Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (7): 1069-1074.doi: 10.3969/j.issn.2095-4344.2174
Previous Articles Next Articles
Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis
Liu Cong, Liu Su
- Department of Plastic Surgery, Affiliated Hospital of Qingdao University, Qingdao 266071, Shandong Province, China
-
Received:2019-01-21Revised:2019-01-30Accepted:2019-05-17Online:2021-03-08Published:2020-12-08 -
Contact:Liu Su, Associate chief physician, Department of Plastic Surgery, Affiliated Hospital of Qingdao University, Qingdao 266071, Shandong Province, China -
About author:Liu Cong, Master, Department of Plastic Surgery, Affiliated Hospital of Qingdao University, Qingdao 266071, Shandong Province, China
CLC Number:
Cite this article
Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074.
share this article
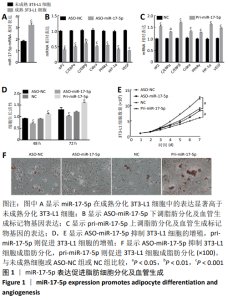
2.1 miR-17-5p表达促进脂肪细胞分化及血管生成相关基因的表达 miR-17-5p在未成熟分化的3T3-L1细胞及成熟分化的3T3-L1细胞中均有表达,在成熟分化的3T3-L1细胞中表达显著高于未成熟分化的3T3-L1细胞(t=-89.12,P < 0.001),见图1A。抑制或过表达miR-17-5p后采用RT-qPCR检测脂肪分化及血管生成标记物基因的表达,见图1B,C,结果显示ASO-miR-17-5p抑制物显著抑制了脂肪分化及血管生成标记物基因的表达(P < 0.05),pri-miR-17-5p模拟物显著上调了脂肪分化及血管生成标记物基因的表达(P < 0.05)。 MTT检测细胞增殖结果显示,ASO-miR-17-5p抑制物显著抑制了3T3-L1细胞的增殖(t=18.97,P < 0.05),而pri-miR-17-5p模拟物则显著促进了3T3-L1细胞的增殖(t=-3.44,P < 0.05),见图1D。通过细胞生长活性进行分析,ASO-miR-17-5p抑制物显著抑制了3T3-L1细胞的生长活性(t=18.34,P < 0.001),而pri-miR-17-5p模拟物则显著提高了3T3-L1细胞的生长活性(t=-21.91,P < 0.001),见图1E。油红O染色结果显示,ASO-miR-17-5p抑制物显著抑制了3T3-L1细胞分化,而pri-miR-17-5p模拟物则能促进3T3-L1细胞分化(t=1.32,P < 0.05,t=-0.92,P < 0.05),见图1F。上述结果表明,miR-17-5p表达促进脂肪细胞分化及血管生成。"
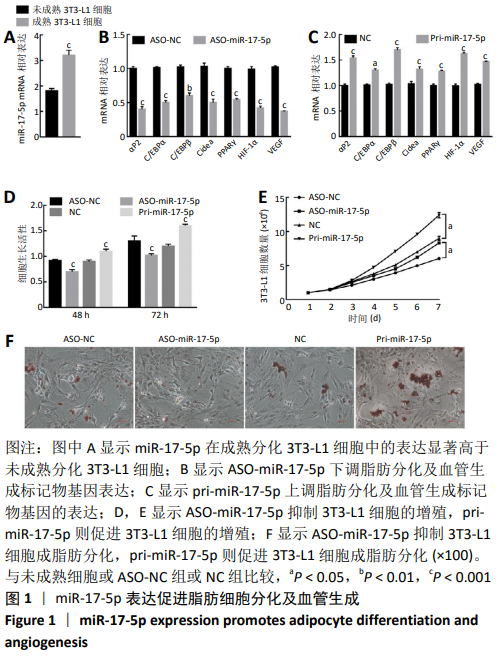
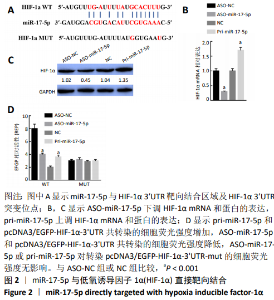
2.2 miR-17-5p与HIF-1α直接靶向结合 通过Targetscan软件分析发现,在miR-17-5p的候选靶基因中,HIF-1α能够与miR-17-5p靶向结合,见图2A;通过RT-qPCR检测抑制miR-17-5p表达显著下调了HIF-1α mRNA的表达,而过表达miR-17-5p则显著上调了HIF-1α mRNA的表达(t=53.45,P < 0.001;t=-55.00,P < 0.001),见图2B;与RT-qPCR检测结果一致,抑制miR-17-5p表达显著下调了HIF-1α蛋白的表达,而过表达miR-17-5p则显著上调了HIF-1α蛋白的表达(t=54.71,P < 0.001;t=-29.09,P < 0.001),见图2C;通过EGFP荧光报告基因检测miR-17-5p与HIF-1α直接靶向关系,首先构建具有miR-17-5p结合位点的HIF-1α 3’UTR EGFP报告基因或HIF-1α 3’UTR突变体的EGFP报告载体,其次pcDNA3/EGFP-HIF-1α-3’UTR或pcDNA3/EGFP-HIF-1α-3’UTR-mut与pri-miR-17-5p或ASO-miR-17-5p共转染到3T3-L1细胞,与空载体相比,与pri-miR-17-5p和pcDNA3/EGFP-HIF-1α-3’UTR共转染的细胞荧光强度显著增加;相反地,与ASO-miR-17-5p和pcDNA3/EGFP-HIF-1α-3’UTR共转染的细胞荧光强度显著降低(t=71.63,P < 0.001;t=-37.50,P < 0.001),然而过表达或抑制miR-17-5p均未影响转染pcDNA3/EGFP-HIF-1α-3’UTR-mut的细胞的荧光强度(t=-0.46,P > 0.05;t=-0.62,P > 0.05),见图2D。结果表明,HIF-1α是miR-17-5p的直接靶基因,并且miR-17-5p可以上调HIF-1α的表达。"
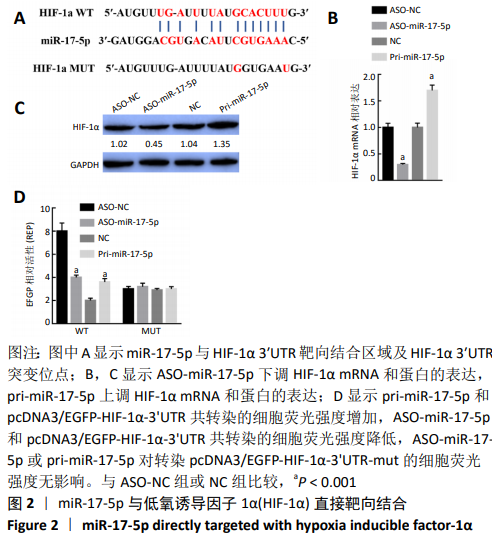
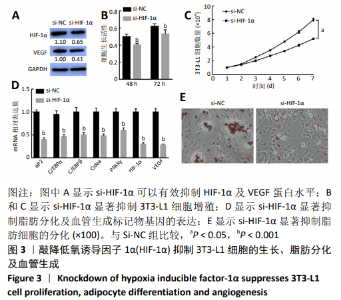
2.3 敲降HIF-1α抑制3T3-L1细胞的生长、脂肪分化及血管生成 构建HIF-1α敲降载体(si-HIF-1α)研究HIF-1α对3T3-L1细胞增殖、脂肪分化及血管生成的影响。Western blot结果显示,si-HIF-1α可以有效抑制HIF-1α的内源性蛋白水平(t=2.64,P < 0.01),此外si-HIF-1α也能够显著抑制VEGF的蛋白水平(t=-1.20,P < 0.01),见图3A;si-HIF-1α显著抑制3T3-L1细胞增殖(t=13.47, P < 0.001;t=11.02,P < 0.001;t=1.57,P < 0.05),见图3B,C;与转染空载体对照组比较,si-HIF-1α显著抑制了脂肪分化及血管生成标记物基因(αP2、C/EBPα、C/EBPβ、Cidea、PPARγ、HIF-1α、VEGF)mRNA的表达(P < 0.001),见图3D;油红O结果也显示si-HIF-1α能够显著抑制脂肪细胞的分化(t=1.68,P < 0.05),见图3E。结果表明,敲降HIF-1α抑制3T3-L1细胞的生长、脂肪分化及血管生成。"
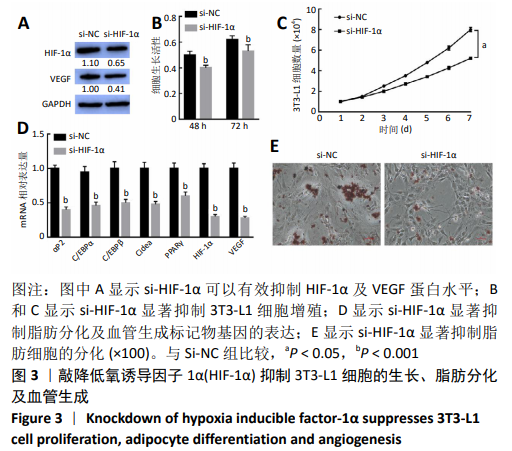
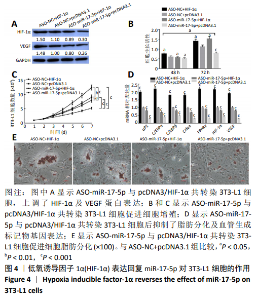
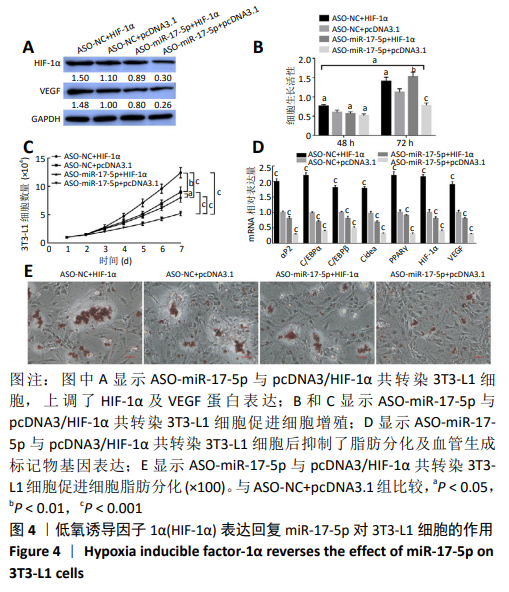
2.4 HIF-1α表达回复miR-17-5p对3T3-L1细胞的作用 通过回复实验验证miR-17-5p对3T3-L1细胞表型的影响由HIF-1α基因介导。Western blot结果显示,pcDNA3/HIF-1α与ASO-NC共转染3T3-L1细胞,HIF-1α及VEGF的蛋白水平显著上调(P < 0.05),ASO-miR-17-5p与pcDNA3空载体共转染3T3-L1细胞后HIF-1α及VEGF的蛋白水平显著下调(P < 0.01),而ASO-miR-17-5p与pcDNA3/HIF-1α共转染3T3-L1细胞后HIF-1α及VEGF的蛋白水平上调(P < 0.05),见图4A;细胞增殖结果也显示,pcDNA3/HIF-1α与ASO-NC共转染3T3-L1细胞显著促进细胞的增殖(P < 0.05),ASO-miR-17-5p与pcDNA3空载体共转染3T3-L1细胞显著抑制了细胞的增殖(P < 0.05),而ASO-miR-17-5p与pcDNA3/HIF-1α共转染3T3-L1细胞促进了细胞增殖(P < 0.05),见图4B和4C;pcDNA3/HIF-1α与ASO-NC共转染3T3-L1细胞显著上调了脂肪分化及血管生成标记物基因表达(P < 0.001),ASO-miR-17-5p与pcDNA3空载体共转染3T3-L1细胞显著抑制脂肪分化及血管生成标记物基因表达(P < 0.001),而ASO-miR-17-5p与pcDNA3/HIF-1α共转染3T3-L1细胞后抑制了脂肪分化及血管生成标记物基因表达(P < 0.001),见图4D;油红O染色结果与上述实验结果一致(P < 0.05),见图4E。结果表明,HIF-1α参与了miR-17-5p调控3T3-L1细胞的增殖、成脂分化及血管形成。"
| [1] MAURIZI G, DELLA GUARDIA L, MAURIZI A, et al. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J Cell Physiol. 2018;233(1):88-97. [2] KONANIAH ES, KUHEL DG, BASFORD JE, et al. Deficiency of LRP1 in Mature Adipocytes Promotes Diet-Induced Inflammation and Atherosclerosis-Brief Report. Arterioscler Thromb Vasc Biol. 2017; 37(6):1046-1049. [3] WEI CK, TSAI YH, KORINEK M, et al. 6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice. Int J Mol Sci. 2017;18(1). pii: E168. [4] ZHENG J, CHEN M, LIU G, et al. Ablation of hephaestin and ceruloplasmin results in iron accumulation in adipocytes and type 2 diabetes. FEBS Lett. 2018;592(3):394-401. [5] STOFKOVA A, KRSKOVA K, VACULIN S, et al. Enhanced activity of hormone sensitive lipase (HSL) in mesenteric but not epididymal fat correlates with higher production of epinephrine in mesenteric adipocytes in rat model of cachectic rheumatoid arthritis. Autoimmunity. 2016;49(4):268-276. [6] FOUBERT P, DOYLE-EISELE M, GONZALEZ A, et al. Development of a combined radiation and full thickness burn injury minipig model to study the effects of uncultured adipose-derived regenerative cell therapy in wound healing. Int J Radiat Biol. 2017;93(3):340-350. [7] CHEN YW, SCUTARU TT, GHETU N, et al. The Effects of Adipose-Derived Stem Cell-Differentiated Adipocytes on Skin Burn Wound Healing in Rats. J Burn Care Res. 2017;38(1):1-10. [8] SCHMID R, WOLF K, ROBERING JW, et al. ADSCs and adipocytes are the main producers in the autotaxin-lysophosphatidic acid axis of breast cancer and healthy mammary tissue in vitro. BMC Cancer. 2018;18(1):1273. [9] SCHEJA L, HEEREN J. Metabolic interplay between white, beige, brown adipocytes and the liver. J Hepatol. 2016;64(5):1176-1186. [10] LAURENT V, GUÉRARD A, MAZEROLLES C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016;7:10230. [11] HANOUN M, ZHANG D, MIZOGUCHI T, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15(3): 365-375. [12] BOYD AL, REID JC, SALCI KR, et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat Cell Biol. 2017;19(11):1336-1347. [13] KARAMAN S, HOLLMÉN M, YOON SY, et al. Transgenic overexpression of VEGF-C induces weight gain and insulin resistance in mice. Sci Rep. 2016;6:31566. [14] ENGIN A. The Pathogenesis of Obesity-Associated Adipose Tissue Inflammation. Adv Exp Med Biol. 2017;960:221-245. [15] SIMENTAL-MENDÍA M, SÁNCHEZ-GARCÍA A, VILCHEZ-CAVAZOS F, et al. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol Int. 2018;38(8):1413-1428. [16] KIHIRA Y, MIYAKE M, HIRATA M, et al. Deletion of hypoxia-inducible factor-1α in adipocytes enhances glucagon-like peptide-1 secretion and reduces adipose tissue inflammation. PLoS One. 2014;9(4):e93856. [17] FAN B, SHEN C, WU M, et al. miR-17-92 cluster is connected with disease progression and oxaliplatin/capecitabine chemotherapy efficacy in advanced gastric cancer patients: A preliminary study. Medicine (Baltimore). 2018;97(35):e12007. [18] HAN H, GU S, CHU W, et al. miR-17-5p Regulates Differential Expression of NCOA3 in Pig Intramuscular and Subcutaneous Adipose Tissue. Lipids. 2017;52(11):939-949. [19] TIAN L, SONG Z, SHAO W, et al. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017;8(1):e2559. [20] LI H, LI T, WANG S, et al. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res. 2013;10(3):313-324. [21] FRIAS FT, ROCHA KCE, DE MENDONÇA M, et al. Fenofibrate reverses changes induced by high-fat diet on metabolism in mice muscle and visceral adipocytes. J Cell Physiol. 2018;233(4):3515-3528. [22] HE JY, WEI XH, LI SJ, et al. Adipocyte-derived IL-6 and leptin promote breast Cancer metastasis via upregulation of Lysyl Hydroxylase-2 expression. Cell Commun Signal. 2018;16(1):100. [23] TABE Y, YAMAMOTO S, SAITOH K, et al. Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating AMPK and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells. Cancer Res. 2017;77(6):1453-1464. [24] ZHANG P, DU J, WANG L, et al. MicroRNA-143a-3p modulates preadipocyte proliferation and differentiation by targeting MAPK7. Biomed Pharmacother. 2018;108:531-539. [25] JIANG J, LI P, LING H, et al. MiR-499/PRDM16 axis modulates the adipogenic differentiation of mouse skeletal muscle satellite cells. Hum Cell. 2018;31(4):282-291. [26] PAN XX, CAO JM, CAI F, et al. Loss of miR-146b-3p Inhibits Perivascular Adipocyte Browning with Cold Exposure During Aging. Cardiovasc Drugs Ther. 2018;32(5):511-518. [27] CHEN C, DENG Y, HU X, et al. miR-128-3p regulates 3T3-L1 adipogenesis and lipolysis by targeting Pparg and Sertad2. J Physiol Biochem. 2018; 74(3):381-393. [28] SHI T, YAN X, QIAO L, et al. MiR-330-5p negatively regulates ovine preadipocyte differentiation by targeting branched-chain aminotransferase 2. Anim Sci J. 2018;89(6):858-867. [29] AL-ANAZI A, PARHAR R, SALEH S, et al. Intracellular calcium and NF-kB regulate hypoxia-induced leptin, VEGF, IL-6 and adiponectin secretion in human adipocytes. Life Sci. 2018;212:275-284. [30] LEE HP, LIN CY, SHIH JS, et al. Adiponectin promotes VEGF-A-dependent angiogenesis in human chondrosarcoma through PI3K, Akt, mTOR, and HIF-α pathway. Oncotarget. 2015;6(34):36746-36761. [31] TANG Q, WU H, LEI J, et al. HIF1α deletion facilitates adipose stem cells to repair renal fibrosis in diabetic mice. In Vitro Cell Dev Biol Anim. 2018;54(4):272-286. [32] BASSE AL, ISIDOR MS, WINTHER S, et al. Regulation of glycolysis in brown adipocytes by HIF-1α. Sci Rep. 2017;7(1):4052. [33] WEISZENSTEIN M, MUSUTOVA M, PLIHALOVA A, et al. Adipogenesis, lipogenesis and lipolysis is stimulated by mild but not severe hypoxia in 3T3-L1 cells. Biochem Biophys Res Commun. 2016;478(2):727-732. [34] KAKUDO N, MORIMOTO N, OGAWA T, et al. Hypoxia Enhances Proliferation of Human Adipose-Derived Stem Cells via HIF-1ɑ Activation. PLoS One. 2015;10(10):e0139890. [35] ZHOU F, DU J, WANG J. Albendazole inhibits HIF-1α-dependent glycolysis and VEGF expression in non-small cell lung cancer cells. Mol Cell Biochem. 2017;428(1-2):171-178. [36] CHEN J, GU Z, WU M, et al. C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1α via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res Ther. 2016;7(1):114. |
| [1] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [2] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [3] | Wang Haiying, Lü Bing, Li Hui, Wang Shunyi. Posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis: prediction of functional prognosis of patients based on spinopelvic parameters [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1393-1397. |
| [4] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [5] | Gu Xia, Zhao Min, Wang Pingyi, Li Yimei, Li Wenhua. Relationship between hypoxia inducible factor 1 alpha and hypoxia signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1284-1289. |
| [6] | Wu Xun, Meng Juanhong, Zhang Jianyun, Wang Liang. Concentrated growth factors in the repair of a full-thickness condylar cartilage defect in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1166-1171. |
| [7] | Shen Jinbo, Zhang Lin. Micro-injury of the Achilles tendon caused by acute exhaustive exercise in rats: ultrastructural changes and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1190-1195. |
| [8] | Chai Le, Lü Jianlan, Hu Jintao, Hu Huahui, Xu Qingjun, Yu Jinwei, Quan Renfu. Signal pathway variation after induction of inflammatory response in rats with acute spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1218-1223. |
| [9] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [10] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [11] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [12] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [13] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [14] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [15] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||