Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (4): 600-606.doi: 10.3969/j.issn.2095-4344.2375
Previous Articles Next Articles
Material selection, theoretical design and biomimetic function of artificial periosteum
Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han
- First People’s Hospital of Changzhou, Changzhou 213000, Jiangsu Province, China
-
Received:2020-03-02Revised:2020-03-07Accepted:2020-04-18Online:2021-02-08Published:2020-11-23 -
Contact:Sun Han, Attending physician, First People’s Hospital of Changzhou, Changzhou 213000, Jiangsu Province, China -
About author:Chang Wenliao, Master candidate, Physician, First People’s Hospital of Changzhou, Changzhou 213000, Jiangsu Province, China -
Supported by:the Basic Applied Research Project of Changzhou, No. CJ20180063; the Youth Program of National Natural Science Foundation of China, No. 31800806
CLC Number:
Cite this article
Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
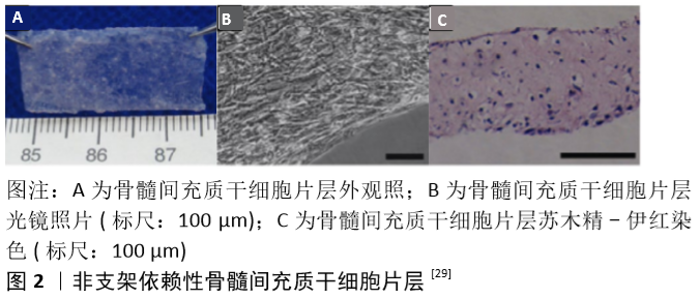
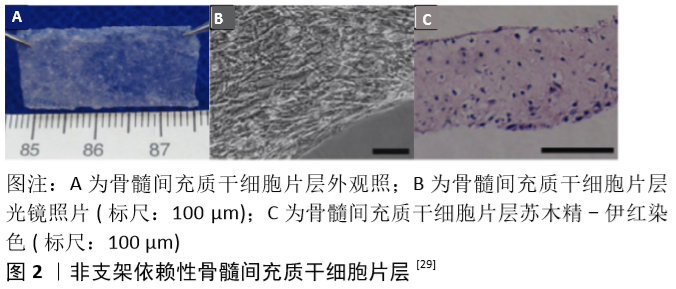
2.1 骨膜和骨折愈合 早期人们认为骨膜由特化的结缔组织组成,通过胶原纤维附着在皮质骨的表面,成为一层薄而坚韧的膜。1739年,DUHAMEL发现骨干的直径与树干相类似,是通过在外表面沉积一层又一层的新生组织进行生长的,而骨膜深面的作用则类似于树的生发层,故他亦将之命名为生发层[6]。不久之后,ITO等[7]证实了骨膜的内层在骨形成过程中起到了至关重要的作用。现在人们已清楚地知晓骨膜大体上可分为2层:外层为纤维层,主要由成纤维细胞胶原纤维组成;内层为生发层,拥有众多细胞及生长因子以用于修复[8-9]。此外,骨膜内还有众多血管穿行[10-11],纤维层内动脉和伴行静脉在骨周围形成密网状血管层,而生发层血管网则相对较细且稀疏,沿长轴走行,二层血管间有吻合支相连,并共同为骨组织供血。 骨折发生后首先会形成血肿,接着趋化作用将通过生长因子、细胞因子和白细胞介素的释放被激活。位于骨膜内层的细胞开始增殖和分化,在血供充足的骨折部位远端形成新生骨。然而在血供不足的骨折部位则首先形成软骨,随后引发新血管生成,通过软骨内成骨作用导致血液供应增加、软骨吸收和新骨形成[12]。数项体内研究表明,当去除骨膜时骨修复受到显著影响,而自体骨膜移植则可促进骨愈合[13-16]。另有研究表明,骨膜细胞在自体骨移植的愈合中起着关键作用[17-18]。由此可见,骨膜除了能提供血供及营养外,还可通过介导膜内成骨及软骨内成骨等机制参与骨再生,因此在天然骨生长、骨折愈合、骨再生过程中均扮演重要的角色。 2.2 人工骨膜材料 已有众多研究表明,损伤或完全切除骨膜将增加骨折不愈合的风险[19]。然而,一些开放性损伤的患者骨折处的骨膜及相关软组织经常缺失;众多骨科医生在进行一些复杂骨折的手术时易过度剥离骨膜以方便暴露及复位;肿瘤及感染等疾病的治疗过程中术者也常为了充分清除病灶而去除骨膜。此外,目前临床治疗骨缺损所常用的非自体移植物,如同种异体骨及人工骨等,也存在忽略了骨膜组织这一弊端[20]。上述诸种情形均易导致伤处较大面积的骨膜缺损,且难以直接修复,最终导致骨质难以完全恢复,成为了临床所面临的一大挑战。 目前临床上较常使用的治疗方法为自体骨膜移植及同种异体骨膜移植,但自体骨膜移植常受限于有限的来源及患者的体质,且易引起采集部位深部组织感染及慢性疼痛[21-22];而同种异体骨膜移植又面临着免疫排斥反应及易传播病原体等弊端[23-25]。为解决这些问题,基于骨膜的组织特性运用工程学方法构建出高效能人工骨膜的技术应运而生。这些人工骨膜材料主要包括支架、生长因子、细胞3大要素,并通过3者间的相互作用最大限度地模拟天然骨膜的功能[26-27]。人造骨膜不仅具有较小的免疫原性,也避免了自体或异体骨膜采集相关并发症,更具有丰富且便利的材料来源。 2.2.1 细胞片层类人工骨膜材料 细胞片层类人工骨膜是较早被研究的一类人工骨膜材料[28],其主要通过体外培养目标细胞使其增殖融合,并诱导产生各种细胞外基质,从而形成含有细胞外基质的一层完整且强健的组织片层[29]。与自体组织移植不同,细胞片层技术仅需要通过微创操作提取少量细胞,对机体损伤较小。这种技术目前主要用于心肌及角膜等组织的修复再生治疗。骨膜是覆盖在骨表面的一层包膜组织,因此在骨缺损部位或再生支架周围包覆多能干细胞片层模拟骨膜组织无疑是一种简单便捷的方法[30]。 常见的细胞片层类材料需要依附于外源性支架进行细胞培养[28,30],但这一方式易受细胞黏附能力的影响。MA等[29]则开发出一种非支架依赖性的骨髓间充质干细胞片层制备技术(图2),其主要是借助体外连续培养系统进行细胞培养,并采用刮取法达成细胞片层的收集。随后他们通过显微镜观察、碱性磷酸酶活性测定及反转录-聚合酶链式反应等测定了骨髓间充质干细胞片层的成骨潜能,并发现其有着出色的兔下颌骨缺损修复能力。 "
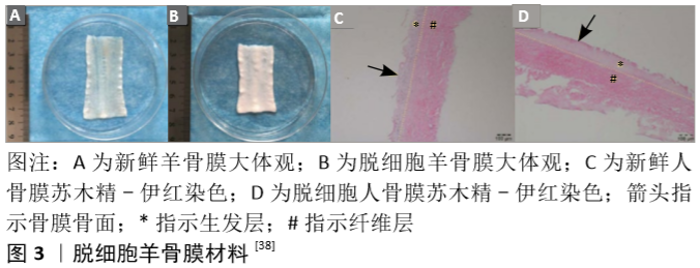
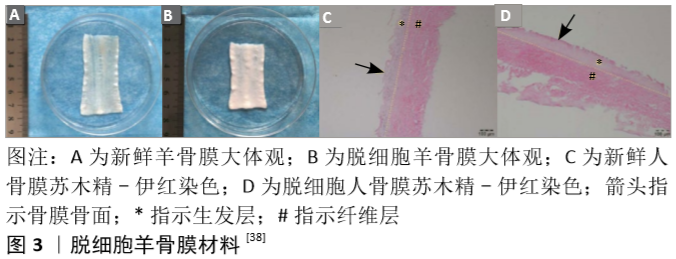
Okuda等[31]则进一步将细胞片层类骨膜材料应用到了牙周骨缺损患者的临床治疗中。他们取患者少量的下颌骨骨膜并提取其细胞在体外进行培养,待形成细胞片层后将其覆盖于负载了富血小板血浆的羟基磷灰石支架表面并植入骨缺损部位。在长达5年的随访中他们发现这种细胞片层类骨膜材料可以帮助患者获得出色的骨缺损愈合率及稳定性[32]。 2.2.2 脱细胞支架类人工骨膜材料 脱细胞组织的细胞外基质材料可在有效去除天然组织中具有免疫原性的细胞成分的基础上,保留其天然发生的内部三维支架结构,或保留有效成分,如结构蛋白成分(如胶原蛋白、弹性蛋白等)、特殊蛋白成分(如纤连蛋白、层粘连蛋白、原纤维蛋白等)、蛋白聚糖成分(如糖胺多糖、硫酸肝素、软骨素等)及各类生长因子成分,这些组织特有的内部结构和天然成分都是当前人工合成材料所无法完美复制的。 羊膜具有抑菌、抗血管生成、减轻疼痛、抑制炎症、抑制瘢痕形成、促进伤口愈合和上皮化等作用,其免疫原性低,富含多种胶原蛋白、血管内皮生长因子和血小板源性生长因子等多种生长因子。目前羊膜已被应用于烧伤创面治疗的临床研究,而脱细胞羊膜也被用于软骨、肌腱或周围神经导管等相关疾病的治疗[33]。此外,由于常被认为是“医疗废物”,羊膜的来源极其丰富,其应用成本也相应变低。GHANMI等[25]探讨了脱细胞新鲜人羊膜作为骨膜代用品对严重骨缺损患者骨再生的影响,他们从脉络膜中分离出羊膜,清除所有血样残留,然后分别置于含有青霉素、链霉素、妥布霉素和两性霉素B的林格液中处理2 h。他们分别用脱细胞新鲜人羊膜和空置法处理40只胫骨干骨缺损的新西兰大白兔,8周后结果显示脱细胞新鲜人羊膜对骨形成在X射线片、CT和组织学等评价指标上均有显著促进意义,但与原生骨膜相比仍不能令人满意。KOUSHAEI等[34]的研究则显示,羊膜治疗骨缺损的成骨效果要优于胶原蛋白膜。这些结果表明,羊膜在骨膜替代材料方面具有潜在的临床应用价值。 为进一步提高材料的骨膜仿生程度,QIU等[35]以猪骨膜为原料,反复冻融3次后剁碎,Triton X-100处理后再加入十二烷基硫酸钠及脱氧核糖核酸酶制得脱细胞骨膜基质,冻干后研磨成粉并加入醋酸溶液及胃蛋白酶粉,并采用NaOH调整pH值至7.40后形成脱细胞骨膜基质水凝胶,这种脱细胞骨膜基质水凝胶具有纤维网状结构,并保留了黏多糖及多种蛋白成分。他们希望这种材料能促进巨噬细胞的趋化和M2极化,从而促进骨修复。M2巨噬细胞可以分泌组织修复信号,如白细胞介素10、转化生长因子β、骨形态发生蛋白2、血管内皮生长因子等,从而促进间充质祖细胞的招募及新生血管生成,诱导成骨和成软骨分化[36]。然而在复杂和严重的骨损伤部位,大量的细胞毒性T细胞和高水平的白细胞介素1、肿瘤坏死因子等促炎细胞因子持续存在,引起严重的促炎反应,继而阻碍了巨噬细胞M1到M2的相变,最终延缓骨再生进程。脱细胞骨膜基质水凝胶则可动态参与骨折修复过程的各个阶段,在骨损伤早期通过优化M1到M2的过渡及时终止促炎反应,并促进血管的形成和迁移,最终诱导成骨分化和矿化,促进体内骨再生。 HE等[37-38]则更为完整地模拟了骨膜的结构和成分。他们综合应用了传统工艺中单独使用的物理、化学及生物酶手段,采用多重冻融循环、离子和非离子洗涤剂、脱氧核糖核酸酶和核糖核酸酶等方法对羊骨膜进行脱细胞处理。由这种改良技术所制得的脱细胞骨膜其胶原纤维完整性没有受到损伤,镜下可以观察到与新鲜骨膜相近的纤维层及生发层结构(图3),同时其胶原和糖胺聚糖的含量也没有明显下降。将其植入兔颅骨缺损部位后不仅有效地阻止了纤维结缔组织的生长,还促进了骨再生。RAPP等[39]则采用传统生物技术将牛骨膜去除所有抗原物质,并在生发层进一步接种脂肪源性基质细胞或骨膜源性基质细胞以模拟天然骨膜的细胞成分,培养14 d后再加入血管内皮生长因子和骨形态发生蛋白2 ;随后对19只大鼠各制造2个5 mm直径的颅骨缺损后并将各组脱细胞骨膜与移植骨一并植入缺损处。他们的研究结果表明,脱细胞的骨膜可有效地作为支架承载脂肪源性基质细胞和骨膜源性基质细胞的黏附增殖,同时与单纯脱细胞骨膜或脱细胞骨膜/干细胞相比,脱细胞骨膜/干细胞/生长因子复合物能最为有效地改善大鼠颅骨缺损的愈合效果,且生长因子对脂肪源性基质细胞的诱导效果更好。 人体来源的脱细胞材料方面,SCH?NMEYR等[40]利用商品化的人脱细胞真皮(AlloDerm,Lifecell公司)作为支架模拟天然骨膜结构。人脱细胞真皮也具有双面结构,其基底面主要由Ⅳ型胶原和层粘连蛋白组成,而表面则由细胞外基质分子组成,并提供多个细胞结合位点。因此,SCH?NMEYR等[40]进一步将人脱细胞真皮和成骨细胞或间充质干细胞相结合,合成类骨膜材料治疗骨缺损。他们在人脱细胞真皮表面接种绿色荧光蛋白阳性的间充质干细胞并转染骨形态发生蛋白2,包裹同系小鼠的内收肌后置于下颌骨缺损处,3周后绿色荧光蛋白阳性的间充质干细胞仍然存在,且新生骨组织已经出现于肌肉和骨膜材料之间的界面;6周后骨缺损已成功愈合。该实验表明人脱细胞真皮可以用来构建新型人工骨膜,且这种骨膜可以释放细胞和具有成骨诱导作用的蛋白。 "

2.2.3 人工合成支架类骨膜材料 脱细胞基质骨膜材料虽免疫原性相对较小,但仍存在发生免疫排斥反应的可能,且增加了病原菌携入的机会,其组织原料获取也相对复杂。因此,更多研究者们正致力于采用人工合成支架制备组织工程骨膜材料。 单层人工合成支架骨膜材料:水凝胶是一类极为亲水的三维网络结构凝胶,其可溶胀和保有大量的水,便于负载细胞及因子,同时也因其特殊的结构被作为血管网络再生的首选材料[41]。水凝胶的制备常采用水溶性或亲水性的高分子作为原料,通过一定的化学交联或物理交联所得。XIN等[42]采用甲基丙烯酸酯与凝胶作为原材料,加入光引发剂后采用紫外线照射交联形成甲基丙烯酸酯凝胶支架,并以SiO2、CaO、P2O5为原料,用改良St?ber法制备生物玻璃纳米微球包封骨形态发生蛋白2后装载于水凝胶中构建出人工骨膜材料。这种材料可以在4周内持续缓释骨形态发生蛋白2,并在4周后继续缓释钙离子及硅离子。体内实验表明,早期释放骨形态发生蛋白2可诱导细胞成骨分化,而随后的钙、硅离子缓释除进一步诱导成骨外还可促进细胞的黏附,最终加速骨再生修复。HOFFMAN等[43-44]亦采用水凝胶作为支架,使用聚乙二醇基三嵌段共聚物作为原料,并加入细胞黏附序列Arg-Gly-Asp-Ser(RGD)通过氨基末端与丙烯酸酯-聚乙二醇-N-羟基琥珀酰亚胺偶联,形成可水解的水凝胶,并进一步包封间充质干细胞,将其作为仿生骨膜材料覆盖于同种异体移植物表面后植入小鼠体内。16周后与未处理的同种异体移植物相比,仿生骨膜组的新生血管量提高了2.4倍,软骨内骨形成提高了2.8倍,生物力学强度提高了1.8倍。尽管取得了良好的治疗效果,但作者发现包覆仿生骨膜的同种异体骨的软骨内成骨过程与自体移植相比有所延迟,若想应用于临床可能还需进一步改进。 除了水凝胶外,静电纺丝纤维也是制备人工骨膜的良好选择。静电纺丝是高分子流体静电雾化的特殊形式,其主要原理是在电场作用下针头处的液滴会由球形变为圆锥形(“泰勒锥”),并从圆锥尖端延展得到纳米级直径的聚合物细丝。这些纳米纤维相互交织成网状,其微观结构与骨膜纤维层相类似,且接近于天然细胞外基质结构,有助于细胞的生长[45]。LYU等[46]发现相同取向排列的静电纺丝纤维甚至能进一步加强骨髓间充质干细胞的成骨分化。此外,静电纺丝膜还是一种常用的载药支架。GONG等[45]采用聚己内酯/明胶混合溶液作为原料制备了静电纺丝膜,并负载中药淫羊藿苷,取得了良好的成骨诱导效果。近年来随着电纺工艺的提升,出现了许多更为复杂的体系,如SHI等[47]采用聚己内酯/明胶纤维及聚己内酯纳米丝混纺使体系能同时负载两种不同的药物,而WU等[48]及GONG等[49]则分别通过分层电纺技术及核壳结构纺丝技术进一步减缓了药物释放速度,这些新技术均进一步提了体系的性能,由此可见静电纺丝膜是一种极富潜力的人工骨膜支架。 然而,前述材料的一大弊端是未能良好地模拟天然骨膜的柔韧性及延展性。移植物良好的力学性能对较大面积骨缺损的重建至关重要。聚醚醚酮的弹性模量与天然骨皮质相仿,但其因具有生物惰性导致应用受限。ZHAO等[4]成功地合成了一系列含氟聚醚醚酮聚合物,并将其电纺成纳米纤维膜用作人工骨膜材料。由于聚醚醚酮类聚合物只能溶解在浓硫酸中,且不具有可纺性,因此他们采用无规磺化反应合成了磺化聚醚醚酮。在此基础上,将聚己内酯和磺化聚醚醚酮相混合后进行常规静电纺丝,制备了一种新型柔性纳米复合膜。磺化聚醚醚酮/聚己内酯复合膜比单纯的磺化聚醚醚酮膜具有更好的亲水性和延展性;蛋白吸附性检测结果表明,磺化聚醚醚酮/聚己内酯复合膜具有较高的生物活性;最后,得益于聚己内酯良好的亲水性,将材料浸没于Ca2+及PO3-富集溶液后磺化聚醚醚酮/聚己内酯复合膜表面沉积的矿化物分布较单纯磺化聚醚醚酮电纺膜更为均匀,预示着其良好的促成骨分化潜能,但遗憾的是文章并未针对这一点进行细胞及体内实验验证。 天然骨膜具有良好延展性的原因之一是干细胞对支架的力学刺激具有高度的敏感性,在应力刺激下它们产生的信号分子可发生显著变化,配合上骨膜内定向的胶原纤维和细胞排列,使得新生骨组织可以向特定的方向生长。为模拟这种结构,SHI等[50]首先在聚乳酸-羟基乙酸纳米薄片上创建了定向微槽结构。他们先以带微槽的聚二甲基硅氧烷作为模板,在其表面用聚乳酸-羟基乙酸-二氯甲烷溶液自旋涂覆聚乳酸-羟基乙酸纳米片,随后将聚乙烯醇浇铸到聚乳酸-羟基乙酸纳米片上,用镊子将聚乙烯醇层联合聚乳酸-羟基乙酸纳米片一并从聚二甲基硅氧烷模具中取出后放入磷酸盐缓冲溶液中溶解掉聚乙烯醇层,得到独立的聚乳酸-羟基乙酸纳米片(图4)。这种定向微槽结构使材料获得了类似天然骨膜调整细胞排列的能力,同时他们还发现该微槽结构能帮助聚乳酸-羟基乙酸薄片牢固地附着在各种组织工程支架上。在接下来的一个实验中,SHI等[51]结合了机械信号和拓扑信号,在体外制备了具有诱导干细胞成骨分化能力的支架。他们采用具有良好柔软性和延展性的石蜡膜,在其表面借助激光雕刻聚二甲基硅氧烷作为模板通过热压法构建定向微槽结构以调节细胞的生长方向和所施加的机械张力方向。随后在表面涂覆一层聚多巴胺层,以提高材料的生物相容性,并使细胞能够顺利附着和增殖。研究发现,在机械拉伸刺激和空间结构诱导的影响下,黏附于膜表面的脂肪间充质干细胞相较于未拉伸组表现出更高的成骨能力,同时也验证了天然骨膜的机械应力有利于干细胞的成骨分化和骨再生。 "

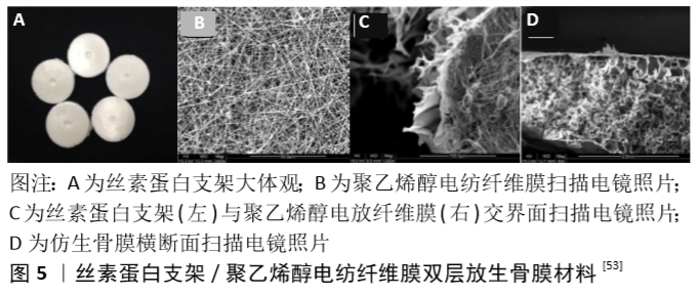
为了更进一步模拟天然的骨膜结构,SHI等[52]还开发了一种以骨膜为模板,在生物材料表面复制其精细的纳米微形态的创新方法。他们首先采用脱氧核糖核酸酶对鸡肱骨骨膜进行脱细胞处理并作为模板,在其表面浇筑聚二甲基硅氧烷获得镜像拓印模板,随后再将胶原溶液覆盖于聚二甲基硅氧烷模板表面并待其固化后获得胶原骨膜材料,其与天然骨膜具有相同表面条索状结构,结果显示这种胶原骨膜拓印材料对间充质干细胞的空间排列和增殖有很强的影响。为了评价其对骨修复的影响,作者将仿生人工骨膜负载干细胞及内皮细胞后包覆在多孔胶原/纳米生物活性玻璃复合支架上进行培养,且支架内载有成骨细胞。结果表明包裹了胶原骨膜拓印材料的支架其表面骨形成要明显优于无胶原骨膜拓印材料包裹组,这为骨膜组织工程提供了一种新的思路。 多层人工合成支架骨膜材料:如前所述,骨膜的天然结构包括纤维层、生发层及穿行血管,单采用一层支架常难以很好地还原骨膜这种多层结构,因此一些研究者尝试采用多层支架构建仿生物骨膜,以尽可能地模拟骨膜固有结构或进一步丰富其功能。 SU等[53]对丝素蛋白溶液进行冻干制备出丝素蛋白支架,并在其表面通过同轴共纺技术覆盖聚乙烯醇/地塞米松电纺纤维膜模拟骨膜天然结构(图5),将人工骨膜与干细胞共培养21 d后,可以检测到成骨基因和蛋白高表达,表明材料成功诱导干细胞成骨分化。ZHANG等[5]采用硅酸钙纳米丝、氧化石墨烯纳米片及壳聚糖作为原料,分别通过溶剂挥发诱导自组装法及冷冻干燥法构建出类纤维样薄膜及多孔状薄膜,以模拟骨膜的双层结构。这种胶原生物膜一侧致密光滑,可防止膜外侧的各类细胞对骨修复产生干扰,另一侧多孔粗糙,有利于骨缺损附近成骨细胞的黏附;此膜还具有出色的机械强度和降解速率,可以防止穿孔和破裂,以及降解过快所导致的骨再生空间无法得到有效维持,并可预防因此产生的炎症等并发症。 对于多层结构的人工骨膜,WANG等[54]提供了一种新的方法,他们利用聚己内酯、胶原蛋白和纳米羟基磷灰石作为原料,加入六氟异丙醇后采用静电纺丝技术制备出纳米纤维片层,并在上面负载骨髓间充质干细胞,随后将这种纳米纤维片层逐层堆叠构建出仿生人工骨膜。将该仿生骨膜包裹同种异体骨后移植修复小鼠股骨4 mm节段性骨缺损,6周后观察到了膜内成骨和软骨内成骨,且该骨膜材料显著改善了同种异体骨移植的愈合率和融合率。WANG等认为这种人工骨膜构建方式的一大优点是可以对每个细胞层进行修饰和调整,以模拟骨膜的特定微环境,从而为构建仿生骨膜替代物提供一个有效的平台,再现天然骨膜独特的细胞、分子、结构和功能特性。 骨膜内含有密集的血管网,为皮质骨供血,并且是骨形成期间细胞运输的通道。聚乙二醇肝素钠水凝胶系统已被发现是一种可供内皮细胞在体外形成毛细血管样结构的平台,该平台可以负载并缓慢释放血管内皮生长因子和纤维母细胞生长因子等多种促血管生成因子。BALDWIN等[55]采用3D打印技术制备聚己内酯多孔网格状支架,再以聚乙二醇肝素钠水凝胶覆盖聚己内酯支架上下两面,在上层水凝胶内负载人类脐静脉内皮细胞,下层水凝胶内负载骨髓间充质干细胞,植入小鼠体内30 d后组织切片结果显示人类脐静脉内皮细胞分化为血管并与宿主血管相连。虽然人类脐静脉内皮细胞的加入并没有增加人类骨髓间充质干细胞的细胞活力,但是增加了总的血管量。人类脐静脉内皮细胞成功地连接到宿主血管系统,成熟为功能血管,并形成了自己的基底膜蛋白。这一材料为人工骨膜的成血管化方向提供了新的思路。 CHOU等[56]认为理想的仿生骨膜在提供成骨诱导生长因子及促血管生成的同时,还应能抑制骨表面微生物感染,从而更好地促进骨愈合。他们将聚己内酯颗粒热熔后通过自制的微注射成型机和模具制作出中间为椭圆结构、两侧连接两个圆环的支架元件,并将支架元件通过圆环相互穿插编织制作成可降解支架模拟纤维层。接着他们将聚乳酸-羟基乙酸、利多卡因、万古霉素、头孢他啶相混合通过静电纺丝制作载药纳米纤维膜模拟生发层,随后对新西兰成年大白兔进行股骨节段性骨折造模,行髓内针固定,在骨折部位覆盖载药纳米纤维膜,然后用聚己内酯支架包裹。12周后结果显示,人工骨膜组骨折愈合率和愈后骨韧性明显高于对照组。该研究开发的载药聚乳酸-羟基乙酸/聚己内酯人工骨膜材料具有持续的药物洗脱能力,能在长时间内持续释放药物,有望为复杂的开放性骨折同时提供止痛、持续杀菌、促骨折愈合等多种治疗。 "

| [1] LIN X, ZHAO C, ZHU P, et al. Periosteum extracellular-matrix-mediated acellular mineralization during bone formation.Adv Healthc Mater. 2018;7(4):10.1002/adhm.201700660. [2] EL BACKLY RM, CHIAPALE D, MURAGLIA A, et al. A modified rabbit ulna defect model for evaluating periosteal substitutes in bone engineering: A pilot study.Front Bioeng Biotechnol.2015;2:80. [3] GUO H, LI X, YUAN X, et al. Reconstruction of radial bone defects using the reinforced tissue-engineered periosteum: An experimental study on rabbit weightbearing segment.J Trauma Acute Care Surg. 2012; 72(2):E94-E100. [4] ZHAO F, HU SH, WANG FJ, et al. A sulfonated peek/pcl composite nanofibrous membrane for periosteum tissue engineering application.J Mater Sci.2019;54(18):12012-12023. [5] ZHANG KR, GAO HL, PAN XF, et al.Multifunctional bilayer nanocomposite guided bone regeneration membrane.Matter.2019;1(3):770-781. [6] BILKAY U, TOKAT C, HELVACI E, et al. Osteogenic capacities of tibial and cranial periosteum: A biochemical and histologic study.J Craniofac Surg. 2008;19(2):453-458. [7] ITO Y, FITZSIMMONS JS, SANYAL A, et al. Localization of chondrocyte precursors in periosteum. Osteoarthritis Cartilage.2001;9(3):215-223. [8] BOMBALDI DE SOUZA RF, BOMBALDI DE SOUZA FC, THORPE A, et al. Phosphorylation of chitosan to improve osteoinduction of chitosan/xanthan-based scaffolds for periosteal tissue engineering. Int J Biol Macromol. 2020;143:619-632. [9] MIZUNO D, KAGAMI H, MIZUNO H, et al. Bone regeneration of dental implant dehiscence defects using a cultured periosteum membrane.Clin Oral Implants Res.2008;19(3):289-294. [10] ZHAO L, ZHAO J, YU J, et al. In vivo investigation of tissue-engineered periosteum for the repair of allogeneic critical size bone defects in rabbits. Regen Med. 2017;12(4):353-364. [11] EL BACKLY RM, ZAKY SH, MURAGLIA A, et al. A platelet-rich plasma-based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: A new concept for bone repair. Tissue Eng Part A. 2013;19(1-2):152-165. [12] HATTORI K, YOSHIKAWA T, TAKAKURA Y, et al. Bio-artificial periosteum for severe open fracture--an experimental study of osteogenic cell/collagen sponge composite as a bio-artificial periosteum.Biomed Mater Eng.2005;15(3):127-136. [13] MOORE SR, HEU C, YU NYC, et al. Translating periosteum’s regenerative power: Insights from quantitative analysis of tissue genesis with a periosteum substitute implant.Stem Cells Transl Med.2016;5(12): 1739-1749. [14] YAMAMIYA K, OKUDA K, KAWASE T, et al. Tissue-engineered cultured periosteum used with platelet-rich plasma and hydroxyapatite in treating human osseous defects.J Periodontol. 2008;79(5):811-818. [15] MIZUNO H, HATA KI, KOJIMA K, et al. A novel approach to regenerating periodontal tissue by grafting autologous cultured periosteum.Tissue Eng.2006;12(5):1227-1335. [16] WEHRHAN F, AMANN K, MOLENBERG A, et al. Critical size defect regeneration using peg-mediated bmp-2 gene delivery and the use of cell occlusive barrier membranes - the osteopromotive principle revisited.Clin Oral Implants Res.2013;24(8):910-920. [17] ZHANG X, AWAD HA, O’KEEFE RJ, et al. A perspective: Engineering periosteum for structural bone graft healing.Clin Orthop Relat Res. 2008;466(8):1777-1787. [18] HOFFMAN MD, BENOIT DSW. Emulating native periosteum cell population and subsequent paracrine factor production to promote tissue engineered periosteum-mediated allograft healing. Biomaterials.2015;52:426-440. [19] XING Q, QIAN Z, KANNAN B, et al. Osteogenic differentiation evaluation of an engineered extracellular matrix based tissue sheet for potential periosteum replacement.ACS Appl Mater Interfaces. 2015;7(41): 23239-23247. [20] ZHANG D, GAO P, LI Q, et al. Engineering biomimetic periosteum with β-tcp scaffolds to promote bone formation in calvarial defects of rats.Stem Cell Res Ther.2017;8(1):134-134. [21] ZHAO L, ZHAO J, WANG S, et al. Comparative study between tissue-engineered periosteum and structural allograft in rabbit critical-sized radial defect model.J Biomed Mater Res B Appl Biomater. 2011;97(1): 1-9. [22] CHUN YY, WANG JK, TAN NS, et al. A periosteum-inspired 3d hydrogel-bioceramic composite for enhanced bone regeneration Macromol Biosci. 2016;16(2):276-287. [23] LI N, SONG J, ZHU G, et al. Periosteum tissue engineering-a review.Biomater Sci. 2016;4(11):1554-1561. [24] FU TS, WANG YC, CHEN CH, et al. Engineered periosteum-bone biomimetic bone graft enhances posterolateral spine fusion in a rabbit model.Spine J.2019;19(4):762-771. [25] GHANMI S, TRIGUI M, BAYA W, et al. The periosteum-like effect of fresh human amniotic membrane on bone regeneration in a rabbit critical-sized defect model.Bone.2018;110:392-404. [26] YANG JW, PARK HJ, YOO KH, et al. A comparison study between periosteum and resorbable collagen membrane on iliac block bone graft resorption in the rabbit calvarium.Head Face Med. 2014;10:15. [27] YIN J, QIU S, SHI B, et al. Controlled release of fgf-2 and bmp-2 in tissue engineered periosteum promotes bone repair in rats.Biomed Mater.2018;13(2):025001-025001. [28] OUYANG HW, CAO T, ZOU XH, et al. Mesenchymal stem cell sheets revitalize nonviable dense grafts: Implications for repair of large-bone and tendon defects. Transplantation. 2006;82(2):170-174. [29] MA D, YAO H, TIAN W, et al. Enhancing bone formation by transplantation of a scaffold-free tissue-engineered periosteum in a rabbit model. Clin Oral Implants Res. 2011;22(10):1193-1199. [30] SYED-PICARD FN, SHAH GA, COSTELLO BJ, et al. Regeneration of periosteum by human bone marrow stromal cell sheets.Oral Maxillofac Surg.2014;72(6):1078-1083. [31] OKUDA K, YAMAMIYA K, KAWASE T, et al. Treatment of human infrabony periodontal defects by grafting human cultured periosteum sheets combined with platelet-rich plasma and porous hydroxyapatite granules: Case series.J Int Acad Periodontol.2009;11(3):206-213. [32] OKUDA K, KAWASE T, NAGATA M, et al. Tissue-engineered cultured periosteum sheet application to treat infrabony defects: Case series and 5-year results.Int J Periodontics Restorative Dent. 2013;33(3):281-287. [33] HE Q, LI Q, CHEN B, et al. Repair of flexor tendon defects of rabbit with tissue engineering method.Chin J Traumatol.2002;5(4):200-208. [34] KOUSHAEI S, SAMANDARI MH, RAZAVI SM, et al. Histological comparison of new bone formation using amnion membrane graft versus resorbable collagen membrane: An animal study.J Oral Implantol. 2018;44(5):335-340. [35] QIU P, LI M, CHEN K, et al. Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis.Biomaterials. 2020;227:119552. [36] JUBAN G, CHAZAUD B. Metabolic regulation of macrophages during tissue repair: Insights from skeletal muscle regeneration.FEBS Lett. 2017;591(19):3007-3021. [37] HE J, LI Z, YU T, et al. Preparation and evaluation of acellular sheep periostea for guided bone regeneration.J Biomed Mater Res A. 2020; 108(1):19-29. [38] HE J, LI Z, YU T, et al. In vitro and in vivo biocompatibility study on acellular sheep periosteum for guided bone regeneration.Biomed Mater.2020;15(1):015013. [39] RAPP SJ, JONES DC, GERETY P, et al. Repairing critical-sized rat calvarial defects with progenitor cell-seeded acellular periosteum: A novel biomimetic scaffold.Surgery. 2012;152(4):595-604,605.e591; discussion:604-595. [40] SCHöNMEYR B, CLAVIN N, AVRAHAM T, et al. Synthesis of a tissue-engineered periosteum with acellular dermal matrix and cultured mesenchymal stem cells.Tissue Eng Part A. 2009;15(7):1833-1841. [41] XIN T, GU Y, CHENG R, et al. Inorganic strengthened hydrogel membrane as regenerative periosteum.ACS Appl Mater Interfaces. 2017;9(47):41168-41180. [42] XIN T, MAO J, LIU L, et al. Programmed sustained release of recombinant human bone morphogenetic protein-2 and inorganic ion composite hydrogel as artificial periosteum.ACS Appl Mater Interfaces.2020;12(6):6840-6851. [43] HOFFMAN MD, XIE C, ZHANG X, et al. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing.Biomaterials. 2013;34(35):8887-8898. [44] HOFFMAN MD, BENOIT DSW. Emerging ideas: Engineering the periosteum: Revitalizing allografts by mimicking autograft healing.Clin Orthop Relat Res.2013;471(3):721-726. [45] GONG M, CHI C, YE J, et al. Icariin-loaded electrospun pcl/gelatin nanofiber membrane as potential artificial periosteum.Colloids Surf B Biointerfaces.2018;170:201-209. [46] LYU S, HUANG C, YANG H, et al. Electrospun fibers as a scaffolding platform for bone tissue repair.J Orthop Res.2013;31(9):1382-1389. [47] SHI R, GONG M, CHI C, et al. Nano twin-fiber membrane with osteogenic and antibacterial dual functions as artificial periosteum for long bone repairing.J Biomed Nanotechnol. 2019;15(2):272-287. [48] WU L, GU Y, LIU L, et al. Hierarchical micro/nanofibrous membranes of sustained releasing vegf for periosteal regeneration.Biomaterials.2020;227:119555-119555. [49] GONG M, HUANG C, HUANG Y, et al. Core-sheath micro/nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications. Nanomedicine. 2019;17:124-136. [50] SHI X, FUJIE T, SAITO A, et al. Periosteum-mimetic structures made from freestanding microgrooved nanosheets.Adv Mater. 2014;26(20): 3290-3296. [51] SHI X, LI L, OSTROVIDOV S, et al. Stretchable and micropatterned membrane for osteogenic differentation of stem cells.ACS Appl Mater Interfaces.2014;6(15):11915-11923. [52] SHI X, CHEN S, ZHAO Y, et al. Enhanced osteogenesis by a biomimic pseudo-periosteum-involved tissue engineering strategy.Adv Healthc Mater.2013;2(9):1229-1235. [53] SU WT, CHIOU WL, YU HH, et al. Differentiation potential of sheds using biomimetic periosteum containing dexamethasone.Mater Sci Eng C Mater Biol Appl.2016;58:1036-1045. [54] WANG T, ZHAI Y, NUZZO M, et al. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction.Biomaterials.2018;182:279-288. [55] BALDWIN JG, WAGNER F, MARTINE LC, et al. Periosteum tissue engineering in an orthotopic in vivo platform. Biomaterials. 2017;121: 193-204. [56] CHOU YC, CHENG YS, HSU YH, et al. A bio-artificial poly([d,l]-lactide-co-glycolide) drug-eluting nanofibrous periosteum for segmental long bone open fractures with significant periosteal stripping injuries.Int J Nanomedicine.2016;11:941-953. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Hu Kai, Qiao Xiaohong, Zhang Yonghong, Wang Dong, Qin Sihe. Treatment of displaced intra-articular calcaneal fractures with cannulated screws and plates: a meta-analysis of 15 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1465-1470. |
| [3] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [4] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [5] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [6] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [7] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [8] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [9] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [10] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [11] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [12] | Zhang Chong, Liu Zhiang, Yao Shuaihui, Gao Junsheng, Jiang Yan, Zhang Lu. Safety and effectiveness of topical application of tranexamic acid to reduce drainage of elderly femoral neck fractures after total hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1381-1386. |
| [13] | Wang Haiying, Lü Bing, Li Hui, Wang Shunyi. Posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis: prediction of functional prognosis of patients based on spinopelvic parameters [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1393-1397. |
| [14] | Lü Zhen, Bai Jinzhu. A prospective study on the application of staged lumbar motion chain rehabilitation based on McKenzie’s technique after lumbar percutaneous transforaminal endoscopic discectomy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1398-1403. |
| [15] | Chen Xinmin, Li Wenbiao, Xiong Kaikai, Xiong Xiaoyan, Zheng Liqin, Li Musheng, Zheng Yongze, Lin Ziling. Type A3.3 femoral intertrochanteric fracture with augmented proximal femoral nail anti-rotation in the elderly: finite element analysis of the optimal amount of bone cement [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1404-1409. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||