Chinese Journal of Tissue Engineering Research
Mechanism of Long-non-coding RNA-mediated Exercise Regulation of Bone tissue Autophagy
-
Online:2019-03-28Published:2019-03-28
CLC Number:
Cite this article
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
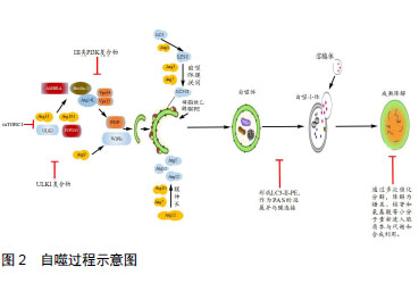
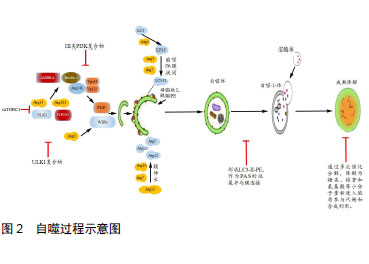
2.1 自噬发生机制 骨自噬分为:微自噬(microautophagy)、分子伴侣介导的自噬(chaperone-mediated autophagy, CMA)和巨自噬(macroautophagy)三种方式。自噬发生过程包括自噬诱导、自噬体形成和成熟降解三个阶段。细胞自噬在正常生理条件下会保持基础水平,而在遇到如运动刺激、饥饿、氧化应激等情况时,将开始自噬诱导。以上条件下,其上游原本活跃的mTOR被抑制,mTORC1活性降低,导致ULK1/2、mATG13的去磷酸化并组成mATG13-ULK1/2复合物,活化ULK1/2,活化的后者又磷酸化FIP200,进而启动自噬[8]。有研究表明,自噬启动时,不同于mTOR通过ULK1Ser757位点抑制ULK1的方式,AMP依赖蛋白激酶(AMP-dependent protein kinase,AMPK)可磷酸化ULK1 Ser317和Ser777位点,进而激活ULK1[9]。自噬诱导后,ATG14、Beclin1、Vps15和mVps34组成复合体启动成核反应,将ATG21、ATG24结合到膜上组成前自噬体(pre-autophagosomal structure, PAS)[10]。此处需要两种泛素化系统:ATG12-ATG5和LC3-II(ATG8同系物)-磷脂酰乙醇胺(phosphatidylethanolamine, PE)参与[11]。形成的自噬体经微管转运到溶酶体,外膜与溶酶体相融合,由水解酶完成整个自噬小体的成熟降解。此阶段中,自噬体内溶胶环境的酸化是自噬体与囊泡融合的必要步骤,且有Lamp2、小GTP蛋白Rab7和紫外线放射抗性相关蛋白(ultraviolet radiation resistance-associated gene protein, UVRAG) 等因子参与[12]。通过多次催化分解,降解为糖类、核苷和氨基酸等小分子重新进入胞质参与代谢和合成利用。"
2.2 骨自噬 2.2.1 BMSCs自噬 BMSCs具有自我更新和多向分化的特性和修复重建受损骨组织的功能,是治疗骨疾病的首选种子细胞,其自噬水平的变化直接影响成骨分化功能的发挥。有学者报道,骨自噬在提高BMSCs氧化应激存活率的同时,还能保持BMSCs活性的稳定[13]。研究显示,5%的低氧条件可通过抑制丝裂原活化蛋白激酶、激活PI3K/AKT/mTOR等通路,联合成骨分化诱导液促进BMSCs向成骨分化[14]。在缺氧模型中,白细胞介素8(Interleukin-8,IL-8)能通过Akt/STAT3通路提高人BMSCs自噬和增殖能力,降低缺氧缺血对人BMSCs造成的损伤[15]。与之相反,通过Western blot分别检测年轻小鼠和衰老小鼠LC3-Ⅱ、Beclin 1、p62蛋白表达发现,与年轻组小鼠相比,老年组小鼠BMSCs凋亡增加,LC3-Ⅱ、Beclin 1表达下降,p62蛋白表达水平升高,自噬信号通路相关蛋白p-Akt、Akt、p-mTOR、mTOR表达升高,骨量减少[16]。这表明在衰老和细胞器受损条件下,老年小鼠BMSCs自噬活性下降,凋亡增加,抑制BMSC成骨分化。有学者研究发现,氧化应激对BMSCs自噬具有双重作用,即在诱导其凋亡的同时亦诱导保护性自噬作用,抑制BMSCs自噬可加速其凋亡,增强其自噬能提高其在Ⅱ型糖尿病移植治疗中的存活率[17]。此外,mTOR能提高自噬活性较低的BMSCs自噬活性,减弱其向成骨细胞分化的潜力[18]。以上均提示自噬可能在BMSCs向OB分化的过程中扮演重要角色。 2.2.2 OB自噬 OB由BMSCs分化而来,通过分泌胶原、基质和酶类启动并主导骨形成过程。研究证明,自噬能正向调控OB分化和成熟。如糖尿病高糖环境过度产生活性氧,加速成骨MC3T3-E1细胞中的蛋白氧化和自噬,而自噬缺陷会导致线粒体形态学缺陷,抑制成骨细胞在高糖环境中的分化[19]。表明自噬是维持OB在高糖环境下活性和功能的重要机制。在观察颅骨成骨分化时,发现自噬流增加显著,这提示在自噬参与颅骨早期成骨分化。此外,OB中NF-kB信号通路激活需要其与巨噬细胞接触,且此过程受p62抑制,即当OB内p62缺陷将导致骨丢失,并影响OB中CCL4表达及造血祖细胞趋化性,最终引发造血干细胞(Hematopoietic stem cells, HSCs)流失[20]。而骨重建是一个连续的生理过程,需BMSCs持续向OB分化。而此过程需增强线粒体呼吸以保持能量源源不断供应,这必然导致内源性活性氧(ROS)堆积。FOXO3作为BMSCs向OB分化的氧化还原平衡器,一旦下降,将导致ROS升高,进而使BMSCs向OB分化减慢甚至停滞。与之相同的是,敲除ATG7抑制自噬后,BMSCs调控ROS能力下降,OB分化也随之降低。这提示FOXO3能通过诱导自噬抑制ROS,进而加速BMSCs向OB分化。后续研究发现,人BMSCs通过AMPK途径前期抑制mTOR,后期激活Akt/mTOR轴介导自噬促BMSCs向OB[21]。 2.2.3 OC自噬 OC是巨大多核细胞,由RANKL与OB产生的M-CSF激活融合而成,主导骨吸收[22]。研究表明,自噬能影响OC活性及功能的发挥。p62这一自噬特异性调控蛋白,是联结RANKL诱导的自噬和OC生成的桥梁,敲除p62不仅抑制OC中RANKL诱导的自噬活化,还抑制OC生成[23]。p62在佩吉特骨病中突变,引发OC体积增大,细胞活性升高。而p62是自噬调节关键mTOR通路的重要参与因子,这提示OC上的p62突变可影响OC自噬,以调节OC分化[24]。在人类类风湿关节炎中,TNFα可激活自噬使OC生成增加,而以药物抑制自噬可抑制OC分化,敲除TNFα转基因小鼠骨髓细胞中ATG7后发现,OC数目减少[25]。表明TNFα在诱导自噬过程中,与OC呈正相关。肿瘤坏死因子受体相关因子3(TNF receptor-associated receptor3,TRAF3)限制RANKL诱导OC生成,而TRAF3自噬性降解能加速OC生成[26]。由多杀巴氏杆菌毒素(Pasteurellamultocidatoxin,PMT)启动的mTOR信号活化在OC形成过程中起重要作用,可能通过下调程序性细胞死亡蛋白4(programmed cell death protein 4,PDCD4)表达,PDCD4是OC特异性基因转录因子c-Jun的抑制物,从而激活c-Jun,启动OC特异性基因的转录[27]。而缺氧应激通过HIF-1/BNIP3激活自噬,进而促进OC分化[28]。 2.3 LncRNA调控骨自噬的作用机制 转录生成非编码RNA(Non-coding RNA,ncRNA)的序列约占人类基因组的绝大部分,其中包括housekeeping非编码RNA、小分子非编码RNA、中等长度非编码RNA和LncRNA。LncRNA在细胞增殖分化、个体发育、信号转导、干细胞维持、代谢等重要生命活动中发挥关键调控作用。其在表观遗传水平、转录水平前后水平等方面具有控制基因表达的作用。此外,肿瘤、糖尿病、精神疾病和神经退行性疾病的发生都与LncRNA的异常表达有关[29]。研究发现,LncRNA在骨细胞的生长发育与分化过程中意义重大,以顺式和反式作用参与并作用于骨细胞分裂、剂量补偿和细胞分化等过程[30]。LncRNA的一级结构——核苷酸排列顺序(碱基互补配对)是LncRNA调节靶基因及其上下游基因转录翻译的基础。其作为ceRNA占据靶基因的结合位点,抑制下游的miRNA或mRNA与靶基因结合(也有学者将此行为称为靶基因模仿)[31]。这种负向调控下游miRNA的方式可能是LncRNA调控细胞自噬的重要途径,如与肿瘤发生相关的LncRNA生长阻滞特异转录物5被证明能结合糖皮质激素的DNA结合域,竞争糖皮质激素反应元件目的基因并调节其表达[32]。这种竞争结合靶基因位点的方式在肌肉分化中也有所体现,Linc-MD1通过碱基互补配对的方式与miR-133和miR-135结合,竞争性抑制二者与靶基因的结合,进而影响肌细胞自噬[33]。 通过查阅整理大量文献发现,有关LncRNA调控细胞自噬的研究多集中在肿瘤细胞、心肌细胞、脑细胞和心肌细胞等,且具体LncRNA如何调控上述细胞机制阐述尚不清晰,见表1。"
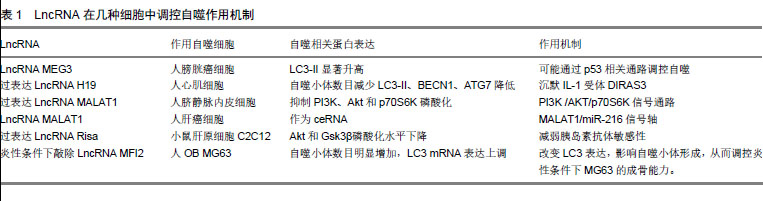

比较正常组织和膀胱癌组织时发现,后者中LncRNA MEG3表达显著降低、LC3-II显著升高。而其过表达则导致自噬水平降低,这表明LncRNA MEG3与LC3-II呈负相关。LncRNA MEG3可与ATG17相互作用以抑制自噬,而自噬蛋白p53作为调控LncRNA MEG3的靶基因。因此推断,LncRNA MEG3可能通过p53相关通路实现对细胞自噬[34]。在糖尿病性心肌病中,LncRNA H19通过沉默IL-1受体拮抗家族GTP酶3(DIRAS family GTPase 3,DIRAS3)在表观遗传水平上抑制自噬的发生[35]。过表达LncRNA H19发现,自噬小体数目减少,且LC3Ⅱ、BECN1、ATG7等自噬相关蛋白水平降低,这提示LncRNA H19参与抑制心肌细胞自噬。过表达LncRNA MALAT1会抑制PI3K、Akt和p70S6K磷酸化,下调重组人富集于脑Ras同系物(Recombinant Human Ras Homolog Enriched in Brain, RHEB)表达,提高低密度脂蛋白诱导的人脐静脉内皮细胞自噬水平[36]。而沉默LncRNA MALAT1能提高PI3K、Akt和p70S6K磷酸化水平,上调RHEB表达并显着抑制人脐静脉内皮细胞自噬,这表明LncRNA MALAT1能通过PI3K /AKT/p70S6K信号通路促进低密度脂蛋白诱导的人脐静脉内皮细胞自噬。而LncRNA MALAT1可作为ceRNA,通过与miR-216b结合抑制由5-FU处理后的miR-216b表达,从而增强自噬以调控肝癌细胞的耐药性[37]。 LncRNA MEG3、LncRNA H19和LncRNA MALAT1在骨组织中均有表达,且与骨代谢关系密切。据此推测,LncRNA可能通过上述的机制:作为ceRNA抢占miRNA结合位点,实现对骨组织细胞自噬的调控,最终影响骨代谢。目前LncRNA直接调控骨自噬的报道还较为鲜见,且为数不多的研究对其发生机制探讨尚不清晰。例如,过表达小鼠肝原细胞C2C12中LncRNA Risa会降低胰岛素抗体敏感性,Akt和Gsk3β磷酸化水平下降。而敲除LncRNA Risa导致C2C12和OB自噬水平升高[38]。这表明LncRNA Risa与OB呈负相关,却未涉及作用机制方面,LncRNA调控骨组织细胞自噬机制尚待补充。 2.4 LncRNA在运动调控骨自噬中的作用机制 骨组织作为力学敏感器官,机械应力刺激可能翻译成结构级联性生化变化,有效刺激骨细胞自噬。运动刺激可提高细胞自噬水平,且不同运动强度对细胞自噬水平影响存在差异,这在心肌细胞、骨骼肌细胞和部分脑细胞中已得到证明,尤以骨骼肌细胞和心肌细胞最为典型。研究发现,老龄大鼠在分别经过8周不同运动强度训练后,其骨骼肌中病理异常均减少,骨骼肌退化情况得以缓解。与此同时,相较于中高耐力训练组大鼠骨骼肌p53基因表达量显著增加而言,低耐力训练组几乎没有增加[39]。此外,离心运动可能通过上调Omi/Beclin-1通路增强骨骼肌自噬[40]。力竭运动可由AMPK/ULK1途径提高小鼠骨骼肌细胞自噬,后续的研究通过Compound C抑制AMPK活性发现,力竭运动运动仍可提高小鼠骨骼肌细胞自噬[41]。提示,除AMPK/ULK1以外,仍存在其它通路影响自噬或ULK1活性。而有氧运动可通过上调心肌细胞AMPK活性,抑制mTOR Ser2448位点磷酸化,进而改 善心肌细胞自噬能力,以防治衰老心肌细胞收缩舒张功能失调[42]。而mTOR已被证明可通过mTOR/ULK1通路增强OC自噬以抑制OC分化,降低骨吸收[43]。此外,在成骨细胞系MC3T3-E1中过表达IL-7可激活mTORC1信号以抑制OB成熟,而mTOR可抑制mTORC1 活性,逆转IL-7过表达导致的成骨细胞系MC3T3-E1成熟障碍[44]。因此,这种运动通过AMPK/mTOR轴调控心肌细胞自噬的机制,可能同样存在于骨组织细胞内,即运动可能通过AMPK先抑制mTOR调节的自噬,而后激活Akt/mTOR信号轴,调控OB或OC分化,最终影响骨代谢。Peng等的研究同样证明了这点, AMPK受miRNA调控,通过蛋白质印迹测定AMPK/PPARα和自噬相关基因蛋白质表达发现,miR-19通过靶向调节PPARα,负向调控AMPK /PPARα轴上AMPK以影响细胞自噬[45]。与此类似,通过基因芯片分析发现,miR-291b-3p在2型瘦素受体缺失的糖尿病模型小鼠肝脏中显著上调。荧光素酶报告载体显示,miR-291b-3p直接靶向AMPKα-1[46]。目前,有关运动调控LncRNA的报道还较为鲜见,且为数不多的研究在作用机制方面探讨尚不明晰。An等[47]研究发现,八段锦运动可通过CXCL8,DUSP2,OSM,CXCR4和NR4A2等mRNA作为关键节点调控LncRNA-mRNA网络,进而上调血清中与之关联的LncRNA RP13-516M14.10、LncRNA NEAT1、LncRNA CTD-2530H12.2、LncRNA CTD-3014M21.1和LncRNA AC068580.5表达。而自主轮转运动能使缺血/再灌注模型小鼠LncRNA MALAT1表达上调,保护其海马神经元免受缺血/再灌注损伤并促进认知恢复[48]。此外,中等强度的跑台运动可显著提高骨质松小鼠的骨密度,提高其骨生成速率和骨强度;同时可以对小鼠骨部分LncRNA产生影响,造成其表达的升高或降低,例如:LncRNA Gm31126、LncRNA Loc105243692、LncRNA Gm11651等上调明显,LncRNA 105247265等下调显著[7]。以上提示,运动可能通过影响LncRNA表达以影响骨自噬水平。此外,Peng等[49]研究发现,LncRNA H19可负向调控miR-675,通过Smad通路调控Runx2进而影响骨形成,或负向调控miR-141,通过Wnt/β-catenin促进骨形成。这为运动影响LncRNA,进而通过相关miRNA及其下游信号通路提供了依据。因此推测,LncRNA能在运动影响下表达上调或下调,负向调控miRNA,再通过AMPK/ULK1或AMPK /PPARα信号轴抑制下游Akt/mTOR信号通路,启动mTOR这一自噬关键因子,最终实现对骨自噬的调控。 "

| [1]Liu Y, Wang N, Zhang S, et al.Autophagy protects bone marrow mesenchymal stem cells from palmitate-induced apoptosis through the ROS-JNK/p38 MAPK signaling pathways[J]. Mol Med Rep, 2018,18(2):1485-1494.[2]Camuzard O, Santucci-Darmanin S, BreuilV, et al.Sex-specific autophagy modulation in osteoblastic lineage: a critical function to counteract bone loss in female [J]. Oncotarget, 2016,7(41):66416-66428.[3]Xu F, Li X, Yan L, et al.Autophagy Promotes the Repair of Radiation-Induced DNA Damage in Bone Marrow Hematopoietic Cells via Enhanced STAT3 Signaling[J]. Radiat Res, 2017,187(3):382-396.[4]Wang M, Han D, Yuan Z, et al.Long non-coding RNA H19 confers 5-Fu resistance in colorectal cancer by promoting SIRT1-mediated autophagy[J]. Cell Death Disease, 2018,9(12):1149.[5]Wang YG, Hu YN, Sun CX, et al.Down-regulation of Risa improves insulin sensitivity by enhancing autophagy[J]. FASEB J, 2016,30(9):3133-3145.[6]Yu X, Pang L, Yang T, et al.lncRNA LINC01296 regulates the proliferation, metastasis and cell cycle of osteosarcoma through cyclin D1[J]. Oncol Rep, 2018,40(5):2507-2514.[7]郭健民.运动对骨质疏松小鼠骨长链非编码RNA表达的影响[D].上海体育学院,2017.[8]Abrahamsen H, Stenmark H, Platta HW.Ubiquitination and phosphorylation of Beclin 1 and its binding partners: Tuning class III phosphatidylinositol 3-kinase activity and tumor suppression[J]. FEBS Lett, 2012,586(11):1584-1591.[9]Kim J, Kundu M, Viollet B, et al.AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1[J]. Nature Cell Biology,2011,13(2):132-141.[10]Feng H, Zhao X, Guo Q, et al. Autophagy resists EMT process to maintain retinal pigment epithelium homeostasis[J]. Int J Biol Sci,2019,15(3):507-521.[11]Zhang W, Chen K, Guo Y, et al.Involvement of PRRSV NSP3 and NSP5 in the autophagy process[J]. Virol J,2019,16(1):13. [12]Yamamoto F, Taniguchi K, Mamada N, et al.TFEB-mediated Enhancement of the Autophagy-lysosomal Pathway Dually Modulates the Process of Amyloid β-Protein Generation in Neurons[J]. Neuroscience,2019,40(2):11-22.[13]Liu Z, Li T, Zhu F, et al.Regulatory roles of miR-22/Redd1-mediated mitochondrial ROS and cellular autophagy in ionizing radiation-induced BMSC injury[J]. Cell Death Dis,2019,10(3):227.[14]Dong Y, Wu Y, Zhao GL, et al.Inhibition of autophagy by 3-MA promotes hypoxia-induced apoptosis in human colorectal cancer cells[J]. Eur Rev Med Pharmacol Sci,2019,23(3):1047-1054.[15]Shologu N, Scully M, Laffey JG, et al.Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury[J]. Int J Mol Sci,2018,19(10):E2996.[16]Akahane M, Shimizu T, Inagaki Y, et al.Implantation of Bone Marrow Stromal Cell Sheets Derived from Old Donors Supports Bone Tissue Formation[J]. Tissue Eng Regen Med,2017,15(1):89-100.[17]Wang H, Wang Z, Tang Q.Reduced expression of microRNA-199a-3p is associated with vascular endothelial cell injury induced by type 2 diabetes mellitus[J]. Exp Ther Med,2018,16(4):3639-3645.[18]Ka M, Smith AL, Kim WY.MTOR controls genesis and autophagy of GABAergic interneurons during brain development[J]. Autophagy,2017,13(8):1348-1363.[19]Bartolomé A, López-Herradón A, Portal-Nú, et al.Autophagy impairment aggravates the inhibitory effects of high glucose on osteoblast viability and function [J]. BiochemJ,2013,455(3):329-337.[20]Chang KH, Sengupta A, Nayak RC, et al. p62 is required for stem cell/progenitor retention through inhibition of IKK/NF- B/Ccl4 signaling at the bone marrow macrophage-osteoblast niche[J]. Cell Rep,2014,9 (6):2084-2097.[21]Gómez-Puerto MC, Verhagen LP, Braat AK, et al. Activation of autophagy by FOXO3 regulates redox homeostasis during osteogenic differentiation[J]. Autophagy, 2016,12(10):1804-1816.[22]Pierrefite-Carle V,Santucci-Darmanin S, BreuilV, et al. Autophagy in bone: Self-eating to stay in balance[J]. Ageing Res Rev,2015, 24 (2): 206-217.[23]Zhu YB, Jia GL, Lu JH, et al. The relationship between autophagy activation in spinal cord and type 2 diabetic neuropathic pain in rats[J]. Acta Physiologica Sinica,2018,34(4):318-323.[24]Helfrich MH, Hocking LJ. Genetics and aetiology of Pagetic disorders of bone[J]. Arch Biochem Biophys, 2008,473(2):172-182.[25]Lin NY, Beyer C, Giessl A, et al. Autophagy regulates TNF-mediated joint destruction in experimental arthritis[J]. Ann RheumDis, 2013,72 (5):761-768.[26]Xiu Y, Xu H, Zhao C, et al. Chloroquine reduced osteoclast genesis in murine osteoporosis by preventingTRAF3degradation[J].Clin Invest,2014,124(1):297.[27]Kloos B, Chakraborty S, Lindner SG, et al. Pasteurellamultocida toxin-induced osteoclast genesis requires mTOR activation[J].Cell Commun Signal,2015,13(1):40.[28]Zhao Y, Chen G, ZhangW, et al. Autophagy regulates hypoxiainduced Osteoclast genesis through the HIF-1/BNIP3 signaling pathway[J]. J Cell Physiol,2012,227(2): 639-648.[29]Yu C, Li L, Xie F, et al. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification[J]. Cardiovasc Res, 2018,114(1):703-711.[30]Ma l, Bajic VB, Zhang Z. On the classification of long noncoding RNAs[J]. RNA Biol,2013,10(6):925-933.[31]Wu DM, Wang S, Wen X, et al. LncRNA SNHG15 acts as a ceRNA to regulate YAP1-Hippo signaling pathway by sponging miR-200a-3p in papillary thyroid carcinoma[J]. Cell Death Dis,2018,9(10):947.[32]Novikova I V, Hennelly S P, Sanbonmatsu K Y. Sizing up long non-coding RNAs: Do LncRNAs have secondary and tertiary structure[J]. Bioarchitecture,2012, 2(6):189-199.[33]Legnini I, Morlando M, Mangiavacchi A, et al. A Feedforward Regulatory Loop between HuR and the Long Noncoding RNA linc-MD1 Controls Early Phases of Myogenesis[J]. Molecular Cell,2014,53(3):506-514.[34]Pawar K, Hanisch C, Palma SE, et al. DownRegulated lncRNA MEG3 eliminates mycobacteria in Macrophages via autophagy[J]. Sci Rep,2016,13(6):19416.[35]Zhuo CJ, Jiang RH, Lin XD, et al. LncRNA H19 inhibits autophagy by epigenetically silencing of DIRAS3 in diabetic cardiomyopathy[J]. Oncotarget,2016,8(1):1429-1437.[36]Li S, Pan X, Yang S, et al. LncRNA MALAT1 promotes oxidized low-density lipoprotein-induced autophagy in HUVECs by inhibiting the PI3K/AKT pathway[J]. J Cell Biochem,2019,120(3):4092-4101.[37]Yuan P, Cao WB, Zang QL, et al. The HIF-2α-MALAT1-miR-216b axis regulates multidrug resistance of hepatocellular carcinoma cells via modulating autophagy[J]. Biochem Biophys Res Comm,2016,478(3):1067-1073.[38]Wang Y, Hu Y, Sun C, et al. Down-regulation of Risa improves insulin sensitivity by enhancing autophagy[J]. FASEB J.,2016,30(9):3133-3145.[39]阮坚.不同强度耐力运动对老龄大鼠骨骼肌自噬的影响[D].湖南师范大学,2018.[40]Xu J, Jiao K, Liu X, et al. Omi/HtrA2 Participates in Age-Related Autophagic Deficiency in Rat Liver[J].Aging Dis,2018,9(6):1031-1042.[41]陈祥和.不同方式运动对Ⅱ型糖尿病小鼠骨代谢的影响及分子机制研究[D]. 华东师范大学,2016.[42]Zhang B, Zhang Y, La Cour KH, et al. Mitochondrial aldehyde dehydrogenase obliterates endoplasmic reticulum stress-induced cardiac contractile dysfunction via correction of autophagy[J]. Biochim Biophys Acta, 2013,1832(4):574-584.[43]Wu H, Wu Z, Li P, et al. Bone Size and Quality Regulation: Concerted Actions of mTOR in Mesenchymal Stromal Cells and Osteoclasts[J]. Stem Cell Reports, 2017,8(6):1600-1616.[44]孙自强. AKT/mTOR/ULK1介导的自噬在骨保护素调控破骨细胞骨吸收活性中的作用机制[D].扬州大学,2017.[45]Liu YM, Ma JH, Zeng QL, et al. MiR-19a Affects Hepatocyte Autophagy via Regulating LncRNA NBR2 and AMPK/PPARα in D-GalN/Lipopoly saccharide-Stimulated Hepatocytes[J].J Cell Biochem, 2018,119(1):358-365. [46]Meng X, Guo J, Fang W, et al. Liver microRNA-291b-3p promotes hepatic lipogenesis through negative regulation of adenosine 5'-monophosphate (AMP)-activated protein kinase[J]. J Biol Chem, 2016,291(20):10625-10634.[47]An T, He ZC, Zhang XQ, et al. Baduanjin exerts anti-diabetic and anti-depression effects by regulating the expression of mRNA, lncRNA, and circRNA[J].Chin Med, 2019,14(1):3.[48]Shang JL, Cheng Q, Duan SJ, et al. Cognitive improvement following ischemia/reperfusion injury induced by voluntary running-wheel exercise is associated with Lnc MALAT1-mediated apoptosis inhibition[J].Int J Mol Med, 2018 ,41(5):2715-2723. [49]Shuping P , Lihua C , Shiwei H , et al. An Overview of Long Noncoding RNAs Involved in Bone Regeneration from Mesenchymal Stem Cells[J]. Stem Cells International, 2018, 2018:1-11. |
| [1] | Shen Jinbo, Zhang Lin. Micro-injury of the Achilles tendon caused by acute exhaustive exercise in rats: ultrastructural changes and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1190-1195. |
| [2] | Chen Jiming, Wu Xiaojing, Liu Tianfeng, Chen Haicong, Huang Chengshuo. Effects of silymarin on liver injury and bone metabolism induced by carbon tetrachloride in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1224-1228. |
| [3] | Wang Mengting, Gu Yanping, Ren Wenbo, Qin Qian, Bai Bingyi, Liao Yuanpeng. Research hotspots of blood flow restriction training for dyskinesia based on visualization analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1264-1269. |
| [4] | Wang Yongsheng, Wu Yang, Li Yanchun. Effect of acute high-intensity exercise on appetite hormones in adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1305-1312. |
| [5] | Zheng Xiaolong, He Xiaoming, Gong Shuidi, Pang Fengxiang, Yang Fan, He Wei, Liu Shaojun, Wei Qiushi. Bone turnover characteristics in patients with alcohol-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 657-661. |
| [6] | Ma Zetao, Zeng Hui, Wang Deli, Weng Jian, Feng Song. MicroRNA-138-5p regulates chondrocyte proliferation and autophagy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 674-678. |
| [7] | Xie Yang, Zhang Shujiang, Liu Menglan, Luo Ying, Yang Yang, Li Zuoxiao. Mechanism by which rapamycin protects spinal cord neurons in experimental autoimmune encephalomyelitis mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 695-700. |
| [8] | Liu Bo, Chen Xianghe, Yang Kang, Yu Huilin, Lu Pengcheng. Mechanism of DNA methylation in exercise intervention for osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 791-797. |
| [9] | Zhao Xiang, Wei Cuilan, Zhang Yeting. Neurogenesis and neuroinflammation under exercise: alteration and regulation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 813-820. |
| [10] | Chen Ziyang, Pu Rui, Deng Shuang, Yuan Lingyan. Regulatory effect of exosomes on exercise-mediated insulin resistance diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 4089-4094. |
| [11] | Jiang Xiaoyan, Zhu Haifei, Lin Haiqi, Lin Wentao. Cold therapy promotes self-limited recovery of delayed-onset muscle soreness [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3609-3613. |
| [12] | Bai Shengchao, Gao Yang, Wang Bo, Li Junping, Wang Ruiyuan. Dynamic changes of mitochondrial function of the skeletal muscle after acupuncture intervention in rats with heavy load exercise-induced injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3648-3653. |
| [13] | Lu Jie, Li Xue, Wang Lu, Fan Jia, Zhang Yeting, Lu Xiaobin, Yuan Qiongjia. Effects of different-intensity swimming exercises on spatial learning and memory ability and the expression of Orexin A in the rat cerebellum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3697-3703. |
| [14] | Wang Zhen, Lin Haiqi, He Fei, Lin Wentao. Exercise activates skeletal muscle satellite cells: exercise prevention and treatment for age-related sarcopenia and muscle injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3752-3759. |
| [15] | Wang Chaoge, Weng Xiquan, Lin Baoxuan, Chen Lina, Xu Guoqin. Exercises under cold exposure change fat type and function in obese rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(20): 3162-3167. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||