Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (1): 130-137.doi: 10.12307/2022.719
Previous Articles Next Articles
Progress of non-coding RNAs in stem cell abnormality of idiopathic pulmonary fibrosis
Huang Bin1, Zheng Jinxu1, Zhang Jun2
- 1Department of Respiratory and Critical Care Medicine, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, Jiangsu Province, China; 2Department of Respiratory and Critical Care Medicine, Affiliated Aoyang Hospital of Jiangsu University, Suzhou 215600, Jiangsu Province, China
-
Received:2021-11-12Accepted:2021-12-24Online:2023-01-08Published:2022-06-14 -
Contact:Zheng Jinxu, MD, Professor, Chief physician, Department of Respiratory and Critical Care Medicine, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, Jiangsu Province, China -
About author:Huang Bin, Master candidate, Department of Respiratory and Critical Care Medicine, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, Jiangsu Province, China -
Supported by:Zhenjiang Major Project, No. SH2018048 (to ZJX); Suzhou Social Development Project, No. SYSD2020010 (to ZJ)
CLC Number:
Cite this article
Huang Bin, Zheng Jinxu, Zhang Jun. Progress of non-coding RNAs in stem cell abnormality of idiopathic pulmonary fibrosis[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 130-137.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

2.1 ncRNA的作用机制 miRNA作为20-24个核苷酸的短单链ncRNA,其通常与Argonaute蛋白等结合形成RNA诱导的沉默复合体,作用于mRNA的3’非翻译区导致靶mRNA降解和翻译的抑制[6],该机制与干细胞功能相关,miR-184通过直接作用于3’UTR抑制细胞角蛋白15和缺氧诱导因子1α抑制因子,诱导Notch通路激活和表皮分化[7]。 lncRNA是超过200个核苷酸的长链ncRNA,基因组序列保守性差,但剪接位点稳定[8]。lncRNA对基因组存在多种调节方式[9-10]:①与miRNA结合:胞质lncRNA充当竞争性内源RNA (competing endogenous RNA,ceRNA),结合miRNA的种子序列发挥海绵作用,抑制miRNA表达,保护靶mRNA免受抑制;②与mRNA配对:lncRNA与mRNA和pre-mRNA互补杂交通过影响mRNA的剪接、编辑及稳定性来参与翻译调控;③与DNA结合:lncRNA与启动子形成三螺旋结构抑制mRNA转录,可招募染色质重塑复合体改变DNA修饰状态;④与蛋白质结合:lncRNA可与蛋白结合将其定位至特定DNA序列,可作为蛋白支架使2个以上蛋白形成复合物,也可作为海绵阻断蛋白表达;⑤编码多肽:少数lncRNA可编码小分子肽。部分机制被发现与干细胞功能相关,lncRNA KLF3-AS1作为miR-185-5p的竞争性内源RNA解除miR-185-5p对Kruppel样因子3的抑制,缓解食管鳞癌细胞的侵袭和迁移[11]。lncRNA HOTTIP介导同源盒A9(homeobox A9,HOXA9)与WD重复域5蛋白结合增强Wnt/β-catenin通路影响胰腺癌干细胞的干细胞化[12]。此外,lncRNA HAND2-AS1将染色质重塑复合物招募到骨形态发生蛋白受体1A的启动子中,诱导骨形态发生蛋白受体1A表达并激活骨形态发生蛋白信号通路,促进肝癌干细胞增殖[13]。 circRNA作为3’-5’端反向剪接形成的闭合环状RNA,在转录或转录后进行多种基因调控[14-15]:①位于胞核的circRNA主要涉及转录、选择性剪接和染色质环化;②胞质中的circRNA可充当 miRNA的竞争性内源RNA,使靶mRNA免于miRNA抑制;③circRNA可作为蛋白质支架和海绵;④部分circRNA可依赖非典型eIF4G蛋白或一种含N6甲基腺苷修饰的基序“RRACH”启动翻译。circRNA通过竞争性内源RNA等方式影响干细胞功能,circRNA PTN通过充当miR-145-5p和miR-330-5p的竞争性内源RNA上调SOX9和ITGA5,促进胶质瘤细胞的生长[16]。circRNA MEG3在肝癌干细胞中阻断m6A甲基转移酶抑制端粒相关蛋白CBF5的表达,抑制端粒酶活性并缩短端粒寿命,抑制细胞增殖[17]。 2.2 ncRNA对特发性肺纤维化中干细胞异常的调控 2.2.1 miRNA调控干细胞异常 特发性肺纤维化的病因尚未明确,首先在环境、氧化应激、衰老和基因等多因素的影响下,产生转化生长因子β、白细胞介素1、结缔组织生长因子等促纤维化介质的增加,并伴随转化生长因子β/SMAD,Notch及ERK等通路的异常激活[18-19]。在正常情况下,胶原蛋白可以诱导机体对损伤的修复。但在特发性肺纤维化进程中,胶原蛋白过度产生,机体呈“异常修复”状态,伴随成纤维细胞异常激活及胞外基质过度沉积。而多种干细胞异常共同构成特发性肺纤维化进展的关键环节,见图3。 "
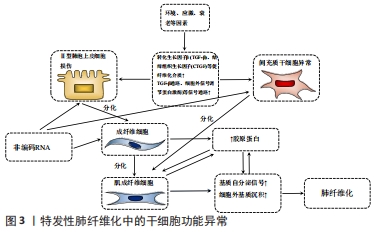

Ⅱ型肺泡上皮细胞是肺内的肺泡干细胞,可分裂产生子代Ⅱ型肺泡上皮细胞及AEC1s。特发性肺纤维化中功能失调的Ⅱ型肺泡上皮细胞在受损后重建力缺失,伴随异常基因表达及蛋白质应激反应,破坏微环境并改变细胞行为而加速衰老[20]。类上皮-间充质转化(epithelial-mesenchymal transition like,EMT-like)是Ⅱ型肺泡上皮细胞因暴露于纤维化介质后发生的最重要的细胞行为,致力于分子重建,Ⅱ型肺泡上皮细胞失去胞间连接并获得间充质表型,从上皮转换到更动态的状态,胞外基质产生增强[21]。与此同时,间充质干细胞也可在特发性肺纤维化初期抵抗肺泡上皮的损伤。然而病理微环境的改变会影响间充质干细胞的行为,表现为促炎因子释放增加,并分化为肌成纤维细胞,促进肺纤维化的发生[22]。肺成纤维细胞具备多细胞分化的潜能,能释放胞外基质致力于伤口的愈合修复,特发性肺纤维化中异常的Ⅱ型肺泡上皮细胞、间充质干细胞等细胞释放促纤维化介质,形成纤维化微环境,受累的肺成纤维细胞被激活,逐渐向肌成纤维细胞的转化(fibroblast-to-myofibroblast transition,FMT),纤维化介质及胞外基质生成进一步加重,构成纤维化的前馈循环[23]。近年证实特发性肺纤维化进展伴随着细胞衰老,衰老是不可避免的时间依赖性功能衰退,伴随生理完整性、体内平衡控制及死亡抗性丧失,其在Ⅱ型肺泡上皮细胞和成纤维细胞常表现为增强的凋亡抵抗或异常分泌模式,发生过程常交织着细胞间的串扰[23-24]。 现有研究揭示了miRNA涉及上述特发性肺纤维化中的干细胞功能障碍过程[25-37],见表1。 "
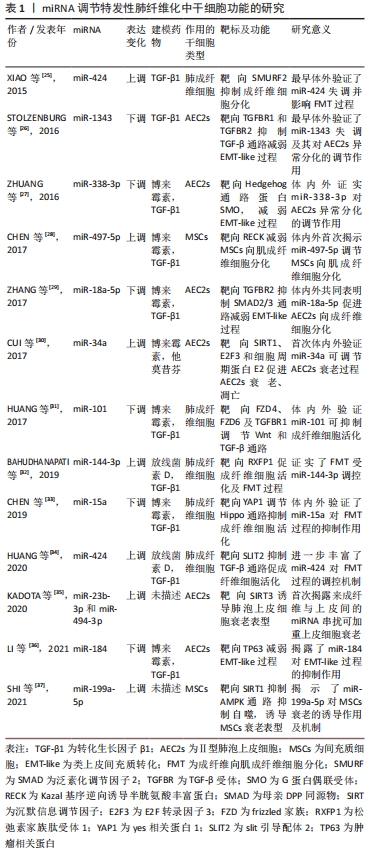

miR-184通过靶向抑癌基因(tumor protein p63,TP63)减弱转化生长因子β所致EMT-like改变,抑制了小鼠肺纤维化[36]。这些研究揭示miRNA可作为特发性肺纤维化诊断、预后以及基因治疗的标志物或靶点选择,通过控制miRNA水平可能成为缓解肺纤维化的直接途径,并且更为重要的是miRNA对靶基因的下调程度是适度的,通常少于50%,可能的原因是一个基因会同时受多个miRNA的调控,也暗示着miRNA变化引发的毒副反应较一般药物可能更小,也拥有更好的前景。 2.2.2 lncRNA调控干细胞异常 尽管对lncRNA调节机制已有部分认识,但其在特发性肺纤维化中的作用仍未明确。迄今LncRNA充当miRNA海绵的功能已被作为研究的切入点,揭示出特发性肺纤维化中存在的lncRNA/miRNA/mRNA功能性调控轴,并构成复杂的竞争性内源RNA网络。表2记录了部分调节特发性肺纤维化中干细胞功能的lncRNA[38-47]。 "
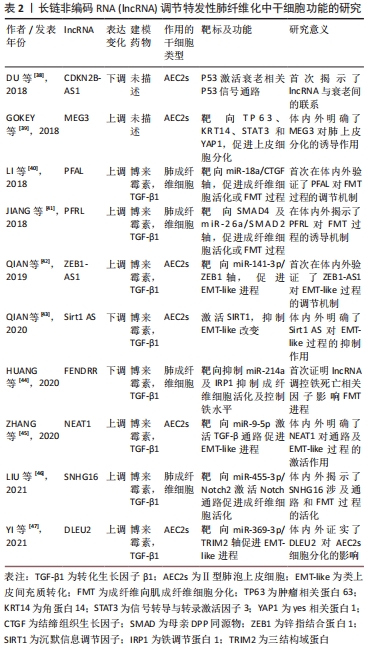

lncRNA PFRL可通过充当miR-26a的竞争性内源RNA靶向SMAD2,并通过靶向SMAD4抑制磷酸化SMAD2/3异位,上调磷酸化SMAD2/3水平,激活SMAD通路促进成纤维细胞的活化及胶原沉积,介导特发性肺纤维化中的前纤维化作用[41]。lncRNA DLEU2沉默后,通过上调miR-369-3p抑制3结构域蛋白2(tripartite motif containing 2,TRIM2)水平,诱导A549细胞增殖及EMT-like过程,进而抑制小鼠肺纤维化[47]。由此看来,LncRNA同样致力于干细胞功能异常,包括上皮细胞功能异常、肺成纤维细胞的活化及细胞衰老等,可以通过靶基因及所在通路的干预调控纤维化进程,对特发性肺纤维化的早期诊断有潜在价值。在干预lncRNA表达后可能直接实现靶mRNA及蛋白丰度的共同调控,这也正是lncRNA区别于miRNA对特发性肺纤维化治疗的独特价值,使lncRNA成为极具吸引力的治疗靶点。 2.2.3 circRNA调控干细胞异常 基于circRNA的组织特异性,circRNA被认为是各疾病的新兴生物标志物,其在特发性肺纤维化中的研究相对匮乏,但也明确了部分与干细胞功能相关的circRNA[48-52],见表3。 "
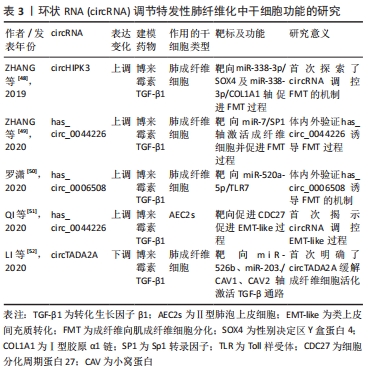

circRNA被报道在吸烟引起的肺纤维化中发挥作用。在吸烟诱导的肺纤维化中,circRNA_0026344靶向外泌体miR-21激活SMAD7,涉及了上皮细胞与成纤维细胞的异常串扰[53]。一方面,circRNA参与了特发性肺纤维化部分发病过程的诱导;另一方面,circRNA可抑制特发性肺纤维化进展,这其中涉及肺上皮或成纤维细胞为主的功能失调过程,可能是新兴生物标志物或药物靶点。circRNA较lncRNA最大的优点是由其共价环状结构带来的高保守性,其用作生物标志物将更具特异性。但限于目前研究水平,其作用机制仍未明确。 2.3 ncRNA与特发性肺纤维化治疗 根据中华医学会最新指南,目前仅吡非尼酮及尼达尼布能够用于治疗特发性肺纤维化,但仅能延缓疾病进展,无法逆转肺纤维化。临床迫切需要新的治疗方法,近年来,干细胞移植疗法受到极大关注。过去几年里,围绕间充质干细胞、Ⅱ型肺泡上皮细胞移植治疗特发性肺纤维化进行了一系列临床试验[54-60],见图4。 "
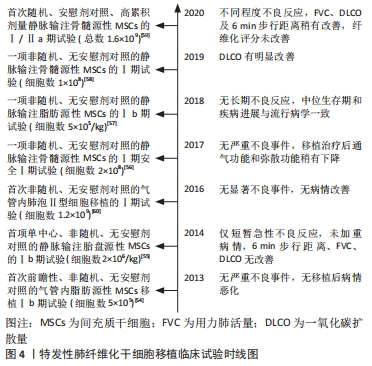

然而就最近2次临床试验结果而言,间充质干细胞移植的疗效不尽人意,其一治疗缓解了一氧化碳扩散力(diffusing lung capacity for carbon monoxide,DLCO)的下降,改善了弥散功能,而在另一个临床试验中,虽然治疗组较安慰剂组6 min步行距离、DLCO及用力肺活量稍有改善,但患者纤维化评分无显著差异,并且伴随不同程度的不良反应[58-59]。间充质干细胞移植的疗效及安全性尚未得到充分的肯定,这在一定程度上与特发性肺纤维化诱导间充质干细胞适应性改变以及剂量过量有关。同时动物实验证明了Ⅱ型肺泡上皮细胞移植的疗效,其安全性目前仅在一次小样本量的临床研究中被验证,但试验的非随机性且无安慰剂对照,对安全性和疗效评估的证据不足,仍需进行大量的随机对照试验[60-61]。目前看来,移植细胞数是决定着干细胞移植安全性及耐受性的最关键因素,且气管内移植和静脉注射的有效剂量可能存在差异。现有证据提示间充质干细胞和Ⅱ型肺泡上皮细胞移植可能是安全的,而疗效有待后续大量的添加安慰剂对照的随机试验加以验证。特发性肺纤维化微环境会造就间充质干细胞、Ⅱ型肺泡上皮细胞的适应性改变,最终向成纤维细胞分化,这可能解释了过量干细胞植入却未能缓解肺纤维化。仅通过干细胞移植可能不足以抵抗肺纤维化对干细胞异常的诱导,这意味着需要对干细胞异常的上游信号加以干预对治疗加以强化。 2.3.1 miRNA与特发性肺纤维化治疗 ncRNA失调贯穿于特发性肺纤维化中干细胞功能异常的多个过程,这意味着通过基因治疗靶向纠正ncRNA水平可能为特发性肺纤维化治疗带来新的希望。而对ncRNA调节无外乎增强或减弱其表达。基因治疗常用的敲减方法包括小干扰RNA(small interfering RNA,siRNA)或反义寡核苷酸以及CRISPR/Cas9技术等,但是敲减效率很大程度上受目的ncRNA亚细胞定位的影响,siRNA作为双链RNA主要在胞浆中发挥作用,而作为单链RNA的反义寡核苷酸对胞核定位的ncRNA敲减效率更高[62]。由于siRNA自身不具备穿透组织和细胞膜的能力且对RNA酶的抵抗力缺乏,其在体内发挥作用常常受阻,近年来通过增加siRNA的化学修饰并借助脂质纳米颗粒等载体,增强了siRNA的稳定性并且解决部分药物的体内运输问题,极大减少了其脱靶效应[63-64]。siRNA易合成且可靶向任何基因,但其在体内半衰期短,仅能实现对目标转录组的短期敲减,这意味着siRNA制剂需长期摄入。 同样的,对反义寡核苷酸的硫代硫酸修饰等也极大提高了其对RNA酶的抗性及其与靶ncRNA的结合亲和力,但与siRNA一样仅可实现短期的沉默表达[65]。而对于ncRNA过表达可通过质粒DNA实现,但效应因细胞分裂代谢逐渐消失,依靠慢病毒或腺病毒载体理论上可将其整合进宿主基因组,实现永久的敲减或过表达,但往往引发不同程度的免疫反应和突变,最终也仅发挥短暂的效应[66-67]。CRISPR/Cas9技术可实现基因表达的永久上调或下调,并且在切口酶配对优化后其脱靶效应极大降低,然而将细胞取出进行基因编辑后移植,细胞的存活率是极大的障碍[68-69]。现阶段siRNA无疑是发展最快的基因治疗手段,由于其几乎能靶向任何基因,且药物研发时间比其他几种方法快的多。在进一步优化药物化学修饰,逐步实现siRNA有效剂量的缩短及半衰期的延长,siRNA疗法也可发挥长期效应。而反义寡核苷酸、病毒载体、基因编辑方法也不可或缺,其共同弥补了siRNA只能实现基因敲减的局限性,且后两者更易获得更高的基因调节效率。 相较于最早靶向mRNA的基因治疗,靶向miRNA疗法的潜在优势是其能实现多基因靶向,这意味着在纠正miRNA失调的同时也可影响miRNA靶基因的水平。与miRNA互补的寡核苷酸可阻断其活性,而模拟miRNA的双链或化学修饰的单链RNA可增强其活性[70]。两者已作为抑制剂和增强剂用于特发性肺纤维化中miRNA靶点的开发。如最近报道在体外实验中采用miR-142-3p模拟物诱导肺泡上皮及成纤维细胞中miR-142-3p过表达后,可降低转化生长因子β受体1(transforming growth factor beta receptor 1,TGFBR1)达到抑制纤维化基因表达的效果[70]。动物实验中miR-155拮抗剂的运用显著抑制纤维化肺组织羟脯氨酸水平,同时降低白细胞介素4、转化生长因子β及干扰素γ水平,并抑制转化生长因子β活化激酶1结合蛋白2(TAB2)的活化达到对纤维化的治疗效果[71]。同样体内外实验证明miR-448模拟物可抑制JNK通路的激活拮抗肺纤维化进展[72]。然而抑制剂或模拟物的效应是短期的,miRNA靶向药物在特发性肺纤维化的研究也暂时止步于体内实验,目前仅在肿瘤等疾病开展了临床试验,如MRX-34和RG-101等[73-74]。此外,现有研究已揭示出miRNA与已知特发性肺纤维化药物靶点的相关性。如双环醇在体外通过促进miR-455-3p抑制凋亡调节因子(BCL2 associated X,Bax)的表达,缓解了纤维化诱导的Ⅱ型肺泡上皮细胞凋亡[75]。TONG等[76]报道阿魏酸联合黄芪甲苷治疗可通过稳定上调miR-29b来改善肺组织氧化应激以及转化生长因子β/SMAD3信号通路,从而抑制肺纤维化及羟脯氨酸合成。上述研究都为探索新的miRNA治疗靶点提供了理论参考,同时也暗示着一种可能性,在综合多种有效的抗纤维化的靶标后,其共同的miRNA可能是最为有效的miRNA靶标。 2.3.2 lncRNA与特发性肺纤维化治疗 由于作用机制的相似性,以lncRNA与circRNA为靶点的基因治疗可能有着共同的优劣势。相较于miRNA疗法,lncRNA与circRNA基因治疗影响的基因更广,因为lncRNA和circRNA表达变化的同时,其靶向的miRNA及miRNA靶基因都会改变。此外,两者还能直接影响靶蛋白的表达。而劣势在于2种疗法对靶miRNA属于间接调控,可能不如直接靶向miRNA的作用强烈。就lncRNA而言,lncRNA过长也影响着其稳定性,且lncRNA是由不够保守的内含子或基因间区域表达的,药物在啮齿类等动物模型的体内评估结果可能与人类细胞相差较大[77]。 lncRNA对应的转录本较多,有些转录本身并不存在失调,靶向疗法可能改变这些转录本水平增加副反应。目前针对特发性肺纤维化中lncRNA基因疗法的研究主要依靠siRNA等常用方法,例如通过腺相关病毒在体内外实现对lncRNA ZFAS1的敲减,通过海绵miR-150-5p下调氨基酸转运载体A系统成员1(solute carrier family 38 member 1,SLC38A1)的表达来减弱铁死亡及脂质过氧化抑制FMT过程,达到肺纤维化的治疗效果[78]。与miRNA类似,lncRNA也活跃于特发性肺纤维化相关药物的研究中。体内外实验表明当归多糖通过下调lncRNA DANCR发挥治疗作用,在机制上,DANCR与RNA结合因子(AU-binding factor,AUF1)结合上调forkhead转录因子O3(forkhead box O3,FOXO3)表达,改善了EMT-like进程,且DANCR、AUF1及FOXO3的过表达逆转了当归多糖对特发性肺纤维化的治疗作用[79]。与此同时,虾青素通过抑制lncRNA ITPF和线粒体相关信号通路阻断活化的成纤维细胞增殖及迁移,从而减轻肺纤维化[80]。这些药物研究不仅肯定了lncRNA在特发性肺纤维化中的作用,也为lncRNA作为药物作用靶点提供了事实依据,通过对已知药物的作用机制挖掘对未来基因靶向药物的研制有一定的借鉴意义。 2.3.3 circRNA与特发性肺纤维化治疗 circRNA由于特殊的闭合环状结构,有着较强的外切酶抗性,而且circRNA大多由外显子组成[81]。这使得circRNA较lncRNA具备更高的稳定性和保守性,其基因治疗特异性可能更高。目前依靠常用的敲减或过表达等方法对特发性肺纤维化中circRNA靶向治疗展开研究,ZHANG等[48]采用腺病毒在体内外沉默circHIPK3,可通过miR-338-3p海绵作用,下调SRY盒转录因子4(SRY-box transcription factor 4,SOX4)及Ⅰ型胶原表达,负调控FMT进而抑制肺纤维化。与此同时, 特发性肺纤维化相关药物的作用机制被发现与circRNA存在联系。ZENG等[82]发现苍术酮治疗可抑制卵清蛋白诱导的肺纤维化,体内实验证实苍术酮可抑制充当miR-211-5p海绵的mmu_circ_0000981的表达,通过调节mmu_circ_0000981/miR-211-5p/TGFBR2轴抑制EMT-like过程,减弱肺纤维化。 总之,这些研究证实circRNA可作为特发性肺纤维化的分子治疗靶点,但目前对circRNA的研究方法还不成熟,已发现的特发性肺纤维化中失调的circRNA数量及功能只是冰山一角,对于circRNA靶向调节剂研制及应用仍面临着众多挑战。同时,特发性肺纤维化中存在circRNA,lncRNA,miRNA为主体的竞争性内源RNA网络被进一步揭露,这也解释了特发性肺纤维化单一疗法疗效欠佳的问题,表明多基因的联合靶向可能有更高的价值。此外,现阶段竞争性内源RNA研究忽略了亚细胞定位这一关键因素,多数实验未明确胞质或胞核定位问题,这一方面关系到作用机制的可能性,一方面也影响着ncRNA干预方式的选择。 "

| [1] RAGHU G, COLLARD HR, EGAN JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. [2] MARTINEZ FJ, COLLARD HR, PARDO A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. [3] DINH PC, PAUDEL D, BROCHU H, et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat Commun. 2020;11(1):1064. [4] LI C, WANG Z, ZHANG J, et al. Crosstalk of mRNA, miRNA, lncRNA, and circRNA and their regulatory pattern in pulmonary fibrosis. Mol Ther Nucleic Acids. 2019;18:204-218. [5] MEMCZAK S, JENS M, ELEFSINIOTI A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441): 333-338. [6] LU TX, ROTHENBERG ME. MicroRNA. J Allergy Clin Immunol. 2018; 141(4):1202-1207. [7] NAGOSA S, LEESCH F, PUTIN D, et al. microRNA-184 induces a commitment switch to epidermal differentiation. Stem Cell Reports. 2017;9(6):1991-2004. [8] XU J, BAI J, ZHANG X, et al. A comprehensive overview of lncRNA annotation resources. Brief Bioinform. 2017;18(2):236-249. [9] KOPP F, MENDELL JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393-407. [10] ZHANG W, TANG G, ZHOU S, et al. LncRNA-miRNA interaction prediction through sequence-derived linear neighborhood propagation method with information combination. BMC Genomics. 2019;20(Suppl 11):946. [11] FU Z, CHEN C, ZHOU Q, et al. LncRNA HOTTIP modulates cancer stem cell properties in human pancreatic cancer by regulating HOXA9. Cancer Lett. 2017;410:68-81. [12] LIU JQ, DENG M, XUE NN, et al. lncRNA KLF3-AS1 Suppresses Cell Migration and Invasion in ESCC by Impairing miR-185-5p-Targeted KLF3 Inhibition. Mol Ther Nucleic Acids. 2020;20:231-241. [13] WANG Y, ZHU P, LUO J, et al. LncRNA HAND2-AS1 promotes liver cancer stem cell self-renewal via BMP signaling. EMBO J. 2019;38(17):e101110. [14] CHEN LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21(8):475-490. [15] YANG Y, FAN X, MAO M, et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27(5):626-641. [16] CHEN J, CHEN T, ZHU Y, et al. circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stemness in glioma. J Exp Clin Cancer Res. 2019;38(1):398. [17] JIANG X, XING L, CHEN Y, et al. CircMEG3 inhibits telomerase activity by reducing Cbf5 in human liver cancer stem cells. Mol Ther Nucleic Acids. 2021;23:310-323. [18] EPSTEIN SHOCHET G, BROOK E, BARDENSTEIN-WALD B, et al. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res. 2020;21(1):56. [19] WANG G, JIAO H, ZHENG JN, et al. HSP27 regulates TGF-β mediated lung fibroblast differentiation through the Smad3 and ERK pathways. Int J Mol Med. 2017;39(1):183-190. [20] SUN T, HUANG Z, ZHANG H, et al. TAZ is required for lung alveolar epithelial cell differentiation after injury. JCI Insight. 2019;5(14): e128674. [21] CHENG F, SHEN Y, MOHANASUNDARAM P, et al. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc Natl Acad Sci U S A. 2016;113(30): E4320-E4327. [22] BONIFAZI M, DI VINCENZO M, CAFFARINI M, et al. How the pathological microenvironment affects the behavior of mesenchymal stem cells in the idiopathic pulmonary fibrosis. Int J Mol Sci. 2020;21(21):8140. [23] SCHAFER MJ, WHITE TA, IIJIMA K, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. [24] NEWTON DA, LOTTES RG, RYAN RM, et al. Dysfunctional lactate metabolism in human alveolar type II cells from idiopathic pulmonary fibrosis lung explant tissue. Respir Res. 2021;22(1):278. [25] XIAO X, HUANG C, ZHAO C, et al. Regulation of myofibroblast differentiation by miR-424 during epithelial-to-mesenchymal transition. Arch Biochem Biophys. 2015;566:49-57. [26] STOLZENBURG LR, WACHTEL S, DANG H, HARRIS A. miR-1343 attenuates pathways of fibrosis by targeting the TGF-β receptors. Biochem J. 2016;473(3):245-256. [27] ZHUANG Y, DAI J, WANG Y, et al. MiR-338* targeting smoothened to inhibit pulmonary fibrosis by epithelial-mesenchymal transition. Am J Transl Res. 2016;8(7):3206-3213. [28] CHEN X, SHI C, WANG C, et al. The role of miR-497-5p in myofibroblast differentiation of LR-MSCs and pulmonary fibrogenesis. Sci Rep. 2017;7: 40958. [29] ZHANG Q, YE H, XIANG F, et al. miR-18a-5p Inhibits Sub-pleural Pulmonary Fibrosis by Targeting TGF-β Receptor II. Mol Ther. 2017; 25(3):728-738. [30] CUI H, GE J, XIE N, et al. miR-34a promotes fibrosis in aged lungs by inducing alveolarepithelial dysfunctions. Am J Physiol Lung Cell Mol Physiol. 2017;312(3):L415-L424. [31] HUANG C, XIAO X, YANG Y, et al. MicroRNA-101 attenuates pulmonary fibrosis by inhibiting fibroblast proliferation and activation. J Biol Chem, 2017;292(40):16420-16439. [32] BAHUDHANAPATI H, TAN J, DUTTA JA, et al. MicroRNA-144-3p targets relaxin/insulin-like family peptide receptor 1 (RXFP1) expression in lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Biol Chem. 2019;294(13):5008-5022. [33] CHEN Y, ZHAO X, SUN J, et al. YAP1/Twist promotes fibroblast activation and lung fibrosis that conferred by miR-15a loss in IPF. Cell Death Differ. 2019;26(9):1832-1844. [34] HUANG Y, XIE Y, ABEL PW, et al. TGF-β1-induced miR-424 promotes pulmonary myofibroblast differentiation by targeting Slit2 protein expression. Biochem Pharmacol. 2020;180:114172. [35] KADOTA T, YOSHIOKA Y, FUJITA Y, et al. Extracellular vesicles from fibroblasts induce epithelial-cell senescence in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2020;63(5):623-636. [36] LI J, PAN C, TANG C, et al. miR-184 targets TP63 to block idiopathic pulmonary fibrosis by inhibiting proliferation and epithelial-mesenchymal transition of airway epithelial cells. Lab Invest. 2021; 101(2):142-154. [37] SHI L, HAN Q, HONG Y, et al. Inhibition of miR-199a-5p rejuvenates aged mesenchymal stem cells derived from patients with idiopathic pulmonary fibrosis and improves their therapeutic efficacy in experimental pulmonary fibrosis. Stem Cell Res Ther. 2021;12(1):147. [38] DU Y, HAO X, LIU X. Low expression of long noncoding RNA CDKN2B-AS1 in patients with idiopathic pulmonary fibrosis predicts lung cancer by regulating the p53-signaling pathway. Oncol Lett. 2018;15(4):4912-4918. [39] GOKEY JJ, SNOWBALL J, SRIDHARAN A, et al. MEG3 is increased in idiopathic pulmonary fibrosis and regulates epithelial cell differentiation. JCI Insight. 2018;3(17):e122490. [40] LI X, YU T, SHAN H, et al. lncRNA PFAL promotes lung fibrosis through CTGF by competitively binding miR-18a. FASEB J. 2018;32(10):5285-5297. [41] JIANG H, CHEN Y, YU T, et al. Inhibition of lncRNA PFRL prevents pulmonary fibrosis by disrupting the miR-26a/smad2 loop. Am J Physiol Lung Cell Mol Physiol. 2018;315(4):L563-L575. [42] QIAN W, CAI X, QIAN Q, et al. lncRNA ZEB1-AS1 promotes pulmonary fibrosis through ZEB1-mediated epithelial-mesenchymal transition by competitively binding miR-141-3p. Cell Death Dis. 2019;10(2):129. [43] QIAN W, CAI X, QIAN Q. Sirt1 antisense long non-coding RNA attenuates pulmonary fibrosis through sirt1-mediated epithelial-mesenchymal transition. Aging (Albany NY). 2020;12(5):4322-4336. [44] HUANG C, LIANG Y, ZENG X, et al. Long noncoding RNA FENDRR exhibits antifibrotic activity in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2020;62(4):440-453. [45] ZHANG Y, YAO XH, WU Y, et al. LncRNA NEAT1 regulates pulmonary fibrosis through miR-9-5p and TGF-β signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(16):8483-8492. [46] LIU P, ZHAO L, GU Y, et al. LncRNA SNHG16 promotes pulmonary fibrosis by targeting miR-455-3p to regulate the Notch2 pathway. Respir Res. 2021;22(1):44. [47] YI H, LUO D, XIAO Y, et al. Knockdown of long non‑coding RNA DLEU2 suppresses idiopathic pulmonary fibrosis by regulating the microRNA‑369‑3p/TRIM2 axis. Int J Mol Med. 2021;47(5):80. [48] ZHANG JX, LU J, XIE H, et al. circHIPK3 regulates lung fibroblast-to-myofibroblast transition by functioning as a competing endogenous RNA. Cell Death Dis. 2019;10(3):182. [49] ZHANG L, CHI X, LUO W, et al. Lung myofibroblast transition and fibrosis is regulated by circ0044226. Int J Biochem Cell Biol. 2020;118:105660. [50] 罗潇.环状RNA的差异性表达及hsa_circ_0006508在特发性肺纤维化中的作用机制研究[D].太原:山西医科大学,2020. [51] QI F, LI Y, YANG X, et al. Hsa_circ_0044226 knockdown attenuates progression of pulmonary fibrosis by inhibiting CDC27. Aging (Albany NY). 2020;12(14):14808-14818. [52] LI J, LI P, ZHANG G, et al. CircRNA TADA2A relieves idiopathic pulmonary fibrosis by inhibiting proliferation and activation of fibroblasts. Cell Death Dis. 2020;11(7):553. [53] BAI J, DENG J, HAN Z, et al. CircRNA_0026344 via exosomal miR-21 regulation of Smad7 is involved in aberrant cross-talk of epithelium-fibroblasts during cigarette smoke-induced pulmonary fibrosis. Toxicol Lett. 2021;347:58-66. [54] TZOUVELEKIS A, PASPALIARIS V, KOLIAKOS G, et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med. 2013;11:171. [55] CHAMBERS DC, ENEVER D, ILIC N, et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19(7):1013-1018. [56] GLASSBERG MK, MINKIEWICZ J, TOONKEL RL, et al. Allogeneic human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis via intravenous delivery (AETHER): a phase i safety clinical trial. Chest. 2017;151(5):971-981. [57] NTOLIOS P, MANOLOUDI E, TZOUVELEKIS A, et al. Longitudinal outcomes of patients enrolled in a phase Ib clinical trial of the adipose-derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Clin Respir J. 2018;12(6):2084-2089. [58] FISHMAN JE, KIM GJ, KYEONG NY, et al. Intravenous stem cell dose and changes in quantitative lung fibrosis and DLCO in the AETHER trial: a pilot study. Eur Rev Med Pharmacol Sci. 2019;23(17):7568-7572. [59] AVERYANOV A, KOROLEVA I, KONOPLYANNIKOV M, et al. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl Med. 2020;9(1):6-16. [60] SERRANO-MOLLAR A, GAY-JORDI G, GUILLAMAT-PRATS R, et al. Safety and tolerability of alveolar type II cell transplantation in idiopathic pulmonary fibrosis. Chest. 2016;150(3):533-543. [61] LOPEZ-RODRIGUEZ E, GAY-JORDI G, KNUDSEN L, et al. Improved alveolar dynamics and structure after alveolar epithelial type II cell transplantation in bleomycin induced lung fibrosis. Front Med (Lausanne). 2021;8:640020. [62] LENNOX KA, BEHLKE MA. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. 2016;44(2):863-877. [63] JANAS MM, SCHLEGEL MK, HARBISON CE, et al. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat Commun. 2018;9(1):723. [64] WAN C, ALLEN TM, CULLIS PR. Lipid nanoparticle delivery systems for siRNA-based therapeutics. Drug Deliv Transl Res. 2014;4(1):74-83. [65] CROOKE ST, VICKERS TA, LIANG XH. Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res. 2020;48(10): 5235-5253. [66] LIU YF, HUANG S, NG TK, et al. Longitudinal evaluation of immediate inflammatory responses after intravitreal AAV2 injection in rats by optical coherence tomography. Exp Eye Res. 2020;193:107955. [67] ROTH TL, PUIG-SAUS C, YU R, et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018; 559(7714):405-409. [68] RAN FA, HSU PD, WRIGHT J, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281-2308. [69] TRAN NT, SOMMERMANN T, GRAF R, et al. Efficient CRISPR/Cas9-Mediated Gene Knockin in Mouse Hematopoietic Stem and Progenitor Cells. Cell Rep. 2019;28(13):3510-3522.e5. [70] GUIOT J, CAMBIER M, BOECKX A, et al. Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p. Thorax. 2020;75(10):870-881. [71] SUN X, KANG Y, XUE S, et al. In vivo therapeutic success of MicroRNA-155 antagomir in a mouse model of pulmonary fibrosis induced by bleomycin. Korean J Intern Med. 2021;36(Suppl 1): S160-S169. [72] XU F, XU F, XIE S, et al. MicroRNA-448 overexpression inhibits fibroblast proliferation and collagen synthesis and promotes cell apoptosis via targeting ABCC3 through the JNK signaling pathway. J Cell Physiol. 2020;235(2):1374-1385. [73] BEG MS, BRENNER AJ, SACHDEV J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35(2):180-188. [74] VAN DER REE MH, DE VREE JM, STELMA F, et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet. 2017; 389(10070):709-717. [75] ZHOU Y, CHAI X. Protective effect of bicyclol against pulmonary fibrosis via regulation of microRNA-455-3p in rats. J Cell Biochem. 2020;121(1): 651-660. [76] TONG J, WU Z, WANG Y, et al. Astragaloside IV Synergizing with Ferulic Acid Ameliorates Pulmonary Fibrosis by TGF-β1/Smad3 Signaling. Evid Based Complement Alternat Med. 2021. doi: 10.1155/2021/8845798. [77] KARNER H, WEBB CH, CARMONA S, et al. Functional Conservation of LncRNA JPX Despite Sequence and Structural Divergence. J Mol Biol. 2020;432(2):283-300. [78] YANG Y, TAI W, LU N, et al. lncRNA ZFAS1 promotes lung fibroblast-to-myofibroblast transition and ferroptosis via functioning as a ceRNA through miR-150-5p/SLC38A1 axis. Aging (Albany NY). 2020;12(10): 9085-9102. [79] QIAN W, CAI X, QIAN Q, et al. Angelica sinensis polysaccharide suppresses epithelial-mesenchymal transition and pulmonary fibrosis via a DANCR/AUF-1/FOXO3 regulatory axis. Aging Dis. 2020,11(1):17-30. [80] CHEN H, WANG J, LI R, et al. Astaxanthin attenuates pulmonary fibrosis through lncITPF and mitochondria-mediated signal pathways. J Cell Mol Med. 2020;24(17):10245-10250. [81] SCHMIDT CA, GIUSTO JD, BAO A, et al. Molecular determinants of metazoan tricRNA biogenesis. Nucleic Acids Res. 2019;47(12):6452-6465. [82] ZENG H, GAO H, ZHANG M, et al. atractylon treatment attenuates pulmonary fibrosis via regulation of the mmu_circ_0000981/miR-211-5p/TGFBR2 axis in an ovalbumin-induced asthma mouse model. Inflammation. 2021. doi: 10.1007/s10753-021-01463-6. |
| [1] | Li Rui, Liu Zhen, Guo Zige, Lu Ruijie, Wang Chen. Aspirin-loaded chitosan nanoparticles and polydopamine modified titanium sheets improve osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 374-379. |
| [2] | Ning Ziwen, Wang Xu, Shi Zhengliang, Qin Yihua, Wang Guoliang, Jia Di, Wang Yang, Li Yanlin. Meniscal injury repair methods for non-blood supply area [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 420-426. |
| [3] | Ma Munan, Xie Jun, Sang Yuchao, Huang Lei, Zhang Guodong, Yang Xiaoli, Fu Songtao. Electroacupuncture combined with bone marrow mesenchymal stem cells in the treatment of chemotherapy-induced premature ovarian insufficiency in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 1-7. |
| [4] | Zhang Yujuan, Yuan Yitong, Du Ruochen, Tian Feng, Fu Yuan, Wang Chunfang. miR-31 promotes the proliferation and migration of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 66-71. |
| [5] | Gao Yixuan, Wang Lingfeng, Ba Te, Li Fang, Cao Shengjun, Li Junliang, Zhou Biao, Chen Qiang. Adipose-derived stem cells overexpressing growth hormone promote proliferation and migration of fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 8-14. |
| [6] | Liu Siqi, Wu Mingrui, Qiao Lingran, Xie Liying, Chen Siyu, Han Zhibo, Zuo Lin. Effects of hydrogel loaded with human umbilical cord mesenchymal stem cells on diabetic wound repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 21-27. |
| [7] | Fu Chunmei, Zhang Pu, Wang Yang, Li Xiaolin, Xue Yan, Fu Jie, Zhang Cixian, Yang Yujuan, Duan Yaya, Feng Kai. Allogeneic hematopoietic stem cell transplantation in the treatment of 24 patients with severe aplastic anemia [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 15-20. |
| [8] | Wei Yanzhao, Zheng Xiaohan, Gao Shijun, Huang Ting, Wei Xufang, Chen Xinxu, Zhao Zhenqiang. Expression of autocrine macrophage migration inhibitory factor and its receptors of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 34-41. |
| [9] | Yang Yan, Wang Jingxian, Zhang Ronghong, Li Chen, Fan Anran, Cui Dongbing, Wu Shumei. Effects of conditioned media of different sources on the proliferation of human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 49-53. |
| [10] | Lan Qian, Gu Yangcong, Xiao Xin, Bi Xueting, Li Na. Human periodontal ligament stem cells-derived exosomes interfere with the proliferation and differentiation of MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 54-58. |
| [11] | Huang Wenjun, Zhou Yafei, Wang Jie, Li Huan, Zhang Yanmin, Zhou Rui. Establishment and identification of a protocol for highly efficient differentiation of hepatocytes from human pluripotent stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 28-33. |
| [12] | Zhao Jufen, Ma Rong, Cao Jia, Yu Chuanyang, Tao Xiang, Wang Jia, Wang Libin. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 beta on stemness expression in breast cancer stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 59-65. |
| [13] | Feng Hao, Zhang Bin, Wang Jianping. Bone marrow mesenchymal stem cell transplantation can improve bone metabolism in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 72-75. |
| [14] | Huang Chuwen, Jiang Hua, Li Minqing. Complications and death causes of peripheral blood stem cell transplantation in the treatment of thalassemia major [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 42-48. |
| [15] | Liu Runyuan, Dong Ming, Han Wenqing, Dong Juhong, Niu Weidong. Application and progress of small extracellular vesicles in periodontal and pulp regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 83-90. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||