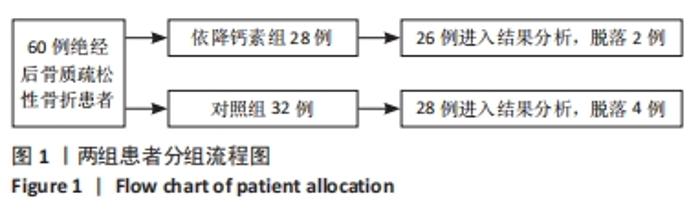
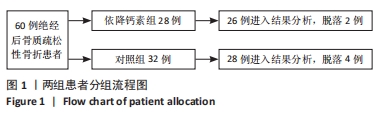
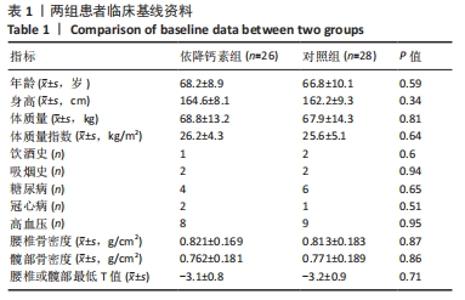
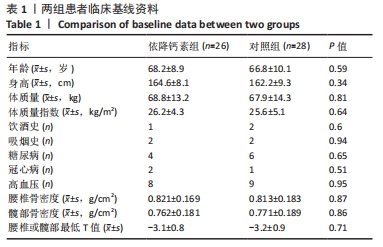
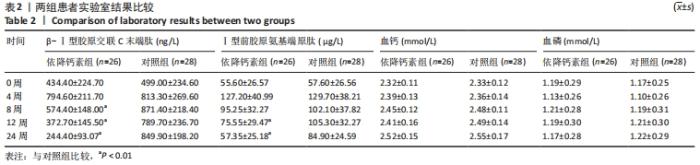
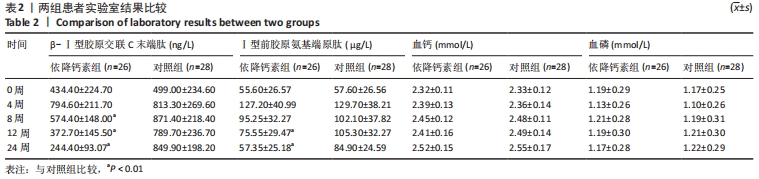
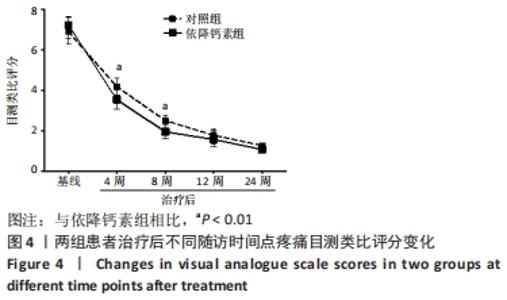
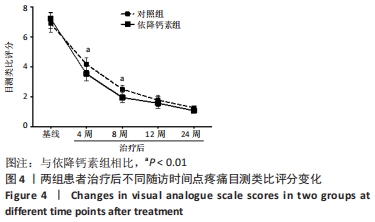
[1] Clynes MA, Harvey NC, Curtis EM, et al. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105-117.
[2] 郭嘉炜, 陈晓, 苏佳灿. 骨质疏松性骨折治疗面临的挑战和解决途径[J]. 中华创伤杂志,2021,37(6):481-487.
[3] 刘驰, 石磊, 王林, 等. 老年骨质疏松性股骨颈骨折住院患者的人口学特征及临床特征分析. 中华老年医学杂志,2021,40(11):1401-1406.
[4] 熊晨, 何贵平, 张堃, 等. 股骨近端防旋髓内钉干骺端扩髓和未扩髓治疗老年严重骨质疏松性股骨转子间骨折的疗效比较[J]. 中华创伤杂志,2021,37(12):1090-1098.
[5] 宋铁楠, 王旭明, 于小均, 等. 老年股骨转子间骨折患者新型股骨近端内固定术后并发症原因分析和处理[J]. 中华老年医学杂志, 2018,37(12):1324-1327.
[6] JI Z, SHI C, HUANG S, et al. Elcatonin attenuates disuse osteoporosis after fracture fixation of tubular bone in rats. J Orthop Surg Res. 2015; 10:103.
[7] CHEN WC, LIN EY, KANG YN. Efficacy and safety of elcatonin in postmenopausal women with osteoporosis: a systematic review with network meta-analysis of randomized clinical trials. Osteoporosi Int. 2019;30(9):1723-1732.
[8] GUTIERREZ-BUEY G, RESTITUTO P, BOTELLA S, et al. Trabecular bone score and bone remodelling markers identify perimenopausal women at high risk of bone loss. Clin Endocrinol. 2019;91(3):391-399.
[9] SRIVASTAVA AK, LIBANATI C, HOHMANN O, et al. Acute effects of calcitonin nasal spray on serum C-telopeptide of type 1 collagen (CTx) levels in elderly osteopenic women with increased bone turnover. Calcif Tissue Int. 2004;75(6):477-481.
[10] TIAN L, YANG R, WEI L, et al. Prevalence of osteoporosis and related lifestyle and metabolic factors of postmenopausal women and elderly men: A cross-sectional study in Gansu province, Northwestern of China. Medicine. 2017;96(43):e8294.
[11] ZHANG C, FENG J, WANG S, et al. Incidence of and trends in hip fracture among adults in urban China: A nationwide retrospective cohort study. PLoS Med. 2020;17(8):e1003180.
[12] SADIC S, CUSTOVIC S, JASAREVUC M, et al. Proximal Femoral Nail Antirotation in Treatment of Intertrochanteric Hip Fractures: a Retrospective Study in 113 Patients. Med Arch. 2015;69(6):352-356.
[13] TAKIGAMI I, MATSUMOTO K, OHARA A, et al. Treatment of trochanteric fractures with the PFNA (proximal femoral nail antirotation) nail system - report of early results. Bull NYU Hosp Joint Dis. 2008;66(4):276-279.
[14] 张智海, 张智若, 刘忠厚, 等. 中国大陆地区以-2.0SD为诊断标准的骨质疏松症发病率回顾性研究[J]. 中国骨质疏松杂志,2016, 22(1):1-8.
[15] 阮文东, 王沛, 雪原, 等. 骨质疏松骨折后再骨折的临床风险因素[J]. 中华骨科杂志,2011,31(7):789-793.
[16] LEE KJ, UM SH, KIM YH. Postoperative Rehabilitation after Hip Fracture: A Literature Review. Hip Pelvis. 2020;32(3):125-131.
[17] 中国康复医学会骨质疏松预防与康复专业委员会, 孟斌, 程黎明, 等. 骨科急性骨丢失防治专家共识[J]. 中华骨与关节外科杂志, 2021,14(7):577-583.
[18] KIMMEL DB, VENNIN S, DESYATOVA A, et al. Bone architecture, bone material properties, and bone turnover in non-osteoporotic post-menopausal women with fragility fracture. Osteoporos Int. 2022;33(5): 1125-1136.
[19] LI N, GONG YC, CHEN J. A meta-analysis of the therapeutic effect of intranasal salmon calcitonin on osteoporosis. Eur J Med Res. 2021; 26(1):140.
[20] YADAV AM, BAGADE MM, GHUMNANI S, et al. The phytochemical plumbagin reciprocally modulates osteoblasts and osteoclasts. Biol Chem. 2022;403(2):211-229.
[21] CHEN JF, YANG KH, ZHANG ZL, et al. A systematic review on the use of daily subcutaneous administration of teriparatide for treatment of patients with osteoporosis at high risk for fracture in Asia. Osteoporosis Int. 2015;26(1):11-28.
[22] CAMACHO PM, PETAK SM, BINKLEY N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2020 Update. Endocr Pract. 2020;26(Suppl 1):1-46.
[23] 中华医学会, 中华医学会杂志社, 中华医学会全科医学分会, 等. 原发性骨质疏松症基层诊疗指南(2019年)[J]. 中华全科医师杂志, 2020,19(4):304-315.
[24] BELLAVIA D, CARADONNA F, DIMARCO E, et al. Terpenoid treatment in osteoporosis: this is where we have come in research. Trends Endocrinol Metabol. 2021;32(11):846-861.
[25] KANG JH, KO HM, MOON JS, et al. Osteoprotegerin expressed by osteoclasts: an autoregulator of osteoclastogenesis. J Dent Res. 2014; 93(11):1116-1123.
[26] XIE Y, ZHANG L, XIONG Q, et al. Bench-to-bedside strategies for osteoporotic fracture: From osteoimmunology to mechanosensation. Bone Res. 2019;7:25.
[27] 王亚军, 陈晓, 智信, 等. 阿仑膦酸盐对骨质疏松性骨折愈合影响的研究进展[J]. 中华创伤杂志,2020,36(1):63-67.
[28] LYRITIS G, BOSCAINOS PJ. Calcitonin effects on cartilage and fracture healing. J Musculoskelet Neuronal Interact. 2001;2(2):137-142.
[29] ITO A, YOSHIMURA M. Mechanisms of the analgesic effect of calcitonin on chronic pain by alteration of receptor or channel expression. Mole Pain. 2017;13:1744806917720316.
[30] TANAKA S, YOSHIDA A, KONO S, et al. Effectiveness of monotherapy and combined therapy with calcitonin and minodronic acid hydrate, a bisphosphonate, for early treatment in patients with new vertebral fractures: An open-label, randomized, parallel-group study. J Orthop Sci. 2017;22(3):536-541.
[31] ELSHEIKH NA, AMR YM. Effect of Adding Calcitonin to Translaminar Epidural Steroid in Degenerative Lumbar Spinal Canal Stenosis. Pain Phys. 2016;19(3):139-146.
[32] SAHIN F, YILMAZ F, KOTEVOGLU N, et al. Efficacy of salmon calcitonin in complex regional pain syndrome (type 1) in addition to physical therapy. Clin Rheumatol. 2006;25(2):143-148.
|