Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (29): 4728-4734.doi: 10.3969/j.issn.2095-4344.1796
Previous Articles Next Articles
Biological characteristics of perivascular cells and their supporting effects on hematopoietic stem/progenitor cells
Yang Xiaoping1, Zheng Bo2
- 1Graduate School of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 2Department of Hematology, General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China
-
Revised:2019-03-19Online:2019-10-18Published:2019-10-18 -
Contact:Zheng Bo, Chief physician, Professor, Master’s supervisor, Department of Hematology, General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
About author:Yang Xiaoping, Master candidate, Graduate School of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81560023 (to ZB)
CLC Number:
Cite this article
Yang Xiaoping, Zheng Bo. Biological characteristics of perivascular cells and their supporting effects on hematopoietic stem/progenitor cells[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(29): 4728-4734.
share this article
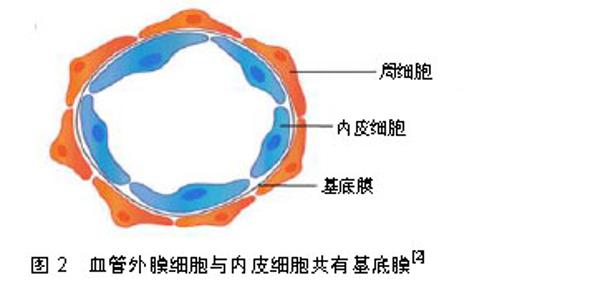
2.1 血管外膜细胞的分布 血管外膜细胞分布广泛,在所有脊椎动物的结缔组织、神经组织、肌肉、腺体、大脑等均有血管外膜细胞的存在[9],但不同组织中血管外膜细胞的胚胎起源存在差异[2,10]。鸡鹌鹑嵌合体分析和遗传谱系追踪实验证明面部、大脑和胸腺中的血管外膜细胞来源于神经嵴[10-13]。在大多数其他器官中,血管外膜细胞来源于中胚层[14]。使用类似的遗传谱系追踪实验显示肠道、肺和肝脏中血管外膜细胞起源于间皮,即单层鳞状上皮[10-11]。以上研究表明血管外膜细胞起源的异质性。 血管外膜细胞嵌入毛细血管壁与内皮细胞共有基底膜[15],见图2,并与内皮细胞共同构成了血管和组织间隙之间的屏障,如血脑屏障、视网膜血液屏障等。不同组织来源的血管外膜细胞具有调节内皮细胞功能的共同特性,但其分布密度随血管外膜细胞的解剖位点和功能差异而不同。中枢神经系统的血管外膜细胞与内皮细胞比例高于其他组织中的外周血管床[15],二者比例范围由1∶1(在视网膜和中枢神经系统中)到1∶100(骨骼肌中),这种分布似乎与血管通透性屏障、内皮细胞转化和血压有关系[16]。"
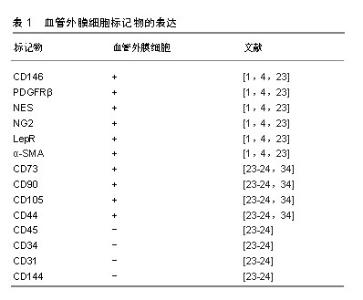
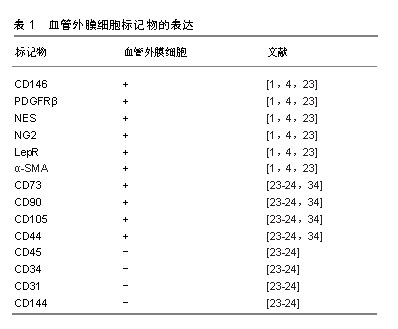
在不同的器官组织中,血管外膜细胞与内皮细胞也有不同的接触关系,如紧密连接、缝隙连接、钉-槽复合体和黏附斑等[3,16-17],这些接触有助于细胞之间信号的传递[18-19]。缝隙连接允许离子和小分子的交换;紧密连接点和黏附斑支持收缩力的传递,从而调节血管直径和血流[3];钉-槽复合体允许分子和离子在2种细胞的细胞质之间扩散[20]。血管外膜细胞与内皮细胞之间通过上述连接对血管的形成、稳定以及渗透性方面起到了至关重要的作用。 2.2 血管外膜细胞标记物 目前有许多标记物已经被应用于血管外膜细胞的鉴定,然而迄今为止,没有一个标记物是血管外膜细胞特异性的,因为大部分血管外膜细胞标记物也存在于其他细胞类型中[10]。目前常用于鉴定血管外膜细胞的典型特征性标记分子包括CD146黏附分子(M-CAM或MUC18)、神经胶质抗原2(neural glial antigen 2,NG2)、血小板源性生长因子β受体(platelet derived growth factor receptors,PDGFRβ)、α-平滑肌肌动蛋白、瘦素受体(leptinReceptor,LepR)、巢蛋白(nestin)[1,4],其他标志物如CD73、血小板源性生长因子α受体、结蛋白、G蛋白信号传导调节因子5(regulator of G protein signaling 5,RGS-5)和细胞表面神经节苷脂3G5也已被用于鉴定血管外膜细胞及其子集[1]。但是,上述这些标记分子的特异性不高,如血小板源性生长因子β受体在成纤维细胞、平滑肌细胞及神经胶质细胞中表达[14];而神经胶质抗原2蛋白聚糖可在巨噬细胞、神经胶质细胞和各种肿瘤细胞上表达[21]。而且,并非所有的血管外膜细胞都表达相同的标记,如神经胶质抗原2仅在动脉系统的血管外膜细胞中表达,而与毛细血管相关的血管外膜细胞缺乏α-平滑肌肌动蛋白[22]。血管外膜细胞在不同物种、组织或发育阶段表达不同的标记。然而,所有血管外膜细胞均表达CD146。因此,需要联合标记才能正确表达血管外膜细胞特征。这种表型异质性表明,围绕不同血管类型的血管外膜细胞也可能发挥不同的功能[23]。 2.3 血管外膜细胞的功能 血管外膜细胞广泛分布于全身组织的微血管壁中,是血管生成早期的主导细胞,可分泌碱性成纤维细胞生长因子和血管内皮生长因子,从而促进内皮细胞的迁移、聚集、增殖和分化,促进血管的芽生、塑形和延长[5,24]。 血管外膜细胞具有收缩性,通过调节毛细血管的管腔大小来控制血流量,防止血管过度扩张,保持血管完整性和渗透性[21],维持内环境的稳定和局部组织的代谢平衡[25]。在中枢神经系统中,血管外膜细胞与内皮细胞、星形胶质细胞构成血脑屏障,限制血液中的神经毒性物质进入中枢神经系统,同时将中枢神经系统中的代谢产物和神经毒性物质排出,维持大脑微环境的稳态,保证神经系统功能的正常发挥[26]。 血管外膜细胞也可通过调节淋巴细胞活化和吞噬活性来影响免疫功能,同时也是抗原递呈细胞,可诱导和调控T淋巴细胞[24,27]。 血管外膜细胞具有间充质细胞及干细胞潜能,从不同组织中分离的血管外膜细胞经体外培养可分化为多种细胞类型[3,5],如成骨细胞、成软骨细胞、脂肪细胞、树突状细胞、星形胶质细胞、少突胶质细胞[28]。在体内,血管外膜细胞也可参与各种组织和器官的修复和再生,并且可以进一步促进组织愈合。有研究者将血管外膜细胞注入急性心肌梗死大鼠心脏,血管外膜细胞的再生能力促进了心脏的修复[29],且血管外膜细胞通过调节心肌毛细血管的收缩性来控制心肌的有效血流灌注,参与心肌梗死后的重建过程[6]。但是,血管外膜细胞也可引发过度的纤维化、脂肪增生和沉积,甚至最终形成肿瘤[30]。 2.4 血管外膜细胞与间充质干细胞的关系 Friedenstein于1974年首先在骨髓中表述了间充质干细胞,这类细胞通常是指胚胎发育期在多种成体间叶组织中存留下来的未分化的原始细胞,其具有长期自我更新和多向分化的潜能,广泛分布于各种不同的组织中。间充质干细胞是在体外培养过程中获得的,其在体内的组织来源尚不明确[31],一直是人们研究的焦点。研究显示,不同组织来源的间充质干细胞主要位于其所在组织的血管壁周围[32-33],而血管外膜细胞分布于全身的毛细血管和微血管的管壁上,且血管外膜细胞亦具有多向分化潜能,部分学者推测,间充质干细胞和血管外膜细胞可能存在共同的起源[34]。在一项通过流式细胞仪进行严格细胞纯化并结合培养和体内分化的实验方法中证明人CD146+血管外膜细胞代表了普遍存在的间充质干细胞的前体细胞[35]。 在分离培养人体多个组织来源的间充质干细胞时发现,它们都表达血管外膜细胞表面标记物:神经胶质抗原2、CD146和血小板源性生长因子β受体,却均不表达任何已知的内皮细胞标记物CD144、vWF、CD31及造血细胞表面标记物CD34、CD45、CD14、CD19[24,36]。Crisan等[34]证实血管外膜细胞在培养前后依然保持间充质干细胞的表面标志物,如CD44、CD73、CD90和CD105,见表1。Corselli等[37]发现血管外膜细胞中有一部分细胞虽然表达神经胶质抗原2、CD146和血小板源性生长因子β受体,但不表达α-平滑肌肌动蛋白,他们推测这些血管外膜细胞或许是更原始的前体细胞,可能是间充质干细胞的前体细胞。Feng等[38]使用遗传谱系追踪技术,首次证实了在牙齿生长和损伤时血管外膜细胞能够分化为间充质干细胞-成牙本质细胞。这些研究提示在其他组织中血管外膜细胞也存在分化为间充质干细胞的可能性。"
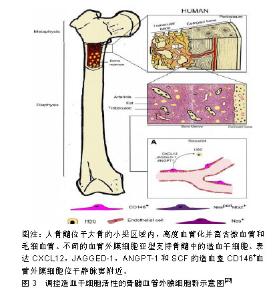
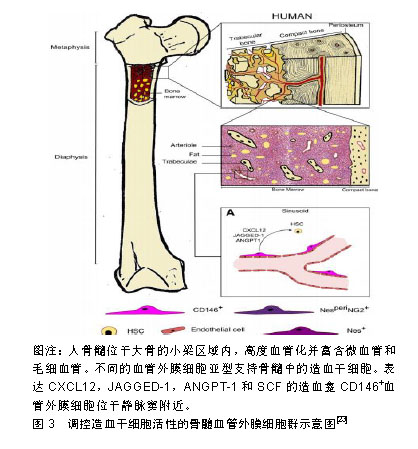
就发育潜能来讲,血管外膜细胞与间充质干细胞具有相似性,即在一定的诱导条件下可以分化成骨、软骨、脂肪细胞等中胚层谱系,在体内和器官培养实验中可以再生肌肉、骨、皮肤。一项研究显示,将牛视网膜血管外膜细胞植入裸鼠体内可分化成软骨细胞和脂肪细胞[34]。血管外膜细胞也可促进成骨再生[34,39],在成骨培养基中培养的血管外膜细胞表达碱性磷酸酶并表现出矿物质沉积,且可造成血管、心脏瓣膜和骨骼肌的病理性钙化。血管外膜细胞在骨骼肌损伤的免疫缺陷小鼠模型中显示出再生能力。将流式细胞术纯化的人骨骼肌源性血管外膜细胞注射到被心脏毒素损伤的NOD/SCID小鼠后肢肌肉中,能再生肌纤维,这种再生能力比CD56+人骨骼肌成肌细胞更显著。来自脂肪组织、胰腺和胎盘的血管外膜细胞注射到mdx/SCID或心脏毒素处理的NOD/SCID小鼠肌肉中时,它们促进血管生成和人血影蛋白或肌营养不良蛋白阳性肌纤维的再生[34,40-42]。将来自人体肌肉的血管外膜细胞移植到急性心肌梗死NOD/SCID小鼠心脏中,与未注射血管外膜细胞的对照组相比显著改善了心脏功能[20,43]。然而,心脏源性的血管外膜细胞不能分化成骨骼肌纤维,但是它们可以分化成其他中胚层谱系-脂肪细胞、成骨细胞和软骨细胞,这表明不同器官血管外膜细胞之间存在异质性[44]。 2.5 对造血干细胞的支持 机体的正常造血依赖造血干/祖细胞以及支持造血细胞生长发育的造血微环境-龛(Niche),这一概念最早由Schofield提出[45],造血干细胞龛是造血组织中造血干/祖细胞定居和调节干细胞活动、静止、自我更新和分化并能产生大量造血祖细胞的特定场所[46]。间充质干细胞作为机体的一种成体干细胞,最早在骨髓基质中发现,除可形成骨髓微环境外,还同基质细胞一样表达多种造血相关因子,促进造血干/祖细胞增殖、分化、成熟及进入血窦,并最终进入血液循环,在造血调控中发挥重要的作用。 在胚胎造血发生过程中,造血干/祖细胞从卵黄囊、主动脉-性腺-中肾区到胎盘、胚胎肝脏、脾脏,最后定位到骨髓,均在血管壁附近发生并发育,且这些组织中都会存在造血干细胞龛[45]。有研究显示,在成年小鼠骨髓中,位于血管壁上的血管外膜细胞参与形成造血干/祖细胞龛,而支持造血的间充质干细胞本身部分来源于血管外膜细胞[35]。研究显示血管外膜细胞是体内间充质干细胞的贮存池,因此越来越多的研究集中在血管外膜细胞在造血干/祖细胞调节中的作用。骨髓位于高度血管化的骨髓腔中,尤其富含由平滑肌细胞包绕且由薄壁神经支配的小动脉,以及分布均匀、管腔大且有孔的血窦,这些血窦允许骨髓和血流之间的细胞运输[47]。目前研究者使用以下不同的标记,如CD146、CXCL12、Nestin和瘦素受体(leptin receptor,LepR)等将小动脉血管外膜细胞和血窦血管外膜细胞群体作为造血龛的一部分进行研究,见图3。"
| [1]Shaw I, Rider S, Mullins J, et al. Pericytes in the renal vasculature: roles in health and disease. Nat Rev Nephrol. 2018;14(8):521-534.[2]Yamazaki T, Mukouyama YS. Tissue Specific Origin, Development, and Pathological Perspectives of Pericytes. Front Cardiovasc Med. 2018;5:78.[3]Bodnar RJ, Satish L, Yates CC, et al. Pericytes: A newly recognized player in wound healing. Wound Repair Regen. 2016;24(2):204-214.[4]Caporali A, Martello A, Miscianinov V, et al. Contribution of pericyte paracrine regulation of the endothelium to angiogenesis. Pharmacol Ther. 2017;171:56-64.[5]陈俊敏,张祥建,刘晓霞,等.周细胞与中枢神经系统疾病[J].中国卒中杂志, 2018,13(1):90-95.[6]毛鑫羽,马惠宁,徐士欣,等.周细胞在缺血性心脏病病理环节中的研究进展[J]. 中华老年心脑血管病杂志, 2016,18(7):756-758.[7]Caplan AI. New MSC: MSCs as pericytes are Sentinels and gatekeepers. J Orthop Res. 2017;35(6):1151-1159.[8]Mravic M, Asatrian G, Soo C, et al. From pericytes to perivascular tumours: correlation between pathology, stem cell biology, and tissue engineering. Int Orthop. 2014;38(9):1819-1824.[9]张弘,张志光.血管周细胞的研究进展[J].国际口腔医学杂志, 2013,40(4): 529-532.[10]Harrell CR, Simovic Markovic B, et al. Molecular mechanisms underlying therapeutic potential of pericytes. J Biomed Sci. 2018;25(1): 21.[11]Trost A, Schroedl F, Lange S, et al. Neural crest origin of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci. 2013;54(13): 7910-7921.[12]Reyahi A, Nik AM, Ghiami M, et al. Foxf2 Is Required for Brain Pericyte Differentiation and Development and Maintenance of the Blood-Brain Barrier. Dev Cell. 2015;34(1):19-32.[13]Trost A, Lange S, Schroedl F, et al. Brain and Retinal Pericytes: Origin, Function and Role. Front Cell Neurosci. 2016;10:20.[14]Dias Moura Prazeres PH, Sena IFG, Borges IDT, et al. Pericytes are heterogeneous in their origin within the same tissue. Dev Biol. 2017; 427(1):6-11.[15]Prazeres PHDM, Almeida VM, Lousado L, et al. Macrophages Generate Pericytes in the Developing Brain. Cell Mol Neurobiol. 2018; 38(4):777-782.[16]Mangialardi G, Cordaro A, Madeddu P. The bone marrow pericyte: an orchestrator of vascular niche. Regen Med. 2016;11(8):883-895.[17]Schrimpf C, Teebken OE, Wilhelmi M, et al. The role of pericyte detachment in vascular rarefaction. J Vasc Res. 2014;51(4):247-258.[18]Fernández-Klett F, Priller J. Diverse functions of pericytes in cerebral blood flow regulation and ischemia. J Cereb Blood Flow Metab. 2015; 35(6):883-887.[19]van Dijk CG, Nieuweboer FE, Pei JY, et al. The complex mural cell: pericyte function in health and disease. Int J Cardiol. 2015;190:75-89.[20]Ahmed TA, El-Badri N. Pericytes: The Role of Multipotent Stem Cells in Vascular Maintenance and Regenerative Medicine. Adv Exp Med Biol. 2018;1079:69-86.[21]Stallcup WB, You WK, Kucharova K, et al. NG2 Proteoglycan- Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression. Microcirculation. 2016;23(2):122-133.[22]Borysova L, Dora KA. The three faces of pericytes. J Physiol. 2018; 596(16):3453-3454.[23]Sá da Bandeira D, Casamitjana J, Crisan M. Pericytes, integral components of adult hematopoietic stem cell niches. Pharmacol Ther. 2017;171:104-113.[24]张磊,吴溯帆.周细胞的研究进展[J].中国美容整形外科杂志, 2017(7): 441-443.[25]徐立霞, 武俏丽.周细胞与阿尔兹海默病的相关研究进展[J]. 继续医学教育, 2018,28(7):127-129.[26]李芮琳,胡利民,王少峡,等.周细胞在脑血管疾病中的细胞功能研究现状[J].中国临床药理学杂志, 2018,34(3):390-392.[27]Hung CF, Mittelsteadt KL, Brauer R, et al. Lung pericyte-like cells are functional interstitial immune sentinel cells. Am J Physiol Lung Cell Mol Physiol. 2017;312(4):L556-L567.[28]Guimarães-Camboa N, Cattaneo P, Sun Y, et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell. 2017;20(3):345-359.e5.[29]Xu J, Gong T, Heng BC, et al. A systematic review: differentiation of stem cells into functional pericytes. FASEB J. 2017;31(5):1775-1786.[30]Underly RG, Levy M, Hartmann DA, et al. Pericytes as Inducers of Rapid, Matrix Metalloproteinase-9-Dependent Capillary Damage during Ischemia. J Neurosci. 2017;37(1):129-140.[31]Murray IR, Péault B. Q&A: Mesenchymal stem cells - where do they come from and is it important. BMC Biol. 2015;13:99.[32]De Souza LE, Malta TM, Kashima Haddad S, et al. Mesenchymal Stem Cells and Pericytes: To What Extent Are They Related. Stem Cells Dev. 2016;25(24):1843-1852.[33]Hardy WR, Moldovan NI, Moldovan L, et al. Transcriptional Networks in Single Perivascular Cells Sorted from Human Adipose Tissue Reveal a Hierarchy of Mesenchymal Stem Cells. Stem Cells. 2017; 35(5):1273-1289.[34]Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.[35]Corselli M, Chin CJ, Parekh C, et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121(15):2891-2901.[36]Fitzsimmons REB, Mazurek MS, Soos A, et al. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018;2018:8031718.[37]Corselli M, Chen CW, Crisan M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30(6): 1104-1109.[38]Feng J, Mantesso A, De Bari C, et al. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A. 2011;108(16):6503-6508.[39]König MA, Canepa DD, Cadosch D, et al. Direct transplantation of native pericytes from adipose tissue: A new perspective to stimulate healing in critical size bone defects. Cytotherapy. 2016;18(1):41-52.[40]Murray IR, Baily JE, Chen WCW, et al. Skeletal and cardiac muscle pericytes: Functions and therapeutic potential. Pharmacol Ther. 2017; 171:65-74.[41]Park TS, Gavina M, Chen CW, et al. Placental perivascular cells for human muscle regeneration. Stem Cells Dev. 2011;20(3):451-463.[42]Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255-267.[43]Chen CW, Okada M, Proto JD, et al. Human pericytes for ischemic heart repair. Stem Cells. 2013;31(2):305-316.[44]Chen WC, Baily JE, Corselli M, et al. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells. 2015;33(2):557-573.[45]Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334.[46]Wei Q, Frenette PS. Niches for Hematopoietic Stem Cells and Their Progeny. Immunity. 2018;48(4):632-648.[47]Itkin T, Gur-Cohen S, Spencer JA, et al. Corrigendum: Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;538(7624):274.[48]Pinho S, Lacombe J, Hanoun M, et al. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7): 1351-1367.[49]Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324-336.[50]He Q, Scott Swindle C, Wan C, et al. Enhanced Hematopoietic Stem Cell Self-Renewal-Promoting Ability of Clonal Primary Mesenchymal Stromal/Stem cells Versus Their Osteogenic Progeny. Stem Cells. 2017;35(2):473-484.[51]Butko E, Pouget C, Traver D. Complex regulation of HSC emergence by the Notch signaling pathway. Dev Biol. 2016;409(1):129-138.[52]Harkness L, Zaher W, Ditzel N, et al. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res Ther. 2016;7:4.[53]Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227-230.[54]Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977-988.[55]Tzeng YS, Li H, Kang YL, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117(2):429-439.[56]Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013; 495(7440):231-235.[57]Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829-834.[58]Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473): 637-643.[59]Han TJ, Wang X. Leptin and its receptor in hematologic malignancies. Int J Clin Exp Med. 2015;8(11):19840-19849.[60]Chen XX, Yang T. Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metab. 2015;33(5):474-485.[61]Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154-168.[62]Hu X, Garcia M, Weng L, et al. Identification of a common mesenchymal stromal progenitor for the adult haematopoietic niche. Nat Commun. 2016;7:13095.[63]Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891-903.[64]Zhou BO, Ding L, Morrison SJ. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1.Elife. 2015;4:e05521. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [4] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [5] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [6] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [7] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [8] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [9] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [10] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [11] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [12] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [13] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [14] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148. |
| [15] | Jiang Xin, Qiao Liangwei, Sun Dong, Li Ming, Fang Jun, Qu Qingshan. Expression of long chain non-coding RNA PGM5-AS1 in serum of renal transplant patients and its regulation of human glomerular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 741-745. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||