症。然而,PLIF手术对椎旁软组织的剥离、椎管的减压容易造成脊柱三柱结构中后柱的结构稳定性遭到破坏,融合后造成活动椎体节段的丢失容易造成相邻未融合节段应力传导的增加,进而导致相邻节段的活动补偿增加[28],增加邻近节段退变的风险。LEE等[29]也有报道,PLIF术后会增加邻近节段退变的风险,可能是因为脊柱固定器械和融合器可以显著增加固定节段硬度和把应力传递到相邻节段有关。 TLIF术通过椎间孔入路进行减压融合,相较于PLIF术具有手术时间短、术中出血少及术后并发症少等优点[30]。对于滑脱病程较长患者而言,滑脱节段的肌肉软组织、韧带发生了适应性改变;TLIF术中没有对椎旁肌进行剥离,从而导致相较于PLIF术更难以复位滑脱椎体,导致脊柱骨盆参数无法恢复至正常水平,在长节段脊柱融合中表现得更为明显[31]。ELMORSY等[32]通过一项前瞻性研究,认为要纠正低级别腰椎滑脱的腰椎前凸,需要使用弯曲杆进行短段固定+融合,而TLIF术更为合适。LOUBEYRE等[30]通过随访发现短期内TLIF组的腰椎滑脱复位效果比PLIF组好,但最终随访时没有维持,且发现TLIF组总并发症发生率高于PLIF组。因此,从长远来看,PLIF在腰椎滑脱手术中具有一定的优势。 LEVEQUE等[33]认为前入路手术或侧方入路手术更容易保持腰椎前凸,避免了后方入路对椎旁肌肉的破坏,而后方放置移植物(PLIF/TLIF)常会在节段和整体上恶化前凸。O’CONNOR等[34]也得出相同结论,认为前方放置移植物可以导致更大的节段性前凸和PI-LL适配改善。显微镜辅助下经椎间孔腰椎间融合(anterior lumbar interbody fusion/Lateral lumbar interbody fusion,ALIF/LLIF)还可以显著减少相邻水平的前凸,从而减少融合水平可能增加的腰椎前凸。 对于仅有单节段腰椎轻度滑脱不伴有脊柱-骨盆矢状面失衡的患者,显微镜辅助下经椎间孔腰椎间融合(minimal invasive surgery-transforaminal lumbar interbody fusion,MIS-TLIF)既能纠正滑脱椎体,还可以减少术中出血及软组织损伤[35];对于单节段滑脱伴有脊柱-骨盆矢状面失衡患者而言,PLIF术或者TLIF术是一个较好的选择;对于多节段滑脱患者而言,PLIF起到的复位椎体、纠正脊柱-骨盆矢状面失衡的作用更显著。对于腰椎滑脱患者术前即存在矢状面失衡或因矢状面失衡而造成的腰椎滑脱患者,行PLIF术式为较好的选择。文章总结了各腰椎术式比较及对矢状面平衡的影响相关研究内容[30,35-40],见表3。"
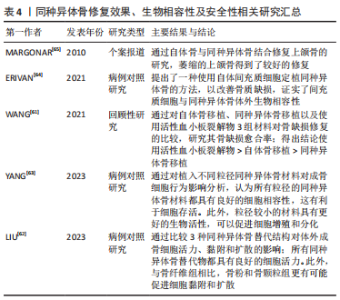
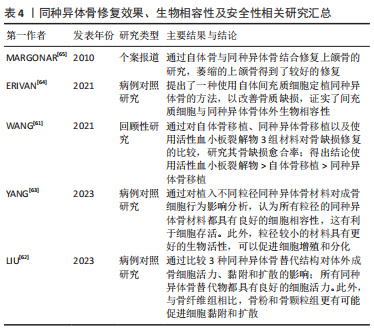
2.4.2 内固定物植入 腰椎融合固定术手术过程需要有椎弓根螺钉、融合器的置入。其中,椎弓根螺钉的选择、插入位置深度、融合器尺寸的选择以及放置深度的差异会对矢状面平衡造成一定的影响。 椎弓根螺钉拥有较强的生物力学强度,PLIF手术中通过椎弓根螺钉以及钛棒系统来维持脊柱三柱之中后柱的稳定,椎弓根螺钉的放置对PLIF术的椎间融合至关重要,并显示出显著的临床结果[41]。有研究表明,椎弓根螺钉插入椎体的深度、角度会显著影响椎弓根螺钉的生物力学稳定性,从而影响脊柱固定节段的三柱平衡[42]。MATSUKAWA等[43]认为更深螺钉的插入以及使用更大直径的螺钉对于维持稳定性是合理的;因为较长、直径较大的螺钉更容易增加骨接触程度,即使没有与前皮质骨接触,但增加了拔出强度。也有学者通过比较行单节段PLIF术手术前后腰椎矢状面各参数的变化得出结论[44],在一定的安全范围内,脊柱外科医生尽可能选择长螺钉,这有利于恢复和保持脊柱-骨盆矢状面平衡。 融合器同样拥有较强的生物力学强度,腰椎融合手术中,融合器作为替代原先被切除的椎间盘,起到连接上下位椎体、撑开椎间隙的作用;同时也起到维持脊柱三柱之中前柱、中柱稳定的作用。椎间融合器可以保持或改善矢状面对齐,这一点很重要,因为脊柱骨盆平衡与退变性腰椎滑脱融合后的功能结果相关[20]。KARIM等[45]证实,当使用椎间融合器时,节段性前凸恶化与术前椎间盘角度指数相关。术前椎间盘后凸的患者通过使用椎间融合器可以获得更多前凸。融合器大小的选择以及置入椎间隙的深度可影响椎间成角,进而影响腰椎矢状面平衡。MELIKIAN等[46]认为,通过侧方经皮入路近30°的椎间融合器的置入以及前纵韧带的松解可以实现较好的前凸,不会对椎体终板和椎体施加过大的压力。对于术前椎间盘后凸的患者通过使用椎间融合器获得更多的节段性前凸[47]。但也不能通过过度撑开椎间隙来增大融合节段成角,有学者认为,椎间隙的过度撑开会加速邻近节段退变的进展,恢复椎间隙至正常水平一定程度上可以恢复腰椎原有的生物力学[48]。然而,在腰椎手术过程中,融合器的选择以及置入的深度在一定程度上受到脊柱外科医生对不同患者的治疗经验的影响,这种经验判断有时会对矢状面平衡的产生难以预料的改变。因此,术前对于脊柱-骨盆矢状面参数的评估显得尤为重要。 2.4.3 融合节段数 手术固定融合节段数是影响脊柱矢状面平衡的一个因素。有学者认为,3个节段以上的长节段脊柱固定融合更容易造成脊柱矢状面失衡,应尽量避免长节段固定融合。腰椎进行的长节段固定融合使腰椎活动度下降,失去了代偿能力,且很难按照腰椎正常生理曲度进行塑性从而失去正常的生理曲度,更容易发生邻近节段退行性改变。CHEH等[49]认为长节段固定融合是腰椎邻近节段退行性改变的因素之一。长节段的固定融合容易导致力臂过长,与相邻节段容易造成应力集中,造成其活动度的增加,更容易导致腰椎失稳[50]。然而,一些学者持相反意见。KUMAR等[51]报道,较长水平的融合固定不会加速邻近节段退变。也有学者通过随访研究发现,对于L5椎体滑脱患者行单节段固定与2个节段的固定,短期而言2个节段的融合固定表现出更好的脊柱矢状位恢复及稳定性[52]。长节段进行腰椎固定使得腰椎活动度下降,使得应力集中分布,很容易造成临近节段退行性改变。因此,应尽量避免超过3个节段以上的长节段固定,如无法避免长节段固定,术前应仔细评估患者矢状面参数。 2.4.4 融合节段平面 由于腰椎各椎体之间排列及结构存在差异,导致其受到的生物力学也存在差异,这就造成了各节段发生腰椎滑脱的发病率也不尽相同。退变性腰椎滑脱多见于L3和L4,这与局部解剖特点以及滑脱机制有关。由于其解剖差异,导致在不同节段行单节段腰椎固定融合后相邻椎体受到的力也不同。一项前瞻性研究显示,对融合节段的邻近节段进行解压也会造成矢状面失衡,进而发展为邻近节段退行性改变[53]。 此外,融合节段的高低是否会影响脊柱-骨盆矢状面平衡也存在一定的争议。BAE等[54]研究发现,融合平面越高的患者发生邻近节段退变的概率高于融合平面低的患者;但也有学者认为邻近节段退变与融合平面高低无关[49]。因此,对于不同平面的融合固定也不能程序式操作,应充分考虑每个节段所受应力情况以及影像学参数的差异。 2.4.5 滑脱复位程度 腰椎滑脱根据Myerding法将其分为4度,其中的Ⅰ,Ⅱ度称为轻度滑脱,Ⅲ,Ⅳ度滑脱称为重度滑脱;对于Ⅲ,Ⅳ度腰椎滑脱行滑脱复位已被国内外学者认同,对于轻度腰椎滑脱,关于术中是否对滑脱椎体进行复位,一直存在争议[55]。有学者认为,对于轻度的腰椎滑脱,有必要进行完全复位,复位程度彻底可建立符合生理力学的内环境;对于滑脱椎体的完全复位,有助于恢复椎间隙高度,增加节段性成角。SOH等[56]认为,当节段性成角大于15°时,有助于延缓邻近节段退行性改变。KAWAKAMI等[57]研究报道,滑脱椎体的恢复有助于矫正腰椎前凸角,并恢复椎体整体的物理排列。另一部分人认为,对于病程较长的腰椎滑脱,原位融合是一种较好的方案[58]。随着病程时间的延长,滑脱节段的肌肉软组织、韧带发生了适应性改变,强行进行复位,势必会对椎旁肌肉及韧带造成损伤,使原有的代偿性矢状面平衡再次发生失衡;为了强求滑移椎体的复位而对肌肉及韧带进行更大程度的剥离,容易造成腰椎后柱的失稳。同时,也有学者研究发现,原位融合与腰椎手术过程中的手法复位相比,尽管会有影像学上角度的差异,但术后并发症的发生率无显著差异,重点在于椎间隙高度以及椎间成角的恢复[59]。目前对于退行性腰椎滑脱复位有效性仍存在争议,需要大样本、多中心随机对照试验来证明。 2.4.6 植骨融合 腰椎融合术中其他方面的因素也可以直接或间接影响脊柱矢状面平衡,如术中植骨。融合手术过程中,通常需要进行椎间植骨或者横突间植骨来促进椎体间的融合。植骨的性质(如自体骨、同种异体骨)、骨诱导的添加与否、植骨数量的选择、椎间植骨还是横突间植骨的选择都会影响椎体融合的进程,从而影响融合节段的稳定性。CAKIR等[60]通过比较分别行自体骨移植与同种异体骨移植的患者数据,得出结论自体骨移植的骨融合率高于同种异体骨患者,同时自体骨移植也更容易维持椎间隙高度。也有报道显示添加骨诱导材料更容易促进椎体间融合,但缺乏大样本研究。近期研究发现,术者进行自体骨移植过程中混合自体动脉血有助于促进椎体间融合,但缺乏远期随访,需要大样本量、长期随访来验证这一结论的真实性。作者总结了关于同种异体骨修复效果、生物相容性及安全性研究[61-65],见表4。"
| [1] KIM J, MOON B, KIM D, et al. Postoperative flat back: contribution of posterior accessed lumbar interbody fusion and spinopelvic parameters. J Korean Neurosurg Soc. 2014;56(4):315-322. [2] DIEBO BG, VARGHESE JJ, LAFAGE R, et al. Sagittal alignment of the spine: what do you need to know? Clin Neurol Neurosurg. 2015;139:295-301. [3] LEGAYE J, DUVAL-BEAUPÈRE G, HECQUET J, et al. Pelvic incidence: a fundamental pelvic parameter for three‐dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7:99-103. [4] FRANK S, PATEL A, UNGAR B, et al. Adult spinal deformity‐postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976). 2010; 35:2224-2231. [5] LIM J, KIM S. Difference of sagittal spinopelvic alignments between degenerative spondylolisthesis and isthmic spondylolisthesis. J Korean Neurosurg Soc. 2013; 53(2):96-101. [6] DUBOUSSET J, HADDAD F, ZELLER R, et al. Ischio-vertebral dysplasia (a dangerous syndrome for the spinal cord). Rev Chir Orthop Reparatrice Appar Mot. 1994; 80(7):610-619. [7] LEGAYE J, DUVAL-BEAUPèRE G, HECQUET J, et al. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7(2):99-103. [8] GELB D, LENKE L, BRIDWELL K, et al. An analysis of sagittal spinal alignment in 100 asymptomatic middle and older aged volunteers. Spine (Phila Pa 1976). 1995; 20(12):1351-1358. [9] GLASSMAN S, BERVEN S, BRIDWELL K, et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976). 2005; 30(6):682-688. [10] BERTHONNAUD E, DIMNET J, HILMI R. Classification of pelvic and spinal postural patterns in upright position. Specific cases of scoliotic patients. Comput Med Imaging Graph. 2009;33(8):634-643. [11] SCHWAB F, LAFAGE V, PATEL A, et al. Sagittal plane considerations and the pelvis in the adult patient. Spine (Phila Pa 1976). 2009;34(17):1828-1833. [12] FENG Y, CHEN L, GU Y, et al. Restoration of the spinopelvic sagittal balance in isthmic spondylolisthesis: posterior lumbar interbody fusion may be better than posterolateral fusion. Spine J. 2015;15(7):1527-1535. [13] LE HUEC J, THOMPSON W, MOHSINALY Y, et al. Sagittal balance of the spine. Eur Spine J. 2019;28(9):1889-1905. [14] LENG Y, TANG C, LIAO Y, et al. Correlation between sacral slope and pedicle morphology of the fourth lumbar vertebra in degenerative lumbar spondylolisthesis. Global Spine J. 2022. doi: 10.1177/21925682221117151. [15] BERVEN S, WADHWA R. Sagittal alignment of the lumbar spine. Neurosurg Clin N Am. 2018;29(3):331-339. [16] CHOI S, SON S, LEE D, et al. L1 incidence reflects pelvic incidence and lumbar lordosis mismatch in sagittal balance evaluation. Medicine (Baltimore). 2018; 97(30):e11668. [17] BOULAY C, TARDIEU C, HECQUET J, et al. Sagittal alignment of spine and pelvis regulated by pelvic incidence: standard values and prediction of lordosis. Eur Spine J. 2006;15(4):415-422. [18] SENTELER M, WEISSE B, SNEDEKER J, et al. Pelvic incidence-lumbar lordosis mismatch results in increased segmental joint loads in the unfused and fused lumbar spine. Eur Spine J. 2014;23(7):1384-1393. [19] TANG C, LIAO Y, TANG Q, et al. What is the difference in pedicle morphology of the fifth lumbar vertebra between isthmic and degenerative L5-S1 spondylolisthesis? An anatomic study of 328 patients via multi-slice spiral computed tomography. Eur Spine J. 2021;30(8):2301-2310. [20] RADOVANOVIC I, URQUHART J, GANAPATHY V, et al. Influence of postoperative sagittal balance and spinopelvic parameters on the outcome of patients surgically treated for degenerative lumbar spondylolisthesis. J Neurosurg Spine. 2017;26(4):448-453. [21] KOBAYASHI H, ENDO K, SAWAJI Y, et al. Global sagittal spinal alignment in patients with degenerative low-grade lumbar spondylolisthesis. J Orthop Surg (Hong Kong). 2019;27(3):2309499019885190. [22] OIKONOMIDIS S, MEYER C, SCHEYERER M, et al. Lumbar spinal fusion of low-grade degenerative spondylolisthesis (Meyerding grade I and II): do reduction and correction of the radiological sagittal parameters correlate with better clinical outcome? Arch Orthop Trauma Surg. 2020;140(9):1155-1162. [23] BERNARD F, MAZERAND E, GALLET C, et al. History of degenerative spondylolisthesis: from anatomical description to surgical management. Neurochirurgie. 2019;65:75-82. [24] KEPLER C, VACCARO A, HILIBRAND A, et al. National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine (Phila Pa 1976). 2014; 39(19):1584-1589. [25] LAN T, HU S, ZHANG Y, et al. Comparison between posterior lumbar interbody fusion and transforaminal lumbar interbody fusion for the treatment of lumbar degenerative diseases: a systematic review and meta-analysis. World Neurosurg. 2018;112:86-93. [26] YE Y, XU H, CHEN D. Comparison between posterior lumbar interbody fusion and posterolateral fusion with transpedicular screw fixation for isthmic spondylolithesis:a meta-analysis. Arch Orthop Trauma Surg. 2013;133(12):1649-1655. [27] FALDINI C, BARILE F, IALUNA M, et al. High-grade dysplastic spondylolisthesis: surgical technique and case series. Musculoskelet Surg. 2022. doi: 10.1007/s12306-022-00763-w. [28] PHAN K, NAZARETH A, HUSSAIN A, et al. Relationship between sagittal balance and adjacent segment disease in surgical treatment of degenerative lumbar spine disease:meta-analysis and implications for choice of fusion technique. Eur Spine J. 2018;27(8):1981-1991. [29] LEE J, KIM Y, SOH J, et al. Risk factors of adjacent segment disease requiring surgery after lumbar spinal fusion:comparison of posterior lumbar interbody fusion and posterolateral fusion. Spine (Phila Pa 1976). 2014;39(5):E339-E345. [30] LOUBEYRE J, FERRERO E, JMAL M, et al. Surgical treatment of degenerative lumbar spondylolisthesis: effect of TLIF and slip reduction on sagittal alignment. Orthop Traumatol Surg Res. 2023. doi: 10.1016/j.otsr.2022.103541. [31] GLASSMAN S, BRIDWELL K, DIMAR J, et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). 2005;30(18):2024-2029. [32] ELMORSY S, ABULNASR H, HASSAN Y, et al. Functional outcome of surgical management of low mid-grade lumbar spondylolisthesis when considering the sagittal balance parameters preoperatively:a prospective study. Chin Neurosurg J. 2022;8(1):35. [33] LEVEQUE J, DROLET C, NEMANI V, et al. The impact of surgical approach on sagittal plane alignment in patients undergoing one- or two- level fusions for degenerative pathology: a multicenter radiographic evaluation 6 months following surgery. World Neurosurg. 2022;164:e311-e317. [34] O’CONNOR B, DROLET C, LEVEQUE J, et al. The impact of interbody approach and lumbar level on segmental, adjacent, and sagittal alignment in degenerative lumbar pathology:a radiographic analysis six months following surgery. Spine J. 2022;22(8):1318-1324. [35] SU K, LUAN J, WANG Q, et al. Radiographic analysis of minimally invasive transforaminal lumbar interbody fusion versus conventional open surgery on sagittal lumbar-pelvic alignment for degenerative spondylolisthesis. World Neurosurg. 2019;124:e733-e739. [36] TANG L, WU Y, JING D, et al. A Bayesian network meta-analysis of 5 different fusion surgical procedures for the treatment of lumbar spondylolisthesis. Medicine (Baltimore). 2020;99(14):e19639. [37] LI G, TONG T, WANG L. Comparative analysis of the effects of OLIF and TLIF on adjacent segments after treatment of L4 degenerative lumbar spondylolisthesis. J Orthop Surg Res. 2022;17(1):203. [38] LI R, SHAO X, LI X, et al. Comparison of clinical outcomes and spino-pelvic sagittal balance in degenerative lumbar spondylolisthesis: minimally invasive oblique lumbar interbody fusion (OLIF) versus transforaminal lumbar interbody fusion (TLIF). Medicine (Baltimore). 2021;100(3):e23783. [39] CHAMPAGNE P, WALSH C, DIABIRA J, et al. Sagittal balance correction following lumbar interbody fusion: a comparison of the three approaches. Asian Spine J. 2019;13(3):450-458. [40] FARROKHI M, YADOLLAHIKHALES G, GHOLAMI M, et al. Clinical outcomes of posterolateral fusion vs. posterior lumbar interbody fusion in patients with lumbar spinal stenosis and degenerative instability. Pain physician. 2018;21(4):383-406. [41] LI Y, WU Z, GUO D, et al. A comprehensive comparison of posterior lumbar interbody fusion versus posterolateral fusion for the treatment of isthmic and degenerative spondylolisthesis: a meta-analysis of prospective studies. Clin Neurol Neurosurg. 2020;188:105594. [42] UEHARA M, TAKAHASHI J, IKEGAMI S, et al. Determination of optimal screw number based on correction angle for main thoracic curve in adolescent idiopathic scoliosis. J J Orthop Sci. 2019;24(3):415-419. [43] MATSUKAWA K, YATO Y, IMABAYASHI H. Impact of screw diameter and length on pedicle screw fixation strength in osteoporotic vertebrae: a finite element analysis. Asian Spine J. 2021;15(5):566-574. [44] ZHOU Q, ZHANG J, ZHENG Y, et al. Effects of different pedicle screw insertion depths on sagittal balance of lumbar degenerative spondylolisthesis, a retrospective comparative study. BMC Musculoskelet Disord. 2021;22(1):850. [45] KARIM S, FISHER C, GLENNIE A, et al. Preoperative patient-reported outcomes are not associated with sagittal and spinopelvic alignment in degenerative lumbar spondylolisthesis. Spine (Phila Pa 1976). 2022;47(16):1128-1136. [46] MELIKIAN R, YOON S, KIM J, et al. Sagittal plane correction using the lateral transpsoas approach: a biomechanical study on the effect of cage angle and surgical technique on segmental lordosis. Spine (Phila Pa 1976). 2016;41(17): E1016-E1021. [47] ALAHMARI A, THORNLEY P, GLENNIE A, et al. Preoperative disc angle is an important predictor of segmental lordosis after degenerative spondylolisthesis fusion. Global Spine J. 2022. doi: 10.1177/21925682221118845. [48] KAITO T, HOSONO N, FUJI T, et al. Disc space distraction is a potent risk factor for adjacent disc disease after PLIF. Arch Orthop Trauma Surg. 2011;131(11):1499-1507. [49] CHEH G, BRIDWELL K, LENKE L, et al. Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation:a minimum 5-year follow-up. Spine (Phila Pa 1976). 2007;32(20):2253-2257. [50] SCHULTE T, LEISTRA F, BULLMANN V, et al. Disc height reduction in adjacent segments and clinical outcome 10 years after lumbar 360 degrees fusion. Eur Spine J. 2007;16(12):2152-2158. [51] KUMAR M, JACQUOT F, HALL H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J. 2001;10(4):309-313. [52] SHAO X, LIU H, WU J, et al. A retrospective comparative study of postoperative sagittal balance in isthmic L5-S1 spondylolisthesis using single segment or two-segment pedicle screw fixation. BMC Musculoskelet Disord. 2022;23(1):145. [53] SEARS W, SERGIDES I, KAZEMI N, et al. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J. 2011; 11(1):11-20. [54] BAE JS, LEE SH, KIM JS, et al. Adjacent segment degeneration after lumbar interbody fusion with percutaneous pedicle screw fixation for adult low-grade isthmic spondylolisthesis: minimum 3 years of follow-up. Neurosurgery. 2010; 67(6):1600-1608. [55] WEGMANN K, GUNDERMANN S, SIEWE J, et al. Correlation of reduction and clinical outcome in patients with degenerative spondylolisthesis. Arch Orthop Trauma Surg. 2013;133(12):1639-1644. [56] SOH J, LEE J, SHIN B. Analysis of risk factors for adjacent segment degeneration occurring more than 5 years after fusion with pedicle screw fixation for degenerative lumbar spine. Asian Spine J. 2013;7(4):273-281. [57] KAWAKAMI M, TAMAKI T, ANDO M, et al. Lumbar sagittal balance influences the clinical outcome after decompression and posterolateral spinal fusion for degenerative lumbar spondylolisthesis. Spine (Phila Pa 1976). 2002;27(1):59-64. [58] JASINSKI J, TONG D, HANSON C, et al. Sagittal adjusting screws for the correction of grade IV spondylolisthesis in a patient with Ehlers-Danlos syndrome:illustrative case. J Neurosurg Case Lessons. 2021;2(2):CASE21196. [59] LAMBRECHTS M, BARBER J, BECKETT N, et al. Surgical reduction of spondylolisthesis during lumbar fusion:are complications associated with slip correction? Clin Spine Surg. 2022;35(1):E1-E6. [60] CAKIR T, YOLAS C. Synthetic bone graft versus autograft obtained from the spinous process in posterior lumbar interbody fusion. Turk Neurosurg. 2021;31(2):199-205. [61] WANG Q, HUANG Z, HUANG X, et al. Reparative effect of super active platelet combined with allogeneic bone for large bone defects. Artif Organs. 2021;45(10): 1219-1228. [62] LIU J, YANG L, ZHANG H, et al. Effects of allogeneic bone substitute configurations on cell adhesion process in vitro. J Orthop Surg. 2023;15(2):579-590. [63] YANG L, ZHANG H, LIU J, et al. Relative influence on cell behaviors of osteoblasts seeded onto demineralized bone matrix with diverse particle size. Cell and tissue banking. 2023; 24(2): 369-385. [64] ERIVAN R, SAMPER N, VILLATTE G, et al. No detectable alteration of inorganic allogeneic bone matrix colonizing mesenchymal cells: a step towards personalized bone grafts. J Bone Metab. 2021;28(2):161-169. [65] MARGONAR R, DOS SANTOS P, QUEIROZ T, et al. Rehabilitation of atrophic maxilla using the combination of autogenous and allogeneic bone grafts followed by protocol-type prosthesis. J Craniofac Surg. 2010;21(6):1894-1896. [66] MATSUMOTO K, SHAH A, KELKAR A, et al. Sagittal imbalance may lead to higher risks of vertebral compression fractures and disc degeneration-a finite element analysis. World Neurosurg. 2022;167:e962-e971. [67] LAFAGE V, SCHWAB F, VIRA S, et al. Spino-pelvic parameters after surgery can be predicted: a preliminary formula and validation of standing alignment. Spine (Phila Pa 1976). 2011;36(13):1037-1045. |
| [1] | Ouyang Beiping, Ma Xiangyang, Luo Chunshan, Zou Xiaobao, Lu Tingsheng, Chen Qiling. Three-dimensional finite element analysis of a new horizontal screw-screw crosslink in posterior atlantoaxial internal fixation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1320-1324. |
| [2] | Niu Hegang, Yang Kun, Zhang Jingjing, Yan Yizhu, Zhang Yinshun. Design of a new posterior atlas fracture reduction and internal fixation system [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1399-1402. |
| [3] | Wang Menghan, Qi Han, Zhang Yuan, Chen Yanzhi. Three kinds of 3D printed models assisted in treatment of Robinson type II B2 clavicle fracture [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1403-1408. |
| [4] | Wang Qiang, Li Shiyun, Xiong Ying, Li Tiantian. Biomechanical changes of the cervical spine in internal fixation with different anterior cervical interbody fusion systems [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 821-826. |
| [5] | Zhang Min, Peng Jing, Zhang Qiang, Chen Dewang. Mechanical properties of L3/4 laminar decompression and intervertebral fusion in elderly osteoporosis patients analyzed by finite element method [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 847-851. |
| [6] | Xue Xiaofeng, Wei Yongkang, Qiao Xiaohong, Du Yuyong, Niu Jianjun, Ren Lixin, Yang Huifeng, Zhang Zhimin, Guo Yuan, Chen Weiyi. Finite element analysis of osteoporosis in proximal femur after cannulated screw fixation for femoral neck fracture [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 862-867. |
| [7] | Zheng Jiafa, Song Xiufeng, Li Hongzhi, Zhou Jinming, Guan Shengyi, Yu He. Open reduction and internal fixation via the para-Achilles tendon approach for the treatment of posterior malleolus sandwich fractures [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 934-938. |
| [8] | Liu Changzhen, Liu Xin, Li Yuefei, Wang Jianye, Feng Zhimeng, Sun Zhaozhong. Imaging landmarks of one-hole split endoscope in the treatment of upper lumbar intervertebral disc herniation under the guidance of three-dimensional reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 939-944. |
| [9] | Yu Zhaoyu, Tan Lixin, Sun Kai, Lu Yao, Li Yong. Meta-analysis of cement-augmented pedicle screw for thoracolumbar degenerative diseases with osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 813-820. |
| [10] | Han Biao, Li Ji, Li Bin, Sun Bo, Zong Shuangle, Wang Hongrun, Li Dongmei, Li Ligeng, Wang Bin. Finite element analysis of optimal fixation method for femoral neck fracture with different reduction conditions [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(12): 1810-1814. |
| [11] | Chen Liuxu, Yang Han, Yang Jian, Yang Linyu, Kang Jianping. Finite element analysis of lumbar vertebra biomechanics after transforaminal lumbar interbody fusion combined with bilateral transpedicular transdiscal lumbar screw fixation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(12): 1815-1822. |
| [12] | Ouyang Beiping, Ma Xiangyang, Luo Chunshan, Zou Xiaobao, Lu Tingsheng, Chen Qiling. Biomechanical analysis of new horizontal screw-screw crosslink in C1-C2 pedicle screw-rod fixation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(12): 1837-1841. |
| [13] | Zhu Ruizhi, Qu Qiang, Cui Pengfei, Liu Dong, Zhang Yongtao, Wang Changyao. Efficacy of joint replacement versus closed reduction and internal fixation in treatment of unstable intertrochanteric fractures combined with osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(12): 1869-1874. |
| [14] | Wang Anquan, Chen Hao, Hua Xingyi, Lu Xiaolin, Zhou Jian, Cui Yiliang, Li Guangyu, Yin Zongsheng. Proximal femoral nail antirotation Asian version for treating femoral intertrochanteric fractures: comparison of the protruding degree of intramedullary nails in Asian population [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(12): 1901-1906. |
| [15] | You Zhengqiu, Zhang Zhongzu, Wang Qunbo. Early symptomatic intervertebral disc pseudocysts after discectomy detected on MRI [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1403-1409. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||