Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (7): 1021-1028.doi: 10.12307/2024.107
Previous Articles Next Articles
miR-20a regulates pressure overload-induced cardiac hypertrophy
Sun Teng, Han Yu, Wang Shuang, Li Jialei, Cao Jimin
- Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Key Laboratory of Cellular Physiology of Shanxi Province, and Department of Physiology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2023-01-16Accepted:2023-02-24Online:2024-03-08Published:2023-07-15 -
Contact:Cao Jimin, MD, Professor, Doctoral supervisor, Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Key Laboratory of Cellular Physiology of Shanxi Province, and Department of Physiology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Sun Teng, MD, Associate professor, Master’s supervisor, Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Key Laboratory of Cellular Physiology of Shanxi Province, and Department of Physiology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
Supported by:National Natural Science Foundation of China, No. 82170294, 81800268 (to ST); National Natural Science Foundation of China, No. 82170523 (to CJM); The Central Leading Local Science and Technology Development Fund Project, No. YDZJSX2022A061 (to ST); Shanxi Province “1331 Project” Key Discipline Construction Plan of Basic Medicine, No. XK201708 (to CJM)
CLC Number:
Cite this article
Sun Teng, Han Yu, Wang Shuang, Li Jialei, Cao Jimin. miR-20a regulates pressure overload-induced cardiac hypertrophy[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1021-1028.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

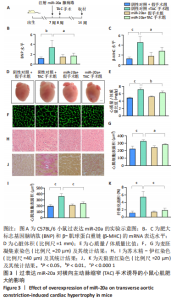
2.1 实验动物数量分析 实验选用C57BL/6J小鼠44只,第1种分组将10只小鼠随机分为2组:假手术组、TAC手术组,每组5只,均进入结果分析;第2种分组将10只小鼠随机分为2组:阴性对照组、miR-20a组,每组5只,均进入结果分析;第3种分组将24只小鼠随机分为阴性对照+假手术组、阴性对照+TAC手术组、miR-20a+假手术组、miR-20a+TAC手术组,每组6只,每组造模成功5只小鼠进入结果分析。 2.2 TAC手术小鼠心脏组织中miR-20a表达水平显著下调 为了研究miR-20a在心肌肥大动物模型中的作用,实验通过TAC手术诱导小鼠心肌肥大。qRT-PCR实验结果显示,与假手术组相比,TAC手术组小鼠术后6周心脏组织中miR-20a的表达水平显著降低(P < 0.05,n=5),见图1。"

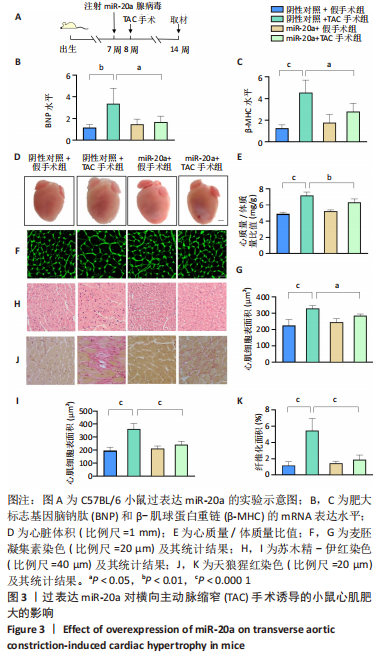
野生型C57BL/6小鼠心脏原位注射miR-20a过表达腺病毒1周后行TAC术,术后6周检测各项指标,见图3A。与阴性对照+TAC手术组相比,miR-20a+TAC手术组心脏组织中肥大标志基因脑钠肽(P < 0.05,n=5)和β-肌球蛋白重链(P < 0.05,n=5)的mRNA表达水平明显著降低,见图3B,C,心脏体积明显减小,见图3D,心质量/体质量比值也显著降低(P < 0.01,n=5),见图3E。同时,心脏组织学染色分析心肌细胞肥大和心肌损伤情况。与阴性对照+TAC手术组相比,miR-20a+TAC手术组麦胚凝集素染色结果(P < 0.05,n=5)和苏木精-伊红染色结果(P < 0.000 1,n=5)均显示心肌细胞横截面积显著减小,见图3F-I;天狼猩红染色结果显示纤维化面积显著减小(P < 0.000 1,n=5),见图3J,K。以上结果说明,过表达miR-20a显著抑制了TAC手术引起的心肌肥大和心肌纤维化。"
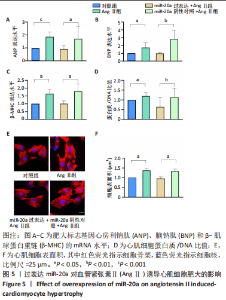
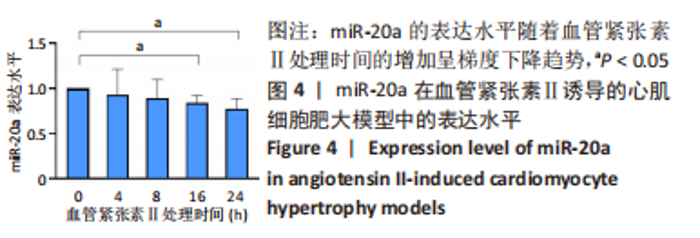
当心脏受到压力超负荷刺激时,心脏中的肾素-血管紧张素系统被激活,导致体内循环血管紧张素Ⅱ水平升高,进而引起心肌肥大[22]。应用血管紧张素Ⅱ刺激H9c2细胞构建心肌细胞肥大模型,于细胞水平探索研究miR-20a在压力超负荷诱导心肌肥大中的作用。文献报道,血管紧张素Ⅱ诱导心肌细胞肥大模型中,心房利钠肽、脑钠肽、β-肌球蛋白重链等肥大标志基因mRNA水平显著升高、蛋白质合成显著增加、心肌细胞表面积显著增大[23]。实验结果也证实,相比于对照组,血管紧张素Ⅱ处理心肌细胞24 h后,肥大标志基因心房利钠肽(P < 0.001)、脑钠肽(P < 0.05)和β-肌球蛋白重链(P < 0.05)的mRNA表达水平显著上调,见图5A-C,同时蛋白质/DNA比值显著升高(P < 0.05),见图5D,F-actin染色实验结果显示细胞表面积也显著增大(P < 0.05),见图5E,F。以上结果表明血管紧张素Ⅱ诱导了心肌细胞肥大。"
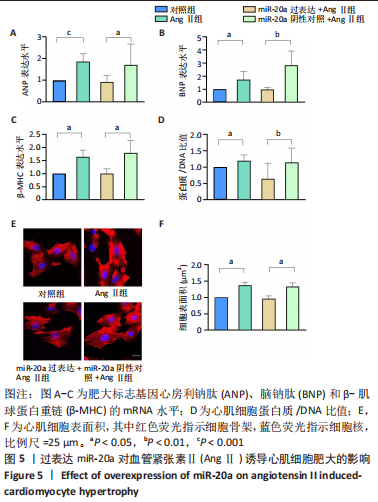
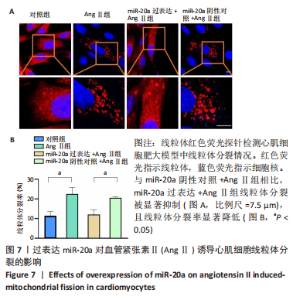
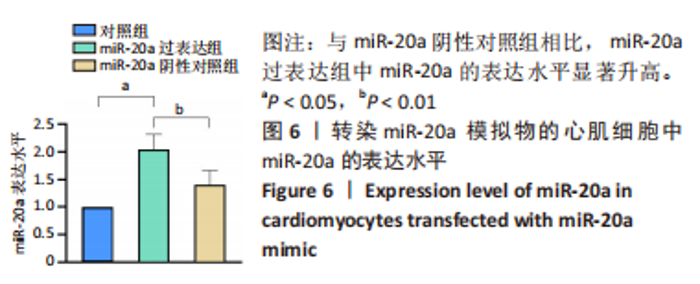
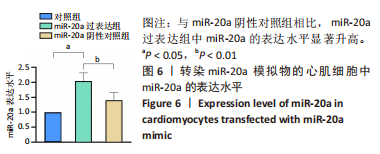
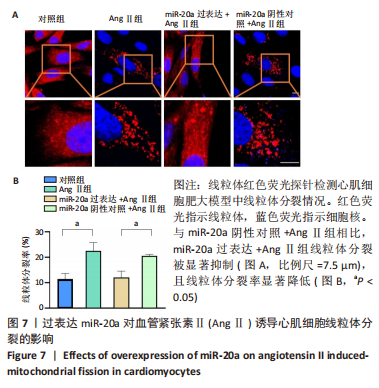
血管紧张素Ⅱ显著下调了miR-20a在心肌细胞中的表达水平,因此作者猜测miR-20a可能参与了血管紧张素Ⅱ诱导的心肌肥大调控,于是开展实验研究。结果显示,与miR-20a阴性对照+血管紧张素Ⅱ组相比,miR-20a过表达+血管紧张素Ⅱ组心房利钠肽(P < 0.05)、脑钠肽(P < 0.01)和β-肌球蛋白重链(P < 0.05)的mRNA表达水平显著降低,见图5A-C,蛋白质/DNA比值显著减小(P < 0.01),见图5D。此外,F-actin染色结果显示,与miR-20a阴性对照+血管紧张素Ⅱ组相比,miR-20a过表达+血管紧张素Ⅱ组细胞表面积显著减小(P < 0.05),见图5E,F。以上结果表明,过表达miR-20a显著抑制了血管紧张素Ⅱ诱导的心肌细胞肥大。 2.6 miR-20a调控线粒体分裂 心肌肥大发生时往往伴随着线粒体分裂,抑制线粒体分裂则抑制了心肌肥大[24]。文献报道,MitoTracker线粒体荧光探针具有细胞通透性,能够特异性地标记细胞中具有生物活性的线粒体,检测线粒体膜电位。探针标记的区域若呈现碎片状、颗粒状则表明线粒体发生分裂[25-26]。因此,实验使用MitoTracker线粒体荧光探针检测线粒体分裂情况,结果显示,与对照组相比,血管紧张素Ⅱ处理后心肌细胞线粒体分裂增多,分裂率显著升高(P < 0.05)。过表达miR-20a后,心肌细胞线粒体的碎片化程度减轻,分裂率显著降低(P < 0.05),见图7。以上结果说明,miR-20a参与了线粒体分裂调控,过表达miR-20a抑制了血管紧张素Ⅱ诱导的线粒体分裂。"
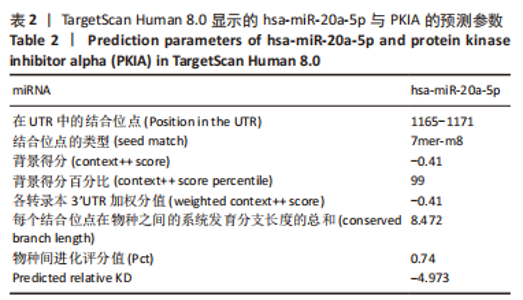
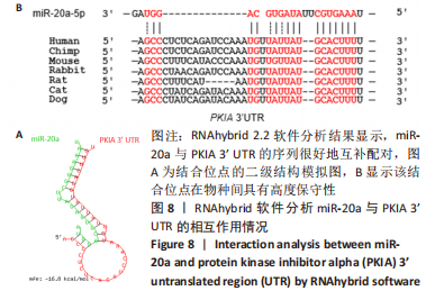
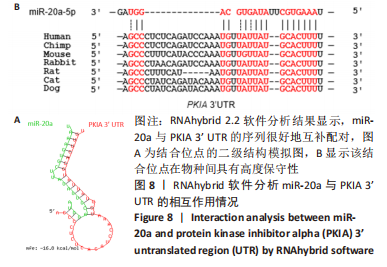
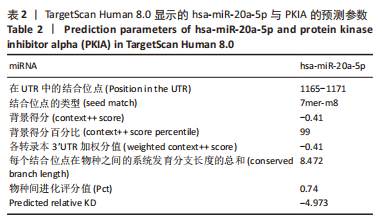
2.7 生物信息学方法预测分析miR-20a靶向心肌肥厚调控分子PKIA 进一步寻找miR-20a调控线粒体分裂的下游靶标。实验使用TargetScan Human 8.0分析预测miR-20a的下游靶基因[27],结果显示,cAMP 依赖性蛋白质活化酶抑制剂 α [protein kinase (cAMP-dependent, catalytic) inhibitor alpha,PKIA]可能是miR-20a的重要靶标,miR-20a的7 mer-m8种子区和PKIA mRNA 的3’UTR的1165-1171位可互补配对,见表2和图8A。同时使用RNAhybrid 2.2软件分析了PKIA的3’UTR序列与miR-20a序列[28],结果显示PKIA的3’UTR区存在miR-20a的潜在结合位点,且该结合位点在多物种间具有高度保守性,见图8A,B。以上结果提示miR-20a在体内可能与PKIA mRNA的3’UTR区相互结合进而抑制PKIA的表达。已有研究表明细胞质中的cAMP可自由扩散到线粒体外膜,激活线粒体表面局部的PKA。活化的PKA磷酸化促裂变动力蛋白相关蛋白1(DRP1)阻断其向线粒体表面的转位,从而导致线粒体分裂减少[29]。而PKIA已被证明可以结合并抑制 PKA的催化亚基的活性,进而促进线粒体分裂,在线粒体动力学中起到重要的作用[30]。因此作者推测miR-20a可能通过靶向结合PKIA并抑制其表达,参与了线粒体分裂的调控途径,进而发挥抵抗心肌肥大的作用。"
| [1] NAKAMURA M, SADOSHIMA J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15(7):387-407. [2] KUWAHARA K, NISHIKIMI T, NAKAO K. Transcriptional regulation of the fetal cardiac gene program. J Pharmacol Sci. 2012;119(3):198-203. [3] WANG K, LONG B, LIU F, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37(33): 2602-2611. [4] TONG XH, LIU QH, XU SH, et al. Changes in RNA, DNA, protein contents and growth of turbot Scophthalmus maximus larvae and juveniles. J Fish Biol. 2010; 77(3):512-525. [5] YANG J, CHEN Y, LI X, et al. New insights into the roles of glucocorticoid signaling dysregulation in pathological cardiac hypertrophy. Heart Fail Rev. 2022;27(4): 1431-1441. [6] LI X, TAN W, ZHENG S, et al. Differential mRNA Expression and Circular RNA-Based Competitive Endogenous RNA Networks in the Three Stages of Heart Failure in Transverse Aortic Constriction Mice. Front Physiol. 2022;13:777284. [7] JIN JY, WEI XX, ZHI XL, et al. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin. 2021;42(5):655-664. [8] WAI T, GARCÍA-PRIETO J, BAKER MJ, et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015; 350(6265):aad0116. [9] FORTE M, SCHIRONE L, AMERI P, et al. The role of mitochondrial dynamics in cardiovascular diseases. Br J Pharmacol. 2021;178(10):2060-2076. [10] AUNG LHH, JUMBO JCC, WANG Y, et al. Therapeutic potential and recent advances on targeting mitochondrial dynamics in cardiac hypertrophy: A concise review. Mol Ther Nucleic Acids. 2021;25:416-443. [11] LIN J, DUAN J, WANG Q, et al. Mitochondrial Dynamics and Mitophagy in Cardiometabolic Disease. Front Cardiovasc Med. 2022;9:917135. [12] RUPAIMOOLE R, SLACK FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203-222. [13] LI J, SHA Z, ZHU X, et al. Targeting miR-30d reverses pathological cardiac hypertrophy. EBioMedicine. 2022;81:104108. [14] UCAR A, GUPTA SK, FIEDLER J, et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012;3:1078. [15] GABISONIA K, PROSDOCIMO G, AQUARO GD, et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature. 2019; 569(7756):418-422. [16] HE D, HU J, LU Y, et al. The Effect of miR-505-5p on Inhibition of Serum Uromodulin Ameliorates Myocardial Inflammation and Apoptosis Induced by Ischemia-Reperfusion. Oxid Med Cell Longev. 2022;2022:3521971. [17] DENG Z, YAO J, XIAO N, et al. DNA methyltransferase 1 (DNMT1) suppresses mitophagy and aggravates heart failure via the microRNA-152-3p/ETS1/RhoH axis. Lab Invest. 2022;102(8):782-793. [18] YANG Y, WANG Z, XU Y, et al. Knockdown of lncRNA H19 alleviates ox-LDL-induced HCAECs inflammation and injury by mediating miR-20a-5p/HDAC4 axis. Inflamm Res. 2022;71(9):1109-1121. [19] WANG D, WANG Y, MA J, et al. MicroRNA-20a participates in the aerobic exercise-based prevention of coronary artery disease by targeting PTEN. Biomed Pharmacother. 2017;95:756-763. [20] SHEU JJ, CHAI HT, SUNG PH, et al. Double overexpression of miR-19a and miR-20a in induced pluripotent stem cell-derived mesenchymal stem cells effectively preserves the left ventricular function in dilated cardiomyopathic rat. Stem Cell Res Ther. 2021;12(1):371. [21] LIU X, GUO B, ZHANG W, et al. MiR-20a-5p overexpression prevented diabetic cardiomyopathy via inhibition of cardiomyocyte apoptosis, hypertrophy, fibrosis and JNK/NF-κB signalling pathway. J Biochem. 2021;170(3):349-362. [22] AN D, ZENG Q, ZHANG P, et al. Alpha-ketoglutarate ameliorates pressure overload-induced chronic cardiac dysfunction in mice. Redox Biol. 2021;46:102088. [23] JU S, PARK S, LIM L, et al. Low density lipoprotein receptor-related protein 1 regulates cardiac hypertrophy induced by pressure overload. Int J Cardiol. 2020; 299:235-242. [24] WANG T, ZHAI M, XU S, et al. NFATc3-dependent expression of miR-153-3p promotes mitochondrial fragmentation in cardiac hypertrophy by impairing mitofusin-1 expression. Theranostics. 2020;10(2):553-566. [25] WANG K, LONG B, JIAO JQ, et al. miR-484 regulates mitochondrial network through targeting Fis1. Nat Commun. 2012;3:781. [26] WANG K, LONG B, ZHOU LY, et al. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun. 2014;5:3596. [27] MCGEARY SE, LIN KS, SHI CY, et al. The biochemical basis of microRNA targeting efficacy. Science. 2019;366(6472):eaav1741. [28] REHMSMEIER M, STEFFEN P, HOCHSMANN M, et al. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10(10):1507-1517. [29] ZHANG F, ZHANG L, QI Y, et al. Mitochondrial cAMP signaling. Cell Mol Life Sci. 2016;73(24):4577-4590. [30] SOTOMAYOR-FLORES C, RIVERA-MEJÍAS P, VÁSQUEZ-TRINCADO C, et al. Angiotensin-(1-9) prevents cardiomyocyte hypertrophy by controlling mitochondrial dynamics via miR-129-3p/PKIA pathway. Cell Death Differ. 2020; 27(9):2586-2604. [31] LUO Y, JIANG N, MAY HI, et al. Cooperative Binding of ETS2 and NFAT Links Erk1/2 and Calcineurin Signaling in the Pathogenesis of Cardiac Hypertrophy. Circulation. 2021;144(1):34-51. [32] LIN QY, ZHANG YL, BAI J, et al. VEGF-C/VEGFR-3 axis protects against pressure-overload induced cardiac dysfunction through regulation of lymphangiogenesis. Clin Transl Med. 2021;11(3):e374. [33] BERNARDO BC, WEEKS KL, PRETORIUS L, et al. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128(1):191-227. [34] CHENG Y, SHEN A, WU X, et al. Qingda granule attenuates angiotensin II-induced cardiac hypertrophy and apoptosis and modulates the PI3K/AKT pathway. Biomed Pharmacother. 2021;133:111022. [35] TAKEZAKO T, UNAL H, KARNIK SS, et al. Current topics in angiotensin II type 1 receptor research: Focus on inverse agonism, receptor dimerization and biased agonism. Pharmacol Res. 2017;123:40-50. [36] ROSENBAUGH EG, SAVALIA KK, MANICKAM DS, et al. Antioxidant-based therapies for angiotensin II-associated cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol. 2013;304(11):R917-928. [37] LIN HB, NAITO K, OH Y, et al. Innate Immune Nod1/RIP2 Signaling Is Essential for Cardiac Hypertrophy but Requires Mitochondrial Antiviral Signaling Protein for Signal Transductions and Energy Balance. Circulation. 2020;142(23):2240-2258. [38] PIQUEREAU J, CAFFIN F, NOVOTOVA M, et al. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res. 2012;94(3):408-417. [39] KONG AS, LAI KS, LIM SE, et al. miRNA in Ischemic Heart Disease and Its Potential as Biomarkers: A Comprehensive Review. Int J Mol Sci. 2022;23(16):9001. [40] GARGIULO P, MARZANO F, SALVATORE M, et al. MicroRNAs: diagnostic, prognostic and therapeutic role in heart failure-a review. ESC Heart Fail. 2022 Nov 8. doi: 10.1002/ehf2.14153. Online ahead of print. [41] PARVAN R, HOSSEINPOUR M, MORADI Y, et al. Diagnostic performance of microRNAs in the detection of heart failure with reduced or preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. 2022;24(12):2212-2225. [42] LUO F, LIU W, BU H. MicroRNAs in hypertrophic cardiomyopathy: pathogenesis, diagnosis, treatment potential and roles as clinical biomarkers. Heart Fail Rev. 2022;27(6):2211-2221. [43] ZHANG Y, WANG Z, GEMEINHART RA. Progress in microRNA delivery. J Control Release. 2013;172(3):962-974. [44] TÄUBEL J, HAUKE W, RUMP S, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J. 2021;42(2):178-188. [45] HU L, DING M, TANG D, et al. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics. 2019;9(13):3687-3706. [46] ZHAO Y, PONNUSAMY M, LIU C, et al. MiR-485-5p modulates mitochondrial fission through targeting mitochondrial anchored protein ligase in cardiac hypertrophy. Biochim Biophys Acta Mol Basis Dis. 2017;1863(11):2871-2881. [47] ZHOU L, LI R, LIU C, et al. Foxo3a inhibits mitochondrial fission and protects against doxorubicin-induced cardiotoxicity by suppressing MIEF2. Free Radic Biol Med. 2017;104:360-370. [48] QI J, WANG F, YANG P, et al. Mitochondrial Fission Is Required for Angiotensin II-Induced Cardiomyocyte Apoptosis Mediated by a Sirt1-p53 Signaling Pathway. Front Pharmacol. 2018;9:176. |
| [1] | Yue Ying, Hao Wenli, Gan Yongkang. Effects of enalapril folic acid on vascular endothelial cell activity and angiotensin II and angiotensin-converting enzyme levels in a rat model of lower extremity venous embolism [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(24): 3877-3882. |
| [2] | Zhang Wei, Liu Ziming, Zhang Nianping, Tian Sixie, Zhang Siyu, Li Yanhua, Ma Cungen. Effects of mitochondrial fission inhibitor-1 on neurotrophic factor and neural inhibitory signaling pathway in a mouse model of multiple sclerosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(17): 2651-2657. |
| [3] | Fan Qinghua, Qi Jie, Zhang Jun. The regulatory role of Hippo-YAP signaling pathway in exercise-induced cardiac hypertrophy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(17): 2708-2715. |
| [4] | Yang Lijuan, Yao Na, Lu Ruiwen, Liu Xiaoyu, Wang Baojun. Bleomycin combined with angiotensin II for establishing a mouse model of aortic dissection [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(14): 2194-2199. |
| [5] | Wu Xiaolei, Han Yu, Li Jialei, Wang Shuang, Cao Jimin, Sun Teng. piRNA-5938 can regulate cardiomyocyte apoptosis and mitochondrial fission [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(11): 1750-1757. |
| [6] | Sun Ying, Xiang Guangda, Xu Xiaoli. Effects of myeloid-derived growth factor on ventricular remodeling in aging mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(31): 5020-5025. |
| [7] | Xie Liying, Liu Siqi, Wu Mingrui, Gao Erhe, Han Zhibo, Zuo Lin. Human umbilical cord mesenchymal stem cell transplantation for myocardial hypertrophy in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4826-4833. |
| [8] | Liu Ya, Liu Xia, Deng Penghui, Ji Wei, Li Jianping. Exercise effects on myocardial type I, III collagen and angiotensin II/transforming growth factor beta1/Smad2 pathway in diabetic myocardial fibrosis rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4173-4179. |
| [9] | Wen Miaomiao, Fan Junbai. Relationship between biological activities of Apelin and Elabela and diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(35): 5735-5740. |
| [10] | Yuan Guoqiang, Qin Yongsheng, Peng Peng. High-intensity interval training for treating pathological cardiac hypertrophy in spontaneously hypertensive rats: effects and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(23): 3708-3715. |
| [11] | Li Qin, Li Yan, Gao Shanshan, Zheng Jintao. Pathways of astragalus polysaccharide inhibiting angiotensin II-induced cardiac hypertrophy [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(19): 3086-3091. |
| [12] | Liu Baoyi1, Li Minde1, Yang Fan2, Li Shaopeng1, Chen Haojie1, Xiao Peng1. Angiotensin II promotes steroid-induced avascular necrosis of the femoral head in mice [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(19): 3044-3049. |
| [13] | Wang Jing, Han Jiang-hong, Li Qiong. 5-Azacytidine combined with angiotensin II induces differentiation of adipose-derived mesenchymal stem cells into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(29): 4629-4634. |
| [14] | Yan Ping, Hou Jing-ying, Zheng Shao-xin, Long Hui-bao, Zhong Ting-ting, Zhou Chang-qing, Guo Tian-zhu, Wu Quan-hua, Wang Tong. Cardiac stem cells improve the electrophysiological stability and ventricular fibrillation threshold via ANGII/AT1R/TGF-beta1/SMAD/CX43 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2016, 20(28): 4226-4233. |
| [15] | Song Yang, Wu Rong, Zeng Yue-can, Zhang Zhen-yong, Du Hong-mei. Protective effect of pioglitazone in rat models of radiation-induced heart injury [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(5): 674-680. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||