Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (29): 4644-4650.doi: 10.3969/j.issn.2095-4344.1803
Previous Articles Next Articles
Scleraxis combined with basic fibroblast growth factor promotes the differentiation of human amniotic mesenchymal stem cells into human ligament fibroblasts in vitro
Sang Peng, Liu Yi
- First Department of Orthopedics, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Revised:2019-04-17Online:2019-10-18Published:2019-10-18 -
Contact:Liu Yi, Professor, Master’s supervisor, First Department of Orthopedics, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Sang Peng, Master, Attending physician, First Department of Orthopedics, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China
CLC Number:
Cite this article
Sang Peng, Liu Yi. Scleraxis combined with basic fibroblast growth factor promotes the differentiation of human amniotic mesenchymal stem cells into human ligament fibroblasts in vitro[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(29): 4644-4650.
share this article

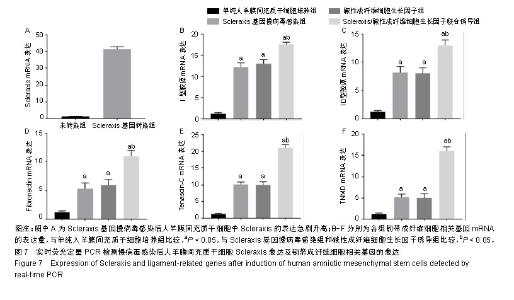
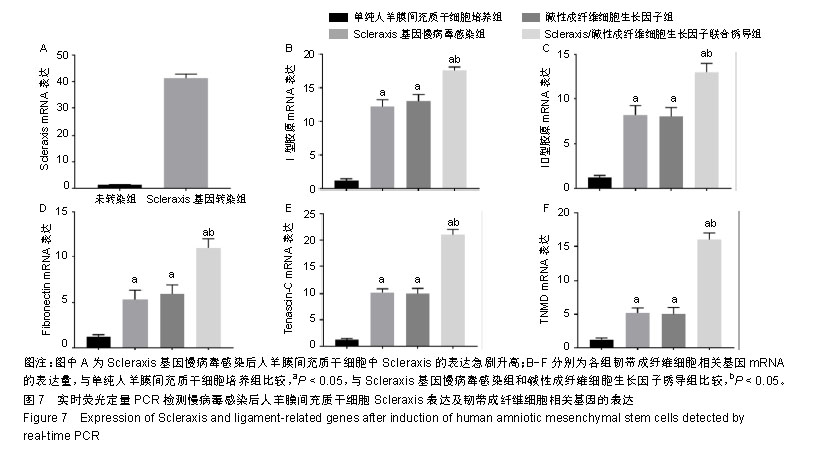
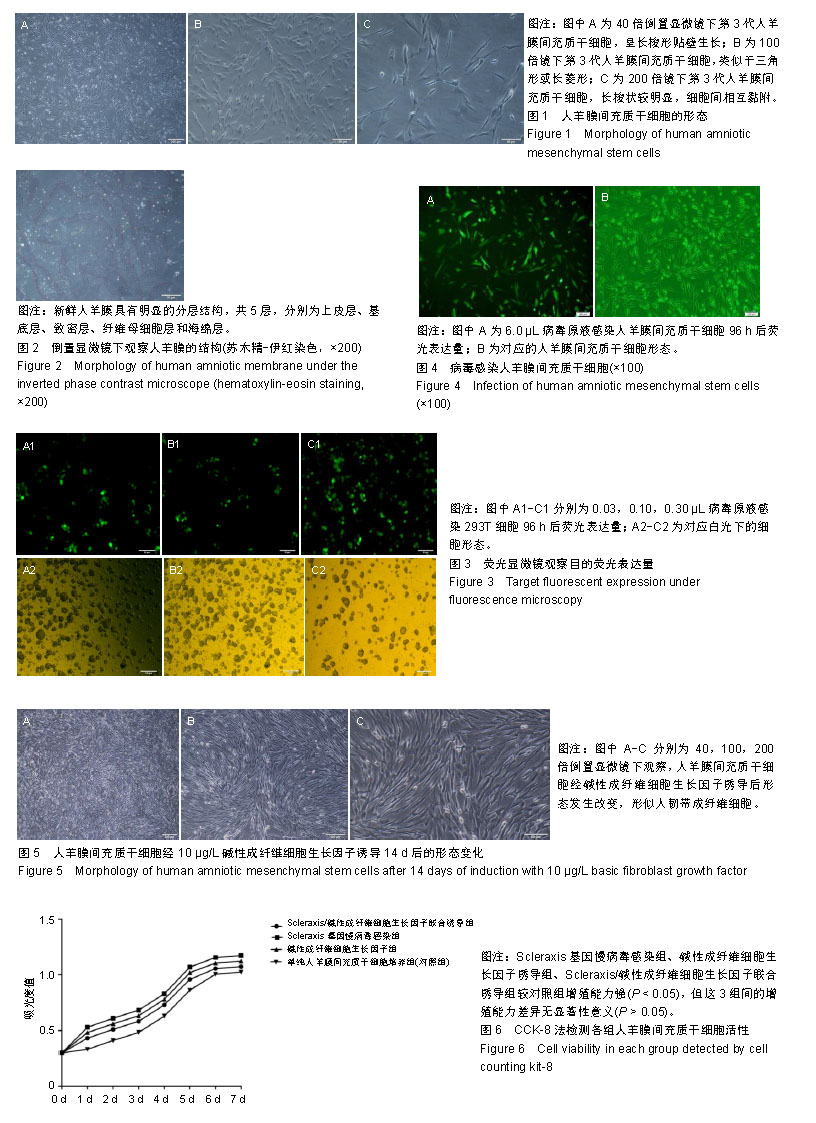
2.1 人羊膜间充质干细胞的形态 倒置相差显微镜显示:40倍镜下第3代人羊膜间充质干细胞呈长梭形贴壁生长,可见漩涡形成,见图1A;100倍镜下可见细胞接触紧密,类似于三角形或长菱形,见图1B;200倍镜下观察细胞长梭状明显,细胞间相互黏附,见图1C。 2.2 倒置显微镜下羊膜的结构 人新鲜羊膜经过切片、苏木精-伊红染色后在倒置显微镜下观察呈现明显的分层结构,一共分为5层,分别为上皮层、基底层、致密层、纤维母细胞层和海绵层,各层均比较明显且很完整,见图2。 2.3 慢病毒包装载体的构建、包装与滴度检测结果 病毒梯度法感染293T细胞96 h后,荧光显微镜观察目的荧光表达量:0.03 μL/孔表达量约50%,0.10 μL/孔表达量约80%,0.30 μL/孔表达量约95%,见图3。 2.4 病毒感染人羊膜间充质干细胞 6.0 μL病毒原液感染人羊膜间充质干细胞96 h后荧光显示感染率良好,见图4。 2.5 碱性成纤维细胞生长因子诱导人羊膜间充质干细胞 经过碱性成纤维细胞生长因子诱导的人羊膜间充质干细胞形态发生改变,14 d时最明显,细胞呈涡旋状,形似人韧带成纤维细胞,见图5。 2.6 CCK-8检测人羊膜间充质干细胞活性 各组细胞的增殖能力均呈S型生长,其中Scleraxis基因慢病毒感染组、碱性成纤维细胞生长因子诱导组、联合诱导组较对照组增殖能力强(P < 0.05),但这3组间的增殖能力差异无显著性意义(P > 0.05),见图6,说明Scleraxis、碱性成纤维细胞生长因子不仅能够促进羊膜间充质干细胞分化,而且还能够促进其增殖。 2.7 实时荧光定量PCR检测慢病毒感染人羊膜间充质干细胞后Scleraxis的表达及韧带成纤维细胞相关基因的表达 Scleraxis基因慢病毒能够成功感染人羊膜间充质干细胞,感染后Scleraxis mRNA的表达量急剧升高,是未转染组的40倍左右,差异有显著性意义(P < 0.05);Scleraxis基因慢病毒感染组、碱性成纤维细胞生长因子诱导组、联合诱导组韧带成纤维细胞相关基因mRNA的表达量均高于单纯人羊膜间充质干细胞培养组,差异有显著性意义(P < 0.05),联合诱导组韧带相关基因的mRNA表达量高于Scleraxis基因慢病毒感染组和碱性成纤维细胞生长因子诱导组,差异有显著性意义(P < 0.05),Scleraxis基因慢病毒感染组、碱性成纤维细胞生长因子诱导组韧带相关基因的表达差异无显著性意义(P > 0.05),见图7。"
| [1]Hinck AP, Mueller TD, Springer TA.Structural Biology and Evolution of the TGF-β Family. Cold Spring Harb Perspect Biol. 2016; 8(12): a022103.[2]Iliadis DP, Bourlos DN, Mastrokalos DS, et al. LARS Artificial Ligament Versus ABC Purely Polyester Ligament for Anterior Cruciate Ligament Reconstruction. Orthop J Sports Med. 2016;4(6):2325967116653359.[3]Spragg L, Chen J, Mirzayan R, et al. The Effect of Autologous Hamstring Graft Diameter on the Likelihood for Revision of Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2016;44(6):1475-1481.[4]Thompson SM, Salmon LJ, Waller A, et al. Twenty-Year Outcome of a Longitudinal Prospective Evaluation of Isolated Endoscopic Anterior Cruciate Ligament Reconstruction With Patellar Tendon or Hamstring Autograft. Am J Sports Med. 2016;44(12):3083-3094.[5]Proffen BL, Sieker JT, Murray MM. Bio-enhanced repair of the anterior cruciate ligament. Arthroscopy. 2015;31(5):990-997.[6]Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7(3):165-197.[7]Brancaleoni GH, Lourenzoni MR, Degrève L. Study of the influence of ethanol on basic fibroblast growth factor structure. Genet Mol Res. 2006;5(2):350-372.[8]Ide J, Kikukawa K, Hirose J, et al. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. J Shoulder Elbow Surg. 2009;18(3):391-398.[9]Yamamoto T, Wakitani S, Imoto K, et al. Fibroblast growth factor-2 promotes the repair of partial thickness defects of articular cartilage in immature rabbits but not in mature rabbits. Osteoarthritis Cartilage. 2004;12(8):636-641.[10]Chuma H, Mizuta H, Kudo S, et al. One day exposure to FGF-2 was sufficient for the regenerative repair of full-thickness defects of articular cartilage in rabbits. Osteoarthritis Cartilage. 2004;12(10):834-842.[11]Li X, Su G, Wang J, et al. Exogenous bFGF promotes articular cartilage repair via up-regulation of multiple growth factors. Osteoarthritis Cartilage. 2013;21(10):1567-1575.[12]Wang H, Zou Q, Boerman OC, et al. Combined delivery of BMP-2 and bFGF from nanostructured colloidal gelatin gels and its effect on bone regeneration in vivo. J Control Release. 2013;166(2):172-181.[13]Li Y, Liu Z, Jin Y, et al. Differentiation of Human Amniotic Mesenchymal Stem Cells into Human Anterior Cruciate Ligament Fibroblast Cells by In Vitro Coculture. Biomed Res Int. 2017;2017:7360354. [14]Carlberg AL, Tuan RS, Hall DJ. Regulation of scleraxis function by interaction with the bHLH protein E47. Mol Cell Biol Res Commun. 2000;3(2):82-86.[15]Hu JS, Olson EN, Kingston RE. HEB, a helix-loop-helix protein related to E2A and ITF2 that can modulate the DNA-binding ability of myogenic regulatory factors. Mol Cell Biol. 1992;12(3):1031-1042.[16]Liu Y, Watanabe H, Nifuji A, et al. Overexpression of a single helix-loop-helix-type transcription factor, scleraxis, enhances aggrecan gene expression in osteoblastic osteosarcoma ROS17/2.8 cells. J Biol Chem. 1997;272(47):29880-29885.[17]Alberton P, Popov C, Prägert M, et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012;21(6):846-858.[18]金瑛,李豫皖,张承昊,等.体外诱导人羊膜间充质干细胞向韧带细胞分化的实验研究[J]. 中国修复重建外科杂志, 2016,30(2):237-244.[19]Filioli Uranio M, Dell'Aquila ME, Caira M, et al. Characterization and in vitro differentiation potency of early-passage canine amnion- and umbilical cord-derived mesenchymal stem cells as related to gestational age. Mol Reprod Dev. 2014;81(6):539-551.[20]丁志,杨松林.间充质干细胞生物学特性及其分化潜能[J].中国组织工程研究与临床康复,2011,15(1):147-150.[21]周文然,李新,王文波,等.人羊膜间充质干细胞生物学特征及向多巴胺能神经元样细胞的分化[J]. 中国组织工程研究, 2014,18(23):3682-3690.[22]Maidhof R, Rafiuddin A, Chowdhury F, et al. Timing of mesenchymal stem cell delivery impacts the fate and therapeutic potential in intervertebral disc repair. J Orthop Res. 2017;35(1):32-40.[23]Nogami M, Kimura T, Seki S, et al. A Human Amnion-Derived Extracellular Matrix-Coated Cell-Free Scaffold for Cartilage Repair: In Vitro and In Vivo Studies. Tissue Eng Part A. 2016;22(7-8):680-688.[24]洪佳琼,高雅,宋洁,等.人羊膜间充质干细胞与骨髓间充质干细胞的生物学特性及免疫抑制作用的比较[J].中国实验血液学杂志, 2016,24(3):858-864. [25]Hong Y. ACL injury: incidences, healing, rehabilitation, and prevention: part of the Routledge Olympic special issue collection. Res Sports Med. 2012;20(3-4):155-156.[26]Zaffagnini S, Signorelli C, Bonanzinga T, et al. Technical variables of ACL surgical reconstruction: effect on post-operative static laxity and clinical implication. Knee Surg Sports Traumatol Arthrosc. 2016;24(11): 3496-3506.[27]Ahn JH, Lee YS, Ko TS, et al. Accuracy and Reproducibility of the Femoral Tunnel With Different Viewing Techniques in the ACL Reconstruction. Orthopedics. 2016;39(6):e1085-e1091. [28]Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128(19):3855-3866.[29]Cserjesi P, Brown D, Ligon KL, et al. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121(4):1099-1110.[30]Pryce BA, Brent AE, Murchison ND, et al. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236(6):1677-1682.[31]Mendias CL, Gumucio JP, Bakhurin KI, et al. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res. 2012;30(4):606-612.[32]Murchison ND, Price BA, Conner DA, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134(14):2697-2708.[33]Alberton P, Popov C, Prägert M, et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012;21(6):846-858.[34]Gulotta LV, Rodeo SA. Emerging ideas: Evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop Relat Res. 2011;469(10):2977-2980.[35]Gulotta LV, Kovacevic D, Packer JD, et al. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.[36]Longo UG, Lamberti A, Maffulli N, et al. Tissue engineered biological augmentation for tendon healing: a systematic review. Br Med Bull. 2011;98:31-59.[37]Durgam SS, Stewart AA, Pondenis HC, et al. Responses of equine tendon- and bone marrow-derived cells to monolayer expansion with fibroblast growth factor-2 and sequential culture with pulverized tendon and insulin-like growth factor-I. Am J Vet Res. 2012;73(1):162-170.[38]Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131(16):3885-3896.[39]陈志达,蔡弢艺,吴进,等. 成纤维细胞生长因子诱导骨髓间充质干细胞分化腱细胞的研究[J]. 中国骨与关节损伤杂志, 2017,32(8):819-822.[40]Chen CH, Cao Y, Wu YF, et al. Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg Am. 2008;33(10):1834-1842.[41]Thomopoulos S, Das R, Sakiyama-Elbert S, et al. bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 2010;38(2):225-234. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [12] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [13] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [14] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| [15] | Bi Qingwei, Liu Chengpu, Li Yan, Zhao Wenwen, Han Mei. Structure analysis of platelet-rich fibrin derived from two centrifugation procedures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3534-3539. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||