Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (32): 5155-5161.doi: 10.12307/2022.879
Previous Articles Next Articles
Emodin promotes fracture healing in osteoporotic fracture rats by inhibiting miR-338-3p expression
Li Haoliang, Li Dongfang
- Department of Traumatology and Orthopaedics, Second Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2021-07-22Accepted:2021-11-29Online:2022-11-18Published:2022-05-12 -
Contact:Li Dongfang, Master, Attending physician, Department of Traumatology and Orthopaedics, Second Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Li Haoliang, Master, Attending physician, Department of Traumatology and Orthopaedics, Second Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
CLC Number:
Cite this article
Li Haoliang, Li Dongfang. Emodin promotes fracture healing in osteoporotic fracture rats by inhibiting miR-338-3p expression[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(32): 5155-5161.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
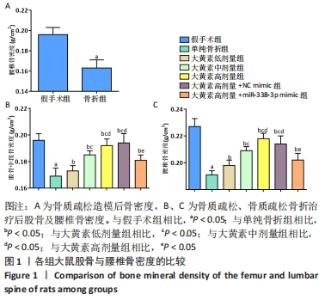
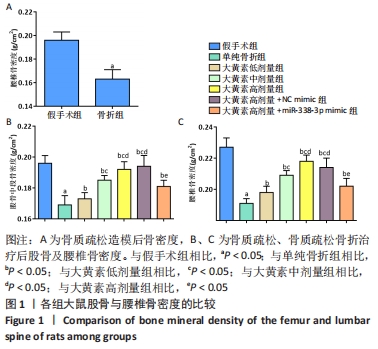
2.1 实验动物数量分析 53只大鼠全部进入结果分析。 2.2 各组大鼠骨密度比较 卵巢切除3个月后检测大鼠右侧股骨中段及L5骨密度,与假手术组大鼠相比,卵巢切除大鼠腰椎骨密度下降(P < 0.05),说明骨质疏松模型造模成功,见图1A。 大鼠经过不同浓度大黄素和miR-338-3p mimic处理,8周后检测大鼠股骨中段及L5骨密度,与单纯骨折组相比,大黄素低、中、高剂量组大鼠股骨中段及L5骨密度升高(P < 0.05),且呈剂量依赖性;大黄素高剂量+miR-338-3p mimic组股骨中段和腰椎骨密度高于骨折组(P < 0.05),低于大黄素高剂量组(P < 0.05),见图1B、C。 "
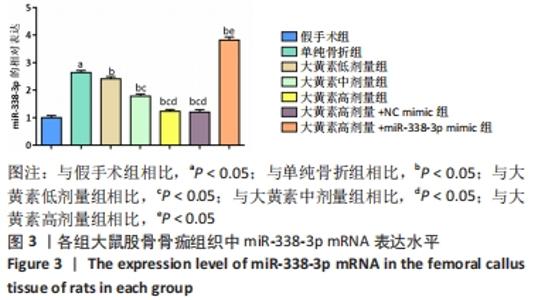
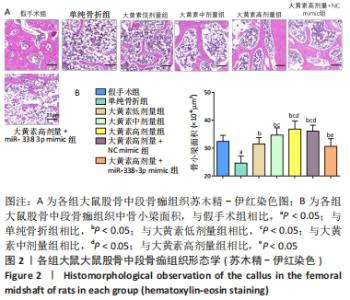
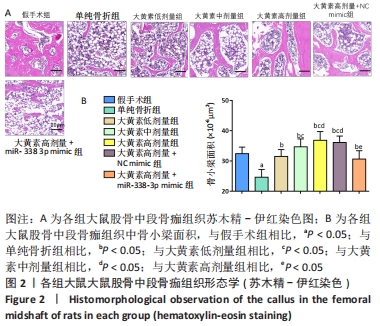
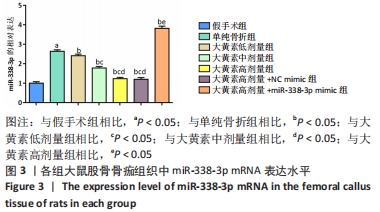
苏木精-伊红染色显示,大黄素高、中、低剂量组中软骨性骨痂组织少于骨性骨痂组织,且骨小梁较粗、致密、数量多,大黄素高剂量组骨小梁结构更密集、排列整齐,单纯骨折组骨小梁较细、间隙大、结构较乱和疏松;大黄素高剂量+miR-338-3p mimic组骨小梁也较细,但间隙较小,结构较为疏松。 定量分析结果显示,单纯骨折组骨小梁面积低于假手术组(P < 0.05),大黄素低、中、高剂量组骨小梁面积高于单纯骨折组(P < 0.05),且呈剂量依赖性;大黄素高剂量+miR-338-3p mimic组骨小梁面积低于大黄素高剂量组P < 0.05),高于单纯骨折组(P < 0.05)。 2.4 各组大鼠骨痂组织中miR-338-3p的表达 RT-qPCR检测结果显示,单纯骨折组miR-338-3p mRNA表达水平高于假手术组(P < 0.05);与单纯骨折组相比,大黄素低、中、高剂量组miR-338-3p mRNA表达水平降低,且呈剂量依赖性(P < 0.05);大黄素高剂量+miR-338-3p mimic组miR-338-3p mRNA表达水平高于大黄素高剂量组和单纯骨折组(P < 0.05),见图3。 "
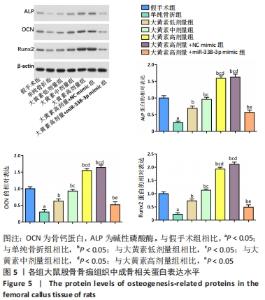
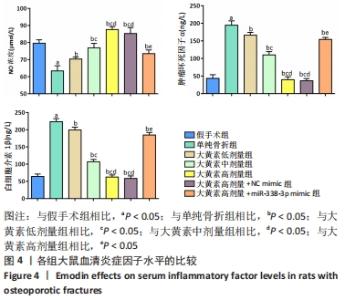
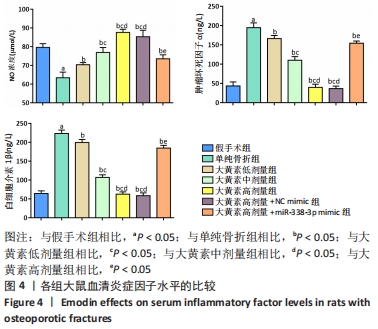
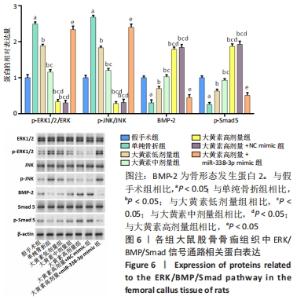
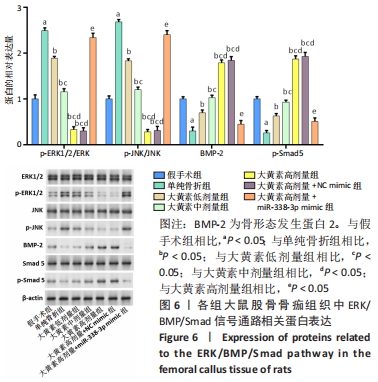
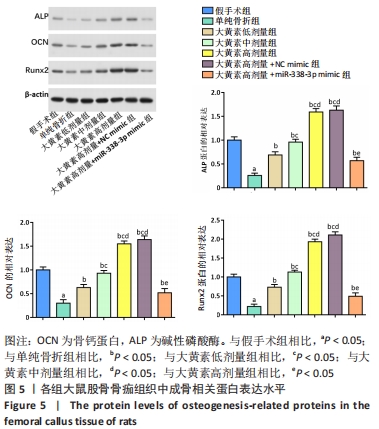
与假手术组相比,单纯骨折组血清NO水平降低(P < 0.05);与单纯骨折组相比,大黄素低、中、高剂量组血清NO水平升高(P < 0.05),且具有剂量依赖性。大黄素高剂量+miR-338-3p mimic组血清NO水平高于单纯骨折组(P < 0.05),低于大黄素高剂量组(P < 0.05)。与假手术组相比,单纯骨折组血清肿瘤坏死因子α和白细胞介素1β水平升高(P < 0.05);与单纯骨折组相比,大黄素低、中、高剂量组血清肿瘤坏死因子α和白细胞介素1β水平降低(P < 0.05),且呈剂量依赖性(P < 0.05)。大黄素高剂量+miR-338-3p mimic组血清肿瘤坏死因子α和白细胞介素1β水平高于大黄素高剂量组(P < 0.05),低于单纯骨折组(P < 0.05)。 2.6 各组大鼠骨痂组织中成骨相关蛋白表达 见图5。 "
| [1] QU XL, ZHENG B, CHEN TY, et al. Bone Turnover Markers and Bone Mineral Density to Predict Osteoporotic Fractures in Older Women: A Retrospective Comparative Study. Orthop Surg. 2020;12(1):116-123. [2] PAIK J, SCOTT LJ. Romosozumab: A Review in Postmenopausal Osteoporosis. Drugs Aging. 2020;37(11):845-855. [3] JOHNSTON CB, DAGAR M. Osteoporosis in Older Adults. Med Clin North Am. 2020;104(5):873-884. [4] KAUSCHKE V, SCHNEIDER M, JAUCH A, et al. Effects of a Pasty Bone Cement Containing Brain-Derived Neurotrophic Factor-Functionalized Mesoporous Bioactive Glass Particles on Metaphyseal Healing in a New Murine Osteoporotic Fracture Model. Int J Mol Sci. 2018;19(11):3531. [5] LIN X, XIONG D, PENG YQ, et al. Epidemiology and management of osteoporosis in the People’s Republic of China: current perspectives. Clin Interv Aging. 2015;25(10):1017-1033. [6] MARSELL R, EINHORN TA. The biology of fracture healing. Injury. 2011; 42(6):551-555. [7] NOH JY, YANG Y, JUNG H. Molecular Mechanisms and Emerging Therapeutics for Osteoporosis. Int J Mol Sci. 2020;21(20):76323. [8] CHENG C, SHOBACK D. Mechanisms Underlying Normal Fracture Healing and Risk Factors for Delayed Healing. Curr Osteoporos Rep. 2019;17(1): 36-47. [9] WANG T, ZHANG X, BIKLE DD. Osteogenic Differentiation of Periosteal Cells During Fracture Healing. J Cell Physiol. 2017;232(5):913-921. [10] BAHNEY CS, ZONDERVAN RL, ALLISON P, et al. Cellular biology of fracture healing. J Orthop Res. 2019;37(1):35-50. [11] WON DY, BYUN SJ, JEONG JS, et al. Association Between Acetylcholinesterase Inhibitors and Osteoporotic Fractures in Older Persons With Alzheimer’s Disease. J Am Med Dir Assoc. 2020;21(8): 1128-1133.e1121. [12] KIM YG, PARK YH, YANG EY, et al. Inhibition of tamoxifen’s therapeutic effects by emodin in estrogen receptor-positive breast cancer cell lines. Ann Surg Treat Res. 2019;97(5):230-238. [13] DONG X, FU J, YIN X, et al. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother Res. 2016;30(8):1207-1218. [14] LEE EH, BAEK SY, PARK JY, et al. Emodin in Rheum undulatum inhibits oxidative stress in the liver via AMPK with Hippo/Yap signalling pathway. Pharm Biol. 2020;58(1):333-341. [15] YANG Y, JIANG Z, ZHUGE D. Emodin Attenuates Lipopolysaccharide-Induced Injury via Down-Regulation of miR-223 in H9c2 Cells. Int Heart J. 2019;60(2):436-443. [16] LI N, WANG C, ZHANG P, et al. Emodin inhibits pancreatic cancer EMT and invasion by up‑regulating microRNA‑1271. Mol Med Rep. 2018; 18(3):3366-3374. [17] MADHYASTHA R, MADHYASTHA H, PENGJAM Y, et al. The pivotal role of microRNA-21 in osteoclastogenesis inhibition by anthracycline glycoside aloin. J Nat Med. 2019;73(1):59-66. [18] LI Z, ZHANG W, HUANG Y. MiRNA-133a is involved in the regulation of postmenopausal osteoporosis through promoting osteoclast differentiation. Acta Biochim Biophys Sin (Shanghai). 2018;50(3):273-280. [19] TONG J, ZHANG M, LI X, et al. MicroRNA-338-3p regulates age‑associated osteoporosis via targeting PCSK5. Mol Med Rep. 2021; 23(2):136. [20] ZHANG XH, GENG GL, SU B, et al. MicroRNA-338-3p inhibits glucocorticoid-induced osteoclast formation through RANKL targeting. Genet Mol Res. 2016;15(3). doi: 10.4238/gmr.15037674. [21] LIU H, SUN Q, WAN C, et al. MicroRNA-338-3p regulates osteogenic differentiation of mouse bone marrow stromal stem cells by targeting Runx2 and Fgfr2. J Cell Physiol. 2014;229(10):1494-1502. [22] SUN Q, ZHANG B, ZHU W, et al. A potential therapeutic target for regulating osteoporosis via suppression of osteoclast differentiation. J Dent. 2019;82:91-97. [23] XIAO L, ZHONG M, HUANG Y, et al. Puerarin alleviates osteoporosis in the ovariectomy-induced mice by suppressing osteoclastogenesis via inhibition of TRAF6/ROS-dependent MAPK/NF-κB signaling pathways. Aging (Albany NY). 2020;12(21):21706-21729. [24] HUANG K, WU G, ZOU J, et al. Combination therapy with BMP-2 and psoralen enhances fracture healing in ovariectomized mice. Exp Ther Med. 2018;16(3):1655-1662. [25] LU X, ZHANG Y, ZHENG Y, et al. The miRNA-15b/USP7/KDM6B axis engages in the initiation of osteoporosis by modulating osteoblast differentiation and autophagy. J Cell Mol Med. 2021;25(4):2069-2081. [26] RUTTEN S, NOLTE PA, KORSTJENS CM, et al. Low- intensitypulsed ultrasound affects RUNX2 immunopositive osteogenic cells in delayed clinical fracture healing. Bone. 2009;(1):862-869. [27] 陈玲,杨云洲,陈继革.接骨七厘片促进大鼠骨折愈合的实验研究[J].湖北中医杂志,2005,27(1):50-52. [28] 张杰,刘广宇,张晓峰,等.香丹注射液促进骨痂生长的生物力学研究[J].中医药信息,2006,23(6):50-51. [29] KLOEN P, DOTY SB, GORDON E, et al. Expression and activation of the BMP-signaling components in human fracture nonunions. J Bone Joint Surg Am. 2002;84 (11):1909-1918. [30] 潘月兴.壮筋续骨汤对骨折大鼠血清 bFGF和IGF-1含量的影响和意义[D].青岛:青岛大学,2013. [31] MARITATO KC, BARNHART MD. Minimally Invasive Fracture Repair. Vet Clin North Am Small Anim Pract. 2020;50(1): xiii-xiv.doi: 10.1016/j.cvsm.2019.10.001. [32] BAECKER H, FRIELER S, SCHILDHAUER TA, et al. Fracture-related infections in traumatology : Current standards and new developments in diagnostics and treatment. Orthopade. 2020;49(8):702-709. [33] VAN DER KLIFT M, DE LAET CD, POLS HA. Assessment of fracture risk: who should be treated for osteoporosis? Best Pract Res Clin Rheumatol. 2005;19(6):937-950. [34] KAVEH S, HOSSEINIFARD H, GHADIMI N, et al. Efficacy and safety of Romosozumab in treatment for low bone mineral density: a systematic review and meta-analysis. Clin Rheumatol. 2020;39(11):3261-3276. [35] CHEN X, REN S, ZHU G, et al. Emodin suppresses cadmium-induced osteoporosis by inhibiting osteoclast formation. Environ Toxicol Pharmacol. 2017;54:162-168. [36] YANG F, YUAN PW, HAO YQ, et al. Emodin enhances osteogenesis and inhibits adipogenesis. BMC Complement Altern Med. 2014;14:74. [37] LEE SU, SHIN HK, MIN YK, et al. Emodin accelerates osteoblast differentiation through phosphatidylinositol 3-kinase activation and bone morphogenetic protein-2 gene expression. Int Immunopharmacol. 2008;8(5):741-747. [38] JIA R, YI Y, LIU J, et al. Cyclic compression emerged dual effects on the osteogenic and osteoclastic status of LPS-induced inflammatory human periodontal ligament cells according to loading force. BMC Oral Health. 2020;20(1):7. [39] ZHENG J, ZHU X, HE Y, et al. CircCDK8 regulates osteogenic differentiation and apoptosis of PDLSCs by inducing ER stress/autophagy during hypoxia. Ann N Y Acad Sci. 2021;1485(1):56-70. [40] KOMORI T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol. 2018;149(4):313-323. [41] 曾宪良,黄军铭.股骨重建髓内钉结合有限切开重建股骨外侧壁治疗不稳定型粗隆下骨折的疗效分析[J].当代医学,2021,27(9):158-160. [42] DONG Y, ZHANG L, JIANG Y, et al. Emodin reactivated autophagy and alleviated inflammatory lung injury in mice with lethal endotoxemia. Exp Anim. 2019;68(4):559-568. [43] REDLICH K, SMOLEN JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11(3):234-250. [44] YANG Y, YUJIAO W, FANG W, et al. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res. 2020;53(1):40. [45] LV PY, GAO PF, TIAN GJ, et al. Osteocyte-derived exosomes induced by mechanical strain promote human periodontal ligament stem cell proliferation and osteogenic differentiation via the miR-181b-5p/PTEN/AKT signaling pathway. Stem Cell Res Ther. 2020;11(1):295. [46] SUN Z, WANG H, WANG Y, et al. MiR-103-3p targets the m(6) A methyltransferase METTL14 to inhibit osteoblastic bone formation. Aging Cell. 2021;20(2):e13298. [47] LI Z, OH H, CUNG M, et al. TAOK3 is a MAP3K contributing to osteoblast differentiation and skeletal mineralization. Biochem Biophys Res Commun. 2020;531(4):497-502. [48] CHEON YH, LEE CH, KIM S, et al. Pitavastatin prevents ovariectomy-induced osteoporosis by regulating osteoclastic resorption and osteoblastic formation. Biomed Pharmacother. 2021;139:111697. [49] XU Y, GU Y, JI W, et al. Activation of the extracellular-signal-regulated kinase (ERK)/c-Jun N-terminal kinase (JNK) signal pathway and osteogenic factors in subchondral bone of patients with knee osteoarthritis. Ann Transl Med. 2021;9(8):663. [50] HUANG D, HOU X, ZHANG D, et al. Two novel polysaccharides from rhizomes of Cibotium barometz promote bone formation via activating the BMP2/SMAD1 signaling pathway in MC3T3-E1 cells. Carbohydr Polym. 2020;231:115732. |
| [1] | Li Xiaomin, Tian Xiangdong, Tan Yetong, Zhu Guangyu, Wang Rongtian, Wang Jian, Xue Zhipeng, Ma Sheng, Hu Yuanyi, Huang Ye, Ding Tiansong. Changes of lower limb force line and knee function after high tibial osteotomy in osteoporotic medial ventricular knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1325-1329. |
| [2] | Wu Taoguang, Nie Shaobo, Chen Hua, Zhu Zhengguo, Qi Lin, Tang Peifu. Biomechanical characteristics of a new multi-dimensional cross locking plate in the treatment of subtrochanteric nonunion [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1330-1334. |
| [3] | Peng Zhixin, Yan Wengang, Wang Kun, Zhang Zhenjiang. Finite element analysis and structural optimization design of 3D printed forearm braces [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1340-1345. |
| [4] | Zheng Hongrui, Zhang Wenjie, Wang Yunhua, He Bin, Shen Yajun, Fan Lei. Femoral neck system combined with platelet-rich plasma in the treatment of femoral neck fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1390-1395. |
| [5] | Zheng Bo, Zhang Xiuli, Zhou Hao, He Zebi, Zhou Jin, Zhou Weiyun, Li Peng. Arthroscopy-assisted locking hollow screw fixation and open reduction plate internal fixation in the treatment of Schatzker II-III tibial plateau fractures: early CT evaluation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1410-1416. |
| [6] | Jiang Xiaocheng, Shi Lu, Wang Yinbin, Li Qiujiang, Xi Chuangzhen, Ma Zefeng, Cai Lijun. Systematical evaluation of bone fusion rate after interbody fusion in patients with osteoporosis and lumbar degenerative disease treated with teriparatide [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1427-1433. |
| [7] | Sun Jiajia, Zhu Haidi, Lu Yun, Zhang Kai. Comparison of bone metabolism markers between type 2 diabetes mellitus and non-type 2 diabetes mellitus patients with hip fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1156-1160. |
| [8] | Zhao Wei, Feng Wei, Yang Tieyi, Ren Wei, Wang Yuxin, Lyu Huicheng, Chang Zhiqiang, Feng Xiaodong, Wang Ziheng, Guo Shibing. Antibiotic bone cement intramedullary nail prepared using 3D printed mold for the treatment of long bone infection in lower limbs [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1023-1030. |
| [9] | Zhang Wei, Huang Zhichao, Zhao Ruifeng, Liang Huan, Ma Yufeng, Shen Yanguang, Zhong Honggang, Chen Zhaojun, Zhang Jichuan, Chen Weiheng. Efficacy of gutta-percha splint on a rabbit fracture model [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1055-1061. |
| [10] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [11] | Huang Guijiang, Ji Yuwei, Zhao Xin, Yang Yi, Zhao Yulan, Wang Peijin, Tang Wei, Jiao Jianlin. Effect and mechanism of different administration routes of placenta-derived mesenchymal stem cells in the treatment of tree shrews with osteoporotic fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 909-914. |
| [12] | Zhang Min, Zhang Xiaoming, Liu Tongbin. Application potential of naringin in bone tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 787-792. |
| [13] | Wang Jinling, Huang Xiarong, Qu Mengjian, Huang Fujin, Yin Lingwei, Zhong Peirui, Liu Jin, Sun Guanghua, Liao Yang, Zhou Jun. Effects of exercise training on bone mass and bone microstructure in aged osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 676-682. |
| [14] | Zhou Changjun, Long Shengli, Zou Wei, Xiao Jie, Long Hao, Feng Mingxing, Zhang Yang, Liu Jie, Zeng Zhongwei. Design and clinical application of coplanar screw guide for percutaneous pedicle screw in the treatment of thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 534-538. |
| [15] | Chen Lei, Jia Yanfei, Lyu Huicheng, Zhang Lifeng. Application of T-shaped plate for fractures involving the quadrilateral region of acetabulum [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 578-582. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||