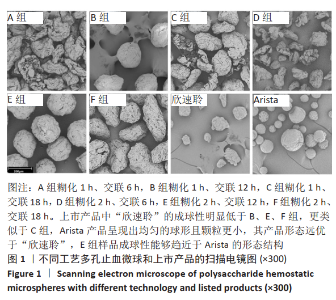
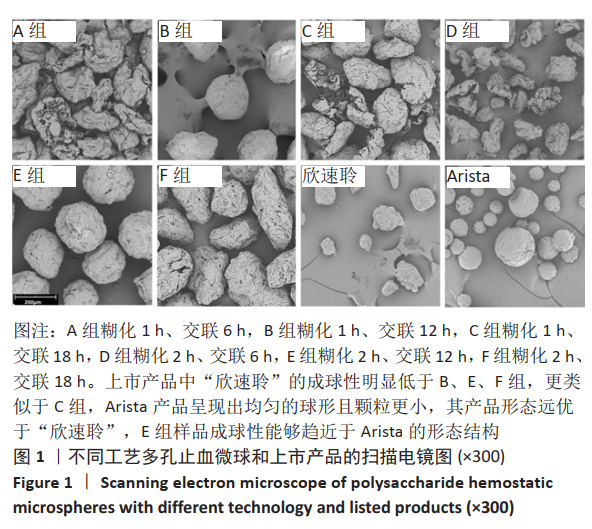
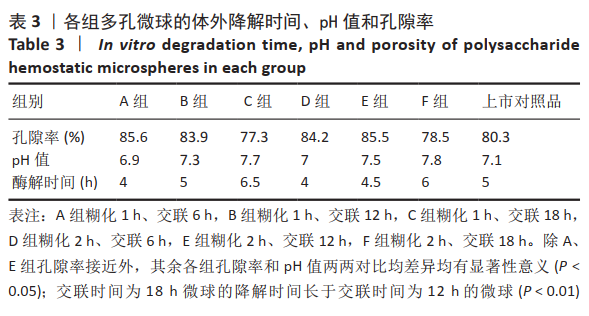
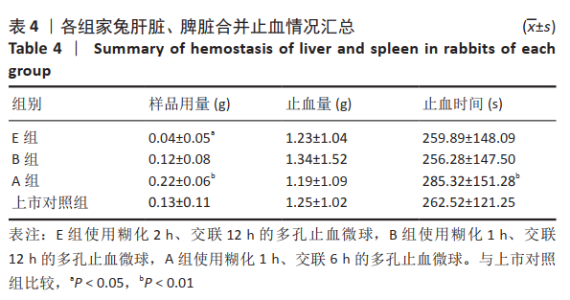
[1] KIM IY, EICHEL L, EDWARDS R, et al. Effects of Commonly Used Hemostatic Agents on the Porcine Collecting System. J Endourol. 2007; 21(6):652-654.
[2] 张少锋, 洪加源.医用生物可吸收止血材料的研究现状与临床应用[J].中国组织工程研究,2012,16(21):3941-3944.
[3] 陈帅,李方义,李剑峰,等.氧化淀粉基复合材料的制备及力学性能[J].高分子材料科学与工程,2017,33(9):177-183.
[4] MALAFAYA PB, ELVIRA C, GALLARDO A ,et al. Porous starch-based drug delivery systems processed by a microwave route. J Biomater Sci Polym Ed. 2001;12(11):1227-1241.
[5] GLENN GM, KLAMCZYNSKI AP, WOODS DF, et al. Encapsulation of Plant Oils in Porous Starch Microspheres. J Agric Food Chem. 2010;58(7): 4180-4184.
[6] KRAAK A. Industrial applications of potato starch products. Ind Crops Prod. 1992;1(2-4):107-112.
[7] 李军,陈存社.酶法制备微孔淀粉的工艺研究[J].北京工商大学学报自然科学版,2005,23(2):9-12.
[8] 刘亚平,侯春林,顾其胜,等.可生物降解止血粉的制备及其止血性能[J].中国修复重建外科杂志,2007,21(8):829-832.
[9] 张治国,索艳格,黄伟,等.一种交联多聚糖淀粉微球的制备方法: 中国,CN201710539216.9[P]. 2017-09-12.
[10] 詹国平,黄可龙,张法旺.阴离子型淀粉微球的制备研究[J].化工新型材料,2005,33(6):44-46.
[11] YANG YT, WEI XZ, SUN P, et al. Preparation, characterization and adsorption performance of a novel anionic starch microsphere. Molecules (Basel, Switzerland). 2010;4(15):2872-2885.
[12] 余雪松,黄赤兵,张银甫,等.负压吸附式止血敷贴对肾脏出血的止血效果观察[J].重庆医学,2008,37(15):1694-1694.
[13] 常贵娟,肖武,李祥村,等.乳液交联法制备多孔淀粉及其吸附性能[J].化工进展,2014(5):229-234.
[14] 马冰洁,唐洪波,马玲.马铃薯淀粉糊的黏度性质[J].东北林业大学学报,2006,34(4):73-75.
[15] 杨毅才.淀粉糊化的过程及影响因素[J].农产品加工,2009(2):18-19.
[16] 原波,陶金轩,刘宏生.显微观察结合目标识别技术观测马铃薯淀粉的糊化特性[J].现代食品科技,2018,34(4):167-171.
[17] Ai YF, Jane Ji. Gelatinization and rheological properties of starch. Starch Starke. 2014;67(3-4):213-224.
[18] 李艳杰.交联淀粉微球的制备及其载药性能的研究[D].济南:齐鲁工业大学,2013.
[19] 刘春秀,章悦庭.反相乳液聚合及其应用[J].金山油化纤,2003,22(2): 26-33.
[20] MORITA T, ITO Y, BROWN IL, et al. In Vitro and In Vivo Digestibility of Native Maize Starch Granules Varying in Amylose Contents.J AOAC Int. 2007;90(6):1628-1634.
[21] 牛雯,刘毅,刘曼玲,等.一种可吸收多聚糖止血材料的生物降解研究[J].中国新药杂志,2003,19(4):336-339.
[22] GURAYA HS, JAMES C, CHAMPAGNE ET. Effect of C ooling, and Freezing on the Digestibility of Debranched Rice Starch and Physical Properties of the Resulting Material. Starch. 2001;53(2):64-74.
[23] 杜宝堂,史跃,何远清,等.微孔真空多聚糖止血微球体内降解与生物安全性[J].中国组织工程研究,2015,19(52):8444-8449.
[24] 黄艳妮,何小华,刘纯,等.微孔多糖止血微球的止血作用及体内降解试验研究[J].医疗卫生装备,2020,41(1):21-24.
[25] 张新义,田俐.腹腔镜应用于胆囊切除术的临床研究[J].中外医疗, 2008,27(25):49.
[26] ERSOY G, KAYNAK MF, YILMAZ O, et al. Hemostatic effects of Microporous Polysaccharide Hemosphere® in a rat model with severe femoral artery bleeding. Adv Ther. 2007;24(3):485-492.
[27] NEUFFER MC, MCDIVITT J, ROSE D, et al. Hemostatic Dressings for the First Responder: A Review. Mil Med. 2004;169(9):716-720.
[28] ERETH MH, DONG Y, SCHRADER LM, et al. Microporous Polysaccharide Hemospheres Do Not Enhance Abdominal Infection in a Rat Model Compared with Gelatin Matrix. Surg Infect. 2009;10(3):273-276.
|