Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (1): 148-152.doi: 10.12307/2022.025
Previous Articles Next Articles
Effect and mechanism of mesenchymal stem cells in the treatment of diabetic nephropathy
Wang Shuyun, Xie Junhui, Yu Xuefeng
- Department of Endocrinology, Tongji Hospital, Tongji Medical College of Huazhong University of Science & Technology, Wuhan 430030, Hubei Province, China
-
Received:2020-07-26Revised:2020-07-28Accepted:2020-09-26Online:2022-01-08Published:2021-10-25 -
Contact:Yu Xuefeng, Professor, Doctoral supervisor, Master’s supervisor, Department of Endocrinology, Tongji Hospital, Tongji Medical College of Huazhong University of Science & Technology, Wuhan 430030, Hubei Province, China Xie Junhui, Associate professor, Associate chief physician, Department of Endocrinology, Tongji Hospital, Tongji Medical College of Huazhong University of Science & Technology, Wuhan 430030, Hubei Province, China -
About author:Wang Shuyun, MD, Department of Endocrinology, Tongji Hospital, Tongji Medical College of Huazhong University of Science & Technology, Wuhan 430030, Hubei Province, China -
Supported by:the National Natural Science Foundation of China for the Youth, No. 81600634 (to XJH)
CLC Number:
Cite this article
Wang Shuyun, Xie Junhui, Yu Xuefeng. Effect and mechanism of mesenchymal stem cells in the treatment of diabetic nephropathy[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 148-152.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
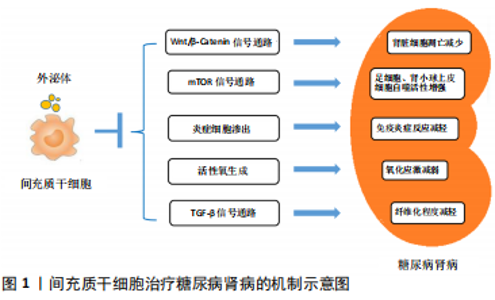
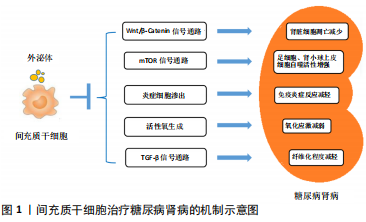
2.1 糖尿病肾病的发病机制与一般治疗策略 2.1.1 糖尿病肾病的发病机制 糖尿病肾病的发病过程与肾脏血流动力学异常、糖脂代谢紊乱、免疫炎症反应、氧化应激、肾脏细胞自噬异常、遗传和表观遗传因素等病理生理机制相关。糖尿病肾病早期即出现肾脏血流动力学异常,表现为肾小球高灌注和高滤过,肾血流量和肾小球滤过率升高。高血糖作为糖尿病肾病发生发展的重要因素,一方面能够加剧葡萄糖氧化,促使线粒体产生过多的活性氧和促进细胞凋亡[4];另一方面,过量的葡萄糖能够通过多元醇途径进一步加剧氧化应激,还能与游离氨基酸或组织蛋白结合并生成不可逆的晚期糖基化终末产物,对肾脏组织造成损伤。除糖代谢紊乱外,在2型糖尿病研究中还发现血清胆固醇水平是糖尿病肾病发展的独立危险因素,证明脂代谢紊乱也参与了糖尿病肾病的发病过程。此外,有研究证实,糖尿病肾病的发生发展还与全身和肾脏局部炎症有关,巨噬细胞、肥大细胞和T细胞等炎症细胞参与其中[5]。大量研究表明,巨噬细胞浸润肾脏组织是慢性肾脏疾病发生发展过程中的显著特征[6],与肾小球滤过率下降、肾小球硬化、蛋白尿增加、血肌酐上升以及肾间质纤维化等病理改变密切相关[7-8]。氧化应激作为参与糖尿病肾病发生发展过程中的重要因素之一,其驱动因素包括NADPH氧化酶、线粒体活性氧生成、内皮型一氧化氮合酶和脂氧合酶等[9]。自噬则是通过溶酶体降解细胞毒蛋白聚集体和受损的细胞器并回收线粒体能量的过程,有报道指出,肾脏细胞(尤其是足细胞和肾小管上皮细胞)的自噬功能受损,参与了各种肾脏疾病的发病过程[10]。此外,针对糖尿病肾病的全基因组研究只发现了一些潜在的基因、位点和单核苷酸多态性。相比之下,越来越多的研究证实表观遗传学与糖尿病肾病之间存在密切联系,包括DNA和染色质修饰、miRNAs和lncRNAs等。组蛋白赖氨酸甲基转移酶、组蛋白翻译后修饰、DNA甲基化以及多种miRNAs、lncRNAs参与了糖尿病肾病相关的纤维化和炎症基因的表达过程[11]。 2.1.2 糖尿病肾病的一般治疗策略 目前针对糖尿病肾病的治疗措施主要是在生活方式干预的基础上控制高血糖、高血压和调节血脂异常等。生活方式干预主要包括戒烟、限制钠的摄入及适当运动,对于肥胖患者还应适当减轻体质量。研究证实,将2型糖尿病患者的糖化血红蛋白控制在7.0%左右能够减少糖尿病微血管并发症的发生[12]。根据中华医学会糖尿病学分会的最新指南建议,糖尿病肾病患者的目标血压应小于130/80 mmHg,详细目标则需依据个体化来制定。临床上推荐的降压药物主要为肾素-血管紧张素-醛固酮系统阻断剂,如血管紧张素转化酶抑制剂、血管紧张素受体拮抗剂、肾素抑制剂和盐皮质激素拮抗剂。血管紧张素转化酶抑制剂和血管紧张素受体拮抗剂在肾脏保护方面的临床疗效有限,可能与其产生的不完全肾素-血管紧张素-醛固酮系统阻断相关。此外,肾脏病临床实践指南推荐糖尿病肾病患者接受他汀类药物治疗来减少发生动脉粥样硬化事件的风险[13]。 2.2 间充质干细胞的特征和临床应用 间充质干细胞是具有自我更新能力和多向分化潜能的多能干细胞,最早从骨髓中分离出来,现如今已经能够从多种组织中分离出来,包括脂肪组织[14-15]、脐带[16]、胎盘[17]、皮肤[18]、脱落牙齿的根部和其他实体器官等[19]。间充质干细胞因其安全易获取,免疫原性低[20],又不涉及重大伦理问题,而被认为是用于干细胞治疗的安全有效的细胞来源。临床前研究证实,间充质干细胞能够发挥多种治疗效果,包括促进血管生成、防止细胞凋亡、抑制炎症和调节细胞外基质动力学等,从而改善组织微环境和促进组织再生[1,20]。 鉴于间充质干细胞在临床前研究中发挥了多重治疗作用,目前全世界正进行着数以百计的临床试验,以探讨单独使用或联合其他药物治疗各种疾病的安全性和临床疗 效[21-24]。这些临床试验涵盖了如下领域:①整形外科,如颅面部创伤;②退行性疾病,如肌萎缩侧索硬化症、青光眼、黄斑变性等;③自身免疫性疾病,如骨关节炎、类风湿性关节炎、多发性硬化、克罗恩病、溃疡性结肠炎、1型糖尿病和系统性红斑狼疮等;④肺部炎症性疾病,如急性呼吸窘迫综合征、慢性阻塞性肺疾病和新型冠状病毒肺炎等;⑤同种异体移植免疫排斥反应,如移植物抗宿主病、实体器官排斥反应;⑥其他疾病,如成骨不全、缺血性心肌病、急性肾损伤和肝硬化等[3,21-23,25]。大多数临床研究都注册于美国国立卫生研究院(National Institutes of Health,NIH)数据库中(https://clinicaltrials.gov/),并在世界各地(主要为中国、欧盟和美国)的医学院校和生物医学机构中开展。然而大多数临床研究都处于第1阶段或第2阶段,晚期阶段的临床研究开展较少。 现有临床研究结果已经证实,应用自体或异体间充质干细胞治疗疾病均具备较高的安全性,并且取得了初步的治疗效果[21]。然而,在临床应用中还需要依据间充质干细胞的特性,更好地协调生产、运输和使用等过程[21,26-30]。尽管现阶段还存在一些亟待解决的问题,间充质干细胞依然是组织修复和疾病治疗领域的新希望。 2.3 间充质干细胞移植对糖尿病肾病的肾脏保护作用 2.3.1 间充质干细胞治疗糖尿病肾病的临床研究 近年来开展了大量的临床试验,以评估间充质干细胞治疗慢性肾脏疾病、急性肾损伤和肾移植等多种肾脏疾病的安全性、可行性和有效性[31-32]。一项针对常染色体显性遗传性多囊肾病的临床研究(NCT02166489)证实静脉输注间充质干细胞具有安全性和耐受性,疗效评估则需进一步开展随机对照试验[33]。间充质前体细胞也是具有自我更新能力并能分化为间充质细胞的多能细胞,与间充质干细胞的特征高度相似。在一项多中心、双盲的随机对照临床试验(NCT01843387)中,2型糖尿病合并晚期糖尿病肾病的患者被随机分为安慰剂组、低剂量组(150×106)和高剂量组(300×106),低剂量与高剂量组患者经单次异体间充质前体细胞移植治疗后12周,肾小球滤过率下降程度显著降低,但尿蛋白、尿蛋白/肌酐、肌酐清除率、血脂、糖化血红蛋白和血压等与安慰剂组相比差异无显著性意义[34]。此外,一项采用间充质干细胞治疗2型糖尿病肾病的临床试验(NCT02585622)已于2017年启动,该研究将评估间充质干细胞对糖尿病肾病患者肾小球滤过率、尿蛋白/肌酐、尿蛋白排泄、血糖、血脂和血压等指标的影响,该项临床试验将于2020年12月结束。 2.3.2 间充质干细胞治疗糖尿病肾病的机制研究 众多针对糖尿病肾病动物模型的研究表明,间充质干细胞能够减少糖尿病肾病动物模型的蛋白尿,改善肾脏功能,以及减轻肾小球硬化和肾小管间质纤维化等肾脏病理改变。据报道,静脉注射间充质干细胞4周后,糖尿病肾病大鼠的24 h尿蛋白减少,血肌酐和尿素水平降低,具有促修复作用的血管内皮生长因子和具有抗凋亡作用的Bcl-2蛋白增加,且肾小管坏死区域缩小[35]。EZQUER等[36]也观察到类似的结果,他们将异体提取的间充质干细胞经静脉输注入糖尿病肾病小鼠体内,与对照组相比,间充质干细胞治疗结束8周后糖尿病肾病小鼠24 h尿蛋白排泄减少了50%,且肾脏组织中仅观察到轻微的肾小管扩张。因此,间充质干细胞能够延缓糖尿病肾病进展,对受损的肾脏组织起到一定的保护作用。间充质干细胞对糖尿病肾病的保护作用机制尚不完全明了,既往研究结果主要涉及以下几个方面,见图1。 (1)减少肾脏细胞凋亡:研究证实,间充质干细胞能够通过保护受损的足细胞和肾小管上皮细胞免于异常凋亡来保护肾脏结构和改善肾脏病理改变。ZHANG等[37]研究发现,多次静脉注射脂肪间充质干细胞能够显著减轻链脲佐菌素诱导的糖尿病大鼠的肾小球肥大并减少尿蛋白排泄,进一步研究证实,脂肪间充质干细胞能够减轻高糖对足细胞的损伤,减少足细胞凋亡。体外研究证实,间充质干细胞条件培养基中所含的高水平表皮生长因子是阻止足细胞损伤和凋亡的关键因素,在间充质干细胞条件培养基中加入抗表皮生长因子抗体能够阻断其对足细胞的保护作用[38]。此外,JIANG等[39]学者从人胚胎胰腺中分离的间充质干细胞能够减少糖尿病肾病大鼠足细胞的融合和缺损,减少肾小球基底膜厚度,维持肾小球滤过屏障的完整性。NI等[40]进一步研究发现,脂肪间充质干细胞能够通过促进成纤维细胞生长因子受体复合物中Klotho蛋白的表达和抑制Wnt/β-Catenin信号通路来抑制高糖诱导的肾小管上皮细胞凋亡。 (2)调节自噬:研究表明,肾脏细胞(尤其是足细胞和肾小管上皮细胞)的自噬功能受损,参与了各种肾脏疾病的发病过程[10]。有报道指出,雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)是一种丝氨酸/苏氨酸激酶,作为最重要的自噬调节因子参与了糖尿病肾病的病理过程。在营养过剩的情况下,葡萄糖、氨基酸和生长因子水平升高能够激活雷帕霉素靶蛋白,引起足细胞和肾小管上皮细胞自噬水平下降,加重肾脏组织损伤。研究发现,采用骨髓间充质干细胞外泌体来治疗糖尿病肾病大鼠模型,能够使糖尿病肾病大鼠肾脏组织中雷帕霉素靶蛋白表达水平明显降低,同时肾小管上皮细胞的自噬活性显著增强[41]。此外,骨髓间充质干细胞能够抑制H2O2培养条件下足细胞内PI3K/Akt/mTOR信号通路激活,逆转H2O2诱导的足细胞异常自噬和凋亡,且转染miRNA-124a后骨髓间充质干细胞的上述效应能够得到进一步增强[42]。因此,间充质干细胞对糖尿病肾病的肾脏保护作用可能与抑制mTOR信号通路进而提高肾脏细胞的自噬活性有关。 (3)改善炎症反应:慢性炎症在糖尿病肾病的发生发展中起到关键作用,而间充质干细胞能够通过改善全身和肾脏局部炎症来延缓糖尿病肾病的进展。近端肾小管上皮细胞受到高糖刺激后高表达白细胞介素6、白细胞介素8和单核细胞趋化蛋白1等促炎细胞因子,体外研究则证实间充质干细胞能够减少高糖条件下近端肾小管上皮细胞中促炎细胞因子的表达[43]。AN等[44]在恒河猴糖尿病肾病模型中证实,间充质干细胞能够显著减少肾脏和血液循环中白细胞介素16的表达,改善全身和肾脏炎症。YU等[45]学者发现,脂肪间充质干细胞能够降低糖尿病肾病大鼠血清中白细胞介素6、白细胞介素1β和肿瘤坏死因子α等促炎细胞因子的水平,同时升高抗炎细胞因子白细胞介素10的水平。此外,炎症细胞浸润(主要为巨噬细胞和T淋巴细胞)是糖尿病肾病病理改变中的一个重要特征。间充质干细胞能够抑制糖尿病肾病动物模型中巨噬细胞的渗出,并且能够诱导具有促炎作用的M1型巨噬细胞向具有抗炎和组织修复作用的M2型巨噬细胞转换,从而改善肾脏炎症。LI等[46]在大鼠1型糖尿病肾病模型中发现,间充质干细胞输注治疗能够减少肾脏组织中CD68与F4/80阳性巨噬细胞,同时白细胞介素1β、白细胞介素6、肿瘤坏死因子α和单核细胞趋化蛋白1等促炎细胞因子的表达下降。最新的一项研究证实,间充质干细胞能够通过促进转录因子EB介导的自噬来诱导糖尿病肾病小鼠肾脏中的巨噬细胞向M2型极化,从而抑制肾脏炎症和保护肾脏组织[47]。除了抑制巨噬细胞渗出和调节巨噬细胞极化外,ZHANG等[48]近期发表的一项研究成果显示,骨髓间充质干细胞还能通过抑制CD103+树突状细胞的成熟来抑制CD8+ T细胞的增殖和活化,从而减少CD8+ T细胞在糖尿病肾病大鼠肾脏组织中的浸润。 (4)改善氧化应激:糖尿病肾病的病理改变机制包括活性氧的过度产生和抗氧化能力不足。KONARI等[49]研究发现,从间充质干细胞中分离提取的线粒体产生的活性氧减少,且异体来源的间充质干细胞能够将自身的线粒体转移给糖尿病肾病大鼠受损的近端肾小管上皮细胞,改善肾小管上皮细胞的氧化应激并抑制细胞凋亡,从而保护肾小管的结构和功能。LEE等[50]通过体外研究发现,间充质干细胞能使巨噬细胞中精氨酸酶1表达增加,高表达精氨酸酶1的巨噬细胞能够进一步改善肾小管上皮细胞的线粒体功能障碍,抑制氧化应激。 (5)抑制纤维化:转化生长因子β(transforming growth factor-β,TGF-β)超家族既是诱导上皮间质转化的关键调控因子[51],又能调控细胞外基质合成从而导致肾脏纤维化[52],TGF-β/Smad信号通路参与了糖尿病肾病的病理过程。上皮间质转化指上皮细胞在特定的生理或病理条件下转化为具有间质表型细胞的过程。在糖尿病肾病的病理改变中,肾小管上皮细胞受到高糖、尿蛋白和氧化应激等刺激后发生表型变化,转变为过度合成细胞外基质的成纤维细胞,导致肾小管间质纤维化[53]。RAO等[54]研究证实,牙髓来源间充质干细胞能够抑制晚期糖基化终末产物诱导的肾小管上皮细胞的上皮间质转化,从而减少糖尿病肾病大鼠尿蛋白排泄和细胞外基质沉积,血清中转化生长因子β的表达水平也显著降低。NAGAISHI等[55]通过链脲佐菌素建立小鼠1型糖尿病肾病模型,以及利用高脂饮食建立小鼠2型糖尿病肾病模型,并采用间充质干细胞或间充质干细胞外泌体进行治疗,两种糖尿病肾病小鼠模型的肾脏组织中转化生长因子β1蛋白表达水平均显著降低,肾脏组织纤维化程度得到明显改善。此外,LV等[56]发现间充质干细胞能够产生并分泌肝细胞生长因子来抑制转化生长因子β的表达,从而减轻糖尿病肾病大鼠的肾小球硬化程度。骨形态发生蛋白7作为一种具有抗纤维化作用的细胞因子,能够拮抗转化生长因子β的表达[57-58]。LV等[59]进一步研究证实,间充质干细胞来源的骨形态发生蛋白7能通过抑制纤维化相关的TGF-β/Smad信号通路来改善糖尿病肾病大鼠的肾小球纤维化。一项最新的研究发现,间充质干细胞分泌的脂氧素A4(Lipoxins A4,LXA4)能在肾脏局部微环境中激动LXA4-ALX/FPR2信号通路,并且抑制TGF-β/Smad信号通路,从而改善糖尿病肾病动物模型的肾脏纤维化[60]。 2.3.3 间充质干细胞外泌体在治疗糖尿病肾病中的作用 间充质干细胞作为一种可以分泌多种营养因子的多能干细胞,还能通过分泌细胞外囊泡来发挥组织修复的作用。外泌体是一种由多泡体分泌的直径30-100 nm的膜囊泡,是细胞外囊泡的常见类型之一,其内充满蛋白质、脂质、mRNA和 miRNAs等。多项研究证实,应用间充质干细胞外泌体治疗糖尿病肾病同样具备安全性和有效性。间充质干细胞外泌体能够将其携带的内容物转移至组织受损部位,在糖尿病肾病模型中发挥肾脏保护作用。ZHONG等[61]发现与细胞分化相关的miRNA-451a在糖尿病肾病小鼠的肾小管上皮细胞中表达减少,而间充质干细胞分泌的细胞外囊泡富含 miRNA-451a,并且能够通过下调α-平滑肌肌动蛋白和增加E-钙黏蛋白的表达来抑制肾小管上皮细胞的上皮间质转化,保护肾脏结构。EBRAHIM等[41]研究发现,骨髓间充质干细胞外泌体能够改善1型糖尿病肾病大鼠肾小管上皮细胞的自噬能力,从而发挥肾脏保护作用。GRANGE等[62]对糖尿病肾病小鼠注射骨髓间充质干细胞来源的细胞外囊泡,显著减少了糖尿病肾病小鼠的尿白蛋白/肌酐,降低了血肌酐和血尿素氮水平,生物信息学分析发现,间充质干细胞来源细胞外囊泡中富含的miRNAs能够作用于转化生长因子β、胰岛素样生长因子1、表皮生长因子受体和血小板衍生生长因子受体等纤维化相关的信号通路,下调促纤维化基因的表达从而抑制糖尿病肾病模型的肾脏纤维化进程。NAGAISHI等[55]通过提取骨髓间充质干细胞来源外泌体来治疗1型糖尿病肾病小鼠,证实骨髓间充质干细胞来源外泌体能够抑制p38-MAPK和转化生长因子β的表达,抑制肾小管上皮细胞中紧密连接蛋白的降解,从而减少尿蛋白排泄。以上研究结果证实间充质干细胞外泌体同样能够通过抑制上皮间质转化、调节自噬和抑制纤维化等机制来发挥改善糖尿病肾病的效果,且避免了间充质干细胞可能引起的肿瘤发生、细胞排斥反应和异常分化等问题。然而,在将间充质干细胞外泌体应用于糖尿病肾病临床治疗之前,还需探究不同来源间充质干细胞外泌体的特性,进一步确认其临床应用的安全性以及疗效的可重复性。此外,间充质干细胞外泌体中所含的有效成分和作用机制也需更加深入的探索。 "
| [1] UCCELLI A, MORETTA L, PISTOIA V. Mesenchymal Stem Cells in Health and Disease. Nat Rev Immunol. 2008;8(9):726-736. [2] ANDRZEJEWSKA A, LUKOMSKA B, JANOWSKI M. Concise Review: mesenchymal stem cells: from roots to boost. Stem Cells. 2019;37(7):855-864. [3] LENG Z, ZHU R, HOU W, et al. Transplantation of Ace2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with Covid-19 Pneumonia. Aging Dis. 2020;11(2):216-228. [4] GIACCO F, BROWNLEE M. Oxidative Stress and Diabetic Complications. Circ Res. 2010;107(9):1058-1070. [5] CHOW F, OZOLS E, NIKOLIC-PATERSON DJ, et al. Macrophages in Mouse Type 2 Diabetic Nephropathy: Correlation with Diabetic State and Progressive Renal Injury. Kidney Int. 2004;65(1):116-128. [6] TIAN S, CHEN SY. Macrophage Polarization in Kidney Diseases. Macrophage (Houst). 2015;2(1):e679. [7] KLESSENS CQF, ZANDBERGEN M, WOLTERBEEK R, et al. Macrophages in Diabetic Nephropathy in Patients with Type 2 Diabetes. Nephrol Dial Transplant. 2017;32(8):1322-1329. [8] NAVARRO-GONZÁLEZ JF, MORA-FERNÁNDEZ C, MUROS DE FUENTES M, et al. Inflammatory Molecules and Pathways in the Pathogenesis of Diabetic Nephropathy. Nat Rev Nephrol. 2011;7(6):327-340. [9] ØSTERGAARD JA, COOPER ME, JANDELEIT-DAHM KAM. Targeting Oxidative Stress and Anti-Oxidant Defence in Diabetic Kidney Disease. J Nephrol. 2020;33(5):917-929. [10] DE RECHTER S, DECUYPERE JP, IVANOVA E, et al. Autophagy in Renal Diseases. Pediatr Nephrol. 2016;31(5):737-752. [11] KATO M, NATARAJAN R. Epigenetics and Epigenomics in Diabetic Kidney Disease and Metabolic Memory. Nat Rev Nephrol. 2019;15(6):327-345. [12] DUCKWORTH W, ABRAIRA C, MORITZ T, et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360(2):129-139. [13] NATIONAL KIDNEY FOUNDATION. Kdoqi Clinical Practice Guideline for Diabetes and Ckd: 2012 Update. Am J Kidney Dis. 2012;60(5):850-886. [14] HALVORSEN YC, WILKISON WO, GIMBLE JM. Adipose-Derived Stromal Cells--Their Utility and Potential in Bone Formation. Int J Obes Relat Metab Disord. 2000;24 Suppl 4:S41-44. [15] ZUK PA, ZHU M, MIZUNO H, et al. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001;7(2):211-228. [16] ROMANOV YA, SVINTSITSKAYA VA, SMIRNOV VN. Searching for Alternative Sources of Postnatal Human Mesenchymal Stem Cells: Candidate Msc-Like Cells from Umbilical Cord. Stem Cells. 2003;21(1):105-110. [17] IN ‘T ANKER PS, SCHERJON SA, KLEIJBURG-VAN DER KEUR C, et al. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells. 2004;22(7):1338-1345. [18] MELO FR, BRESSAN RB, FORNER S, et al. Transplantation of Human Skin-Derived Mesenchymal Stromal Cells Improves Locomotor Recovery after Spinal Cord Injury in Rats. Cell Mol Neurobiol. 2017;37(5):941-947. [19] MARTINEZ SAEZ D, SASAKI RT, NEVES AD, et al. Stem Cells from Human Exfoliated Deciduous Teeth: A Growing Literature. Cells Tissues Organs. 2016;202(5-6):269-280. [20] WANG Y, CHEN X, CAO W, et al. Plasticity of Mesenchymal Stem Cells in Immunomodulation: Pathological and Therapeutic Implications. Nat Immunol. 2014;15(11):1009-1016. [21] GALIPEAU J, SENSÉBÉ L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22(6):824-833. [22] SQUILLARO T, PELUSO G, GALDERISI U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25(5):829-848. [23] TROUNSON A, MCDONALD C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17(1):11-22. [24] NAJI A, ROUAS-FREISS N, DURRBACH A, CAROSELLA ED, et al. Concise Review: Combining Human Leukocyte Antigen G and Mesenchymal Stem Cells for Immunosuppressant Biotherapy. Stem Cells. 2013;31(11):2296-2303. [25] WEI X, YANG X, HAN ZP, et al. Mesenchymal Stem Cells: A New Trend for Cell Therapy. Acta Pharmacol Sin. 2013;34(6):747-754. [26] CHINNADURAI R, COPLAND IB, GARCIA MA, et al. Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNγ Licensing. Stem Cells. 2016;34(9):2429-2442. [27] GALIPEAU J. The Mesenchymal Stromal Cells Dilemma--Does a Negative Phase Iii Trial of Random Donor Mesenchymal Stromal Cells in Steroid-Resistant Graft-Versus-Host Disease Represent a Death Knell or a Bump in the Road? Cytotherapy. 2013;15(1):2-8. [28] GALIPEAU J, KRAMPERA M. The Challenge of Defining Mesenchymal Stromal Cell Potency Assays and Their Potential Use as Release Criteria. Cytotherapy. 2015;17(2):125-127. [29] GALIPEAU J, KRAMPERA M, BARRETT J, et al. International Society for Cellular Therapy Perspective on Immune Functional Assays for Mesenchymal Stromal Cells as Potency Release Criterion for Advanced Phase Clinical Trials. Cytotherapy. 2016;18(2):151-159. [30] PHINNEY DG, GALIPEAU J, KRAMPERA M, et al. Mscs: Science and Trials. Nat Med. 2013;19(7):812. [31] PEIRED AJ, SISTI A, ROMAGNANI P. Mesenchymal Stem Cell-Based Therapy for Kidney Disease: A Review of Clinical Evidence. Stem Cells Int. 2016; 2016:4798639. [32] ROVIRA J, DIEKMANN F, CAMPISTOL JM, et al. Therapeutic Application of Extracellular Vesicles in Acute and Chronic Renal Injury. Nefrologia. 2017; 37(2):126-137. [33] MAKHLOUGH A, SHEKARCHIAN S, MOGHADASALI R, et al. Safety and Tolerability of Autologous Bone Marrow Mesenchymal Stromal Cells in Adpkd Patients. Stem Cell Res Ther. 2017;8(1):116. [34] PACKHAM DK, FRASER IR, KERR PG, et al. Allogeneic Mesenchymal Precursor Cells (Mpc) in Diabetic Nephropathy: A Randomized, Placebo-Controlled, Dose Escalation Study. EBioMedicine. 2016;12:263-269. [35] ABDEL AZIZ MT, WASSEF MA, AHMED HH, et al. The Role of Bone Marrow Derived-Mesenchymal Stem Cells in Attenuation of Kidney Function in Rats with Diabetic Nephropathy. Diabetol Metab Syndr. 2014;6(1):34. [36] EZQUER F, GIRAUD-BILLOUD M, CARPIO D, et al. Proregenerative Microenvironment Triggered by Donor Mesenchymal Stem Cells Preserves Renal Function and Structure in Mice with Severe Diabetes Mellitus. Biomed Res Int. 2015;2015:164703. [37] ZHANG L, LI K, LIU X, et al. Repeated Systemic Administration of Human Adipose-Derived Stem Cells Attenuates Overt Diabetic Nephropathy in Rats. Stem Cells Dev. 2013;22(23):3074-3086. [38] LI D, WANG N, ZHANG L, et al. Mesenchymal Stem Cells Protect Podocytes from Apoptosis Induced by High Glucose Via Secretion of Epithelial Growth Factor. Stem Cell Res Ther. 2013;4(5):103. [39] JIANG Y, ZHANG W, XU S, et al. Transplantation of Human Fetal Pancreatic Progenitor Cells Ameliorates Renal Injury in Streptozotocin-Induced Diabetic Nephropathy. J Transl Med. 2017;15(1):147. [40] NI W, FANG Y, XIE L, et al. Adipose-Derived Mesenchymal Stem Cells Transplantation Alleviates Renal Injury in Streptozotocin-Induced Diabetic Nephropathy. J Histochem Cytochem. 2015;63(11):842-853. [41] EBRAHIM N, AHMED IA, HUSSIEN NI, et al. Mesenchymal Stem Cell-Derived Exosomes Ameliorated Diabetic Nephropathy by Autophagy Induction through the Mtor Signaling Pathway. Cells. 2018;7(12):226. [42] SUN J, LV J, ZHANG W, et al. Combination with Mir-124a Improves the Protective Action of Bmscs in Rescuing Injured Rat Podocytes from Abnormal Apoptosis and Autophagy. J Cell Biochem. 2018;119(9):7166-7176. [43] ISLAM MN, GRIFFIN TP, SANDER E, et al. Human Mesenchymal Stromal Cells Broadly Modulate High Glucose-Induced Inflammatory Responses of Renal Proximal Tubular Cell Monolayers. Stem Cell Res Ther. 2019;10(1):329. [44] AN X, LIAO G, CHEN Y, et al. Intervention for Early Diabetic Nephropathy by Mesenchymal Stem Cells in a Preclinical Nonhuman Primate Model. Stem Cell Res Ther. 2019;10(1):363. [45] YU S, CHENG Y, ZHANG L, et al. Treatment with Adipose Tissue-Derived Mesenchymal Stem Cells Exerts Anti-Diabetic Effects, Improves Long-Term Complications, and Attenuates Inflammation in Type 2 Diabetic Rats. Stem Cell Res Ther. 2019;10(1):333. [46] LI Y, LIU J, LIAO G, et al. Early Intervention with Mesenchymal Stem Cells Prevents Nephropathy in Diabetic Rats by Ameliorating the Inflammatory Microenvironment. Int J Mol Med. 2018;41(5):2629-2639. [47] YUAN Y, LI L, ZHU L, et al. Mesenchymal Stem Cells Elicit Macrophages into M2 Phenotype Via Improving Transcription Factor Eb-Mediated Autophagy to Alleviate Diabetic Nephropathy. Stem Cells. 2020;38(5):639-652. [48] ZHANG F, WANG C, WEN X, et al. Mesenchymal Stem Cells Alleviate Rat Diabetic Nephropathy by Suppressing Cd103(+) Dcs-Mediated Cd8(+) T Cell Responses. J Cell Mol Med. 2020;24(10):5817-5831. [49] KONARI N, NAGAISHI K, KIKUCHI S, et al. Mitochondria Transfer from Mesenchymal Stem Cells Structurally and Functionally Repairs Renal Proximal Tubular Epithelial Cells in Diabetic Nephropathy in Vivo. Sci Rep. 2019;9(1):5184. [50] LEE SE, JANG JE, KIM HS, et al. Mesenchymal Stem Cells Prevent the Progression of Diabetic Nephropathy by Improving Mitochondrial Function in Tubular Epithelial Cells. Exp Mol Med. 2019;51(7):77. [51] HILLS CE, SQUIRES PE. The Role of Tgf-Beta and Epithelial-to Mesenchymal Transition in Diabetic Nephropathy. Cytokine Growth Factor Rev. 2011;22(3): 131-139. [52] ZIYADEH FN. Mediators of Diabetic Renal Disease: The Case for Tgf-Beta as the Major Mediator. J Am Soc Nephrol. 2004;15 Suppl 1:S55-57. [53] TONOLO G, CHERCHI S. Tubulointerstitial Disease in Diabetic Nephropathy. Int J Nephrol Renovasc Dis. 2014;7:107-115. [54] RAO N, WANG X, XIE J, et al. Stem Cells from Human Exfoliated Deciduous Teeth Ameliorate Diabetic Nephropathy in Vivo and in Vitro by Inhibiting Advanced Glycation End Product-Activated Epithelial-Mesenchymal Transition. Stem Cells Int. 2019;2019:2751475. [55] NAGAISHI K, MIZUE Y, CHIKENJI T, et al. Mesenchymal Stem Cell Therapy Ameliorates Diabetic Nephropathy Via the Paracrine Effect of Renal Trophic Factors Including Exosomes. Sci Rep. 2016;6:34842. [56] LV SS, LIU G, WANG JP, et al. Mesenchymal Stem Cells Transplantation Ameliorates Glomerular Injury in Streptozotocin-Induced Diabetic Nephropathy in Rats Via Inhibiting Macrophage Infiltration. Int Immunopharmacol. 2013;17(2):275-282. [57] MOTAZED R, COLVILLE-NASH P, KWAN JT, et al. Bmp-7 and Proximal Tubule Epithelial Cells: Activation of Multiple Signaling Pathways Reveals a Novel Anti-Fibrotic Mechanism. Pharm Res. 2008;25(10):2440-2446. [58] LUO DD, PHILLIPS A, FRASER D. Bone Morphogenetic Protein-7 Inhibits Proximal Tubular Epithelial Cell Smad3 Signaling Via Increased Snon Expression. Am J Pathol. 2010;176(3):1139-1147. [59] LV S, LIU G, SUN A, et al. Mesenchymal Stem Cells Ameliorate Diabetic Glomerular Fibrosis in Vivo and in Vitro by Inhibiting Tgf-Beta Signalling Via Secretion of Bone Morphogenetic Protein 7. Diab Vasc Dis Res. 2014; 11(4):251-261. [60] BAI Y, WANG J, HE Z, et al. Mesenchymal Stem Cells Reverse Diabetic Nephropathy Disease Via Lipoxin A4 by Targeting Transforming Growth Factor Beta (Tgf-Beta)/Smad Pathway and Pro-Inflammatory Cytokines. Med Sci Monit. 2019;25:3069-3076. [61] ZHONG L, LIAO G, WANG X, et al. Mesenchymal Stem Cells-Microvesicle-Mir-451a Ameliorate Early Diabetic Kidney Injury by Negative Regulation of P15 and P19. Exp Biol Med (Maywood). 2018;243(15-16):1233-1242. [62] GRANGE C, TRITTA S, TAPPARO M, et al. Stem Cell-Derived Extracellular Vesicles Inhibit and Revert Fibrosis Progression in a Mouse Model of Diabetic Nephropathy. Sci Rep. 2019;9(1):4468. |
| [1] | Wang Qin, Shen Cheng, Liao Jing, Yang Ye. Dapagliflozin improves renal injury in diabetic nephropathy rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1278. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1316-1322. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1323-1329. |
| [4] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1033-1039. |
| [5] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1053-1059. |
| [6] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1068-1074. |
| [7] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1086-1092. |
| [8] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1098. |
| [9] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1133-1140. |
| [10] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1141-1150. |
| [11] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [12] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [13] | Cao Wei, Mao Furong, Hu Xiaohua, Yang Xiaohong. N-6 methyladenosine RNA methylation regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 266-270. |
| [14] | Fan Danyang, Fu Runze, Mi Jiajing, Liu Chunyan. Expression and role of cannabinoid receptors during bone remodeling [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 283-288. |
| [15] | Zhou Hongbo, Yu Zhengwen, Liu Jianguo. Molecular mechanism of bone regeneration promoted by medical metal implant materials [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1661-1670. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||