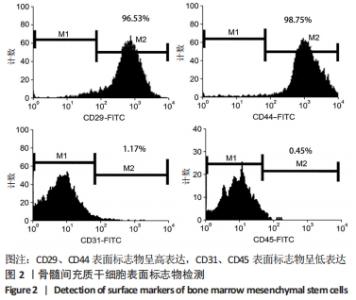
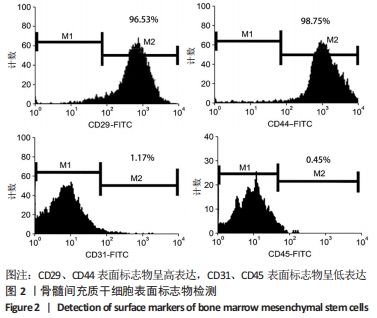
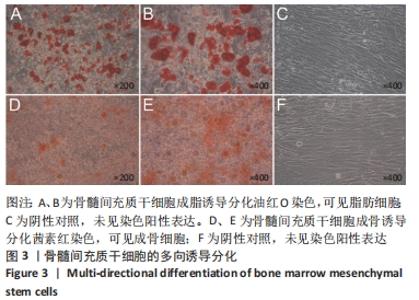
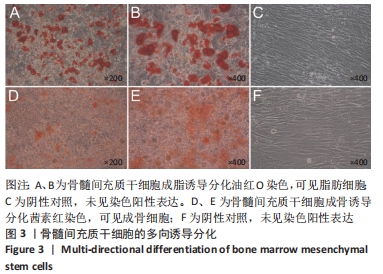
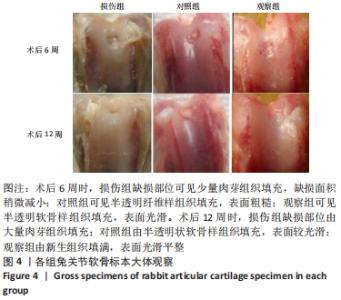
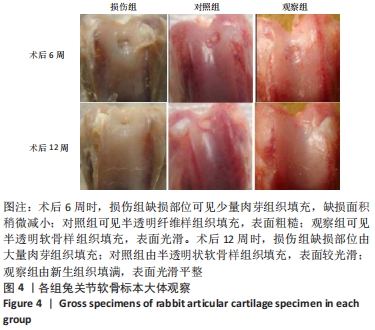
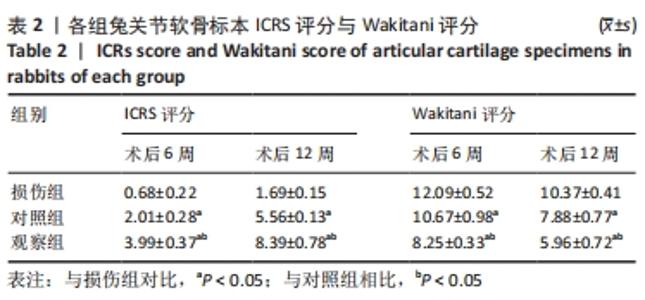
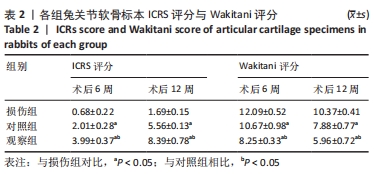
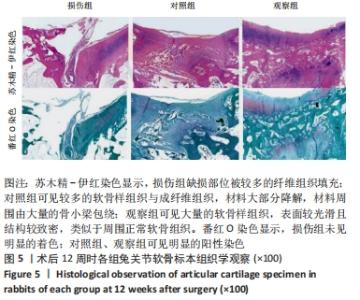
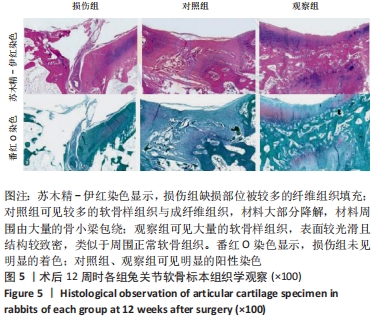
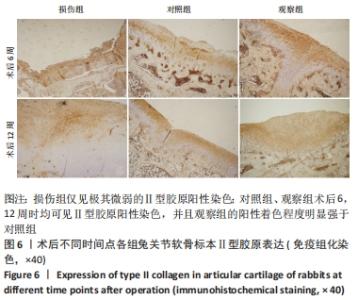
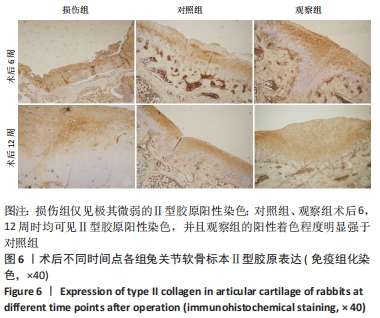
[1] ARMIENTO AR, STODDART MJ, ALINI M, et al.Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018; 65:1-20.
[2] PARK IS, JIN RL, OH HJ, et al. Sizable Scaffold-Free Tissue-Engineered Articular Cartilage Construct for Cartilage Defect Repair. Artif Organs. 2019; 43(3):278-287.
[3] GAO L, GOEBEL LKH, ORTH P, et al. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Dis Model Mech. 2018;11(6):dmm034280.
[4] HUANG Y, WANG XL, QIU H, et al. Subchondral drilling method combined with gum-bletilla complex to repair articular cartilage defects. Zhongguo Zhong Yao Za Zhi. 2018;43(4):813-819.
[5] BECHER C, MALAHIAS MA, ALI MM, et al. Arthroscopic microfracture vs. arthroscopic autologous matrix-induced chondrogenesis for the treatment of articular cartilage defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2731-2736.
[6] ZHANG Y, LIU S, GUO W, et al. Human umbilical cord Wharton’s jelly mesenchymal stem cells combined with an acellular cartilage extracellular matrix scaffold improve cartilage repair compared with microfracture in a caprine model. Osteoarthritis Cartilage. 2018;26(7):954-965.
[7] 董岩,崔鹏,周敬滨,等.关节镜测量尺指导下微骨折术治疗膝关节软骨损伤的临床近期疗效观察[J].中国运动医学杂志,2016,35(5):478-480.
[8] REDONDO ML, BEER AJ, YANKE AB. Cartilage Restoration: Microfracture and Osteochondral Autograft Transplantation. J Knee Surg. 2018;31(3):231-238.
[9] 张鹏,荆琳,邸冬雪,等.关节镜下自体骨软骨移植并服温阳益髓方修复膝骨关节炎的软骨缺损[J].中国组织工程研究,2020,24(26):4135-4140.
[10] 马巧稚,张仲文,刘腾腾,等.3D-T1-VIBE评价膝关节纤维蛋白凝胶型自体软骨细胞移植术后软骨修复[J].中国医学影像技术,2020,36(2): 271-275.
[11] 王晶,塔依尔·阿力甫,姚志涛,等.探究慢病毒介导BMP-2及VEGF-165转染山羊骨髓间充质干细胞向软骨方向分化的影响[J].口腔医学研究,2020, 36(6):528-533.
[12] 刘杨,张啸,罗春阳,等.应用3D生物打印的软骨支架修复关节软骨缺损[J].中华骨科杂志,2020,40(6):344-352.
[13] 肖红利,邓江,韩子冀,等.渐进性梯度孔径骨软骨支架的制备及细胞相容性研究[J].中华老年医学杂志,2020,39(4):456-461.
[14] HOLZAPFEL BM, RUDERT M, HUTMACHER DW. Scaffold-based Bone Tissue Engineering. Orthopade. 2017;46(8):701-710.
[15] SHAFIEE A, ATALA A. Tissue Engineering: Toward a New Era of Medicine. Annu Rev Med. 2017;68:29-40.
[16] 易鹏 .水凝胶在软骨组织工程中的设计与应用进展[J].疑难病杂志, 2020,19(2):202-206
[17] 李云洁,滕彬宏,赵艳红,等.软骨组织工程用羧甲基壳聚糖/氧化海藻酸钠复合水凝胶的制备及体外评估[J].华西口腔医学杂志,2019, 37(3):253-259
[18] 解光越,孙振,刘冠华,等.人脐带Wharton胶来源间充质干细胞复合藻酸钠水凝胶构建组织工程软骨修复兔膝关节软骨缺损[J].中国老年学杂志,2019,39(5):1170-1173
[19] THIELEN NGM, VAN DER KRAAN PM, VAN CAAM APM. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells. 2019;8(9):969.
[20] 黄冬娥,秦茵,林木南,等.不同波型电针治疗膝骨关节炎及对关节液转化生长因子-β1的影响[J].中国针灸,2020,40(4):370-374.
[21] 许颖捷,邵博,曾雪敏,等.慢病毒介导TGF-β3转染兔脂肪间充质干细胞对其向成软骨细胞分化的影响[J].新疆医科大学学报,2019,42(7): 842-847.
[22] 邓明,谢萍,吴飞,等.负载转化生长因子β3的藻酸钙凝胶复合脂肪间充质干细胞修复兔关节软骨缺损的实验研究[J].中国医师杂志, 2018,20(1):54-59.
[23] 刘洋,吕洋,王浩宇,等.1,25-维生素-D3体外诱导骨髓间充质干细胞向心肌样细胞分化的最适浓度[J].解剖学报,2019,50(5):580-588.
[24] 邢丹,刘伟,赵昱,等.3D干细胞微载体修复兔膝关节软骨缺损的实验研究[J].中华骨科杂志,2020,40(14):945-952.
[25] 马春涛,谭昱,肖育志,等.右归饮诱导的骨髓间充质干细胞/纤维蛋白胶复合物对膝关节软骨缺损兔软骨修复的影响[J].中医杂志,2019, 60(14):1225-1231.
[26] MA Q, LIAO J, CAI X. Different Sources of Stem Cells and their Application in Cartilage Tissue Engineering. Curr Stem Cell Res Ther. 2018;13(7):568-575.
[27] JING H, GAO B, GAO M, et al. Restoring tracheal defects in a rabbit model with tissue engineered patches based on TGF-beta3-encapsulating electrospun poly(l-lactic acid-co-epsilon-caprolactone)/collagen scaffolds. Artif Cells Nanomed Biotechnol. 2018;46(sup1):985-995.
[28] YANG Q, TENG BH, WANG LN, et al. Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of TGF-β3 for chondrogenic differentiation of adipose-derived stem cells. Int J Nanomedicine. 2017; 12:6721-6733.
[29] LEE C H, COOK J L, MENDELSON A, et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376(9739): 440-448.
[30] 刘登榜,韩小松,黄文良,等.TGF-β3诱导大鼠骨髓间充质干细胞成软骨细胞分化的实验观察[J].中华医学杂志,2017,97(36):2860-2865.
[31] BARRY F, BOYNTON RE, LIU B, et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268(2):189-200.
[32] 陈臻浩.Sox9对软骨细胞分化和基质产生的调控机制[J].复旦学报(医学版),2019,46(6):824-828.
|