Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (4): 597-605.doi: 10.3969/j.issn.2095-4344.1042
Previous Articles Next Articles
Application and research progress of platelet-rich plasma in bone tissue regeneration
Tang Qiyuan1, Ma Yaping2, 3, Zhang Bin4, Luo Nanning1, Wang Xin2, 3, Zhang Yi5
-
Online:2019-02-08Published:2019-02-08 -
Contact:Wang Xin, PhD, Associate chief physician, Department of Orthopedic Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Joint Orthopedics Research Center of Zunyi Medical University & University of Rochester Medical Center, Zunyi 563000, Guizhou Province, China Zhang Yi, PhD, Associate professor, School of Public Health, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Tang Qiyuan, The First Clinical Institute, Zunyi Medical University, Zunyi 563000, Guizhou Province, China Ma Yaping, Department of Orthopedics, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Joint Orthopedics Research Center of Zunyi Medical University & University of Rochester Medical Center, Zunyi 563000, Guizhou Province, China Tang Qiyuan and Ma Yaping contributed equally to this work. -
Supported by:the National Natural Science Foundation of China, No. 31760266 (to WX); the College Students Innovation Training Program of Zunyi Medical University, No. 201751052 (to TQY)
CLC Number:
Cite this article
Tang Qiyuan, Ma Yaping, Zhang Bin, Luo Nanning, Wang Xin, Zhang Yi. Application and research progress of platelet-rich plasma in bone tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(4): 597-605.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

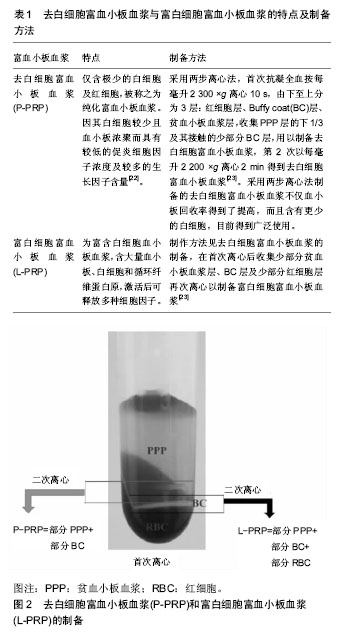
2.1 富血小板血浆制备方法、主要分类及特点 2.1.1 富血小板血浆制备方法及其作用 富血小板血浆由Harke等[9]于1977年首次分离,其制备过程十分简便,经过离心即可获得。方法为:取10 mL外周全血加入到10%柠檬酸钠溶液中,在室温下160×g离心 20 min,血液分为血清层和血细胞层,移取两者分界线下6 mm以上的溶液至新的无抗凝剂试管中,400×g离心15 min,得到上层的贫血小板血浆(platelet poor plasma,PPP)血清层和下层的富血小板血浆[10]。 富血小板血浆是经自体外周血离心获得的第一代血小板浓聚物,显著增加了所含血小板的浓度,其浓度可达全血所含浓度的四五倍,经激活后即可释放多种高浓度的生长因子[11-12],并快速形成一个富集生长因子的纤维血凝块。一方面填充骨缺损部位达到快速止血的作用,另一方面它是各种生长因子的自体来源,在促进细胞增殖和迁移方面具有积极的作用,从而促进修复骨缺损[13-14]。然而,在兔颅骨骨缺损模型中研究未激活富血小板血浆、CaCl2激活富血小板血浆以及骨形态发生蛋白2对颅骨的再生作用中发现,未激活的富血小板血浆更有利于伤口愈合、血管生成和纤维母细胞成熟[15],但是这些作用的分子机制尚不清楚。有研究为了证明局部提供的富血小板血浆是否能调节参与早期肌肉再生过程所涉及的特定分子,应用Wistar大鼠构建右屈肌机械性损伤模型,分为富血小板血浆治疗组和未治疗组,在术后第2天和第5天处死,取肌肉样本在分子水平上进行评估。结果显示富血小板血浆治疗组显著增加了促炎细胞因子白细胞介素1β和转化生长因子β1的mRNA水平,继而诱导了如肌原性调节因子(MyoD1、Myf5和Pax7)、胰岛素样生长因子1肌肉同种型(Insulin-like growth factors,IGF-1Eb)等的mRNA和(或)蛋白质水平的增加,也显著改善了NF-kB-p65和caspase 3凋亡参数等。结果表明富血小板血浆对骨骼肌损伤修复的影响是通过炎症分子和肌原性途径的调控,以及调控受myomiRNAs和热休克蛋白调控的二级通路,从而有助于适当的和有效的组织再生[16]。 富血小板血浆能刺激细胞增殖分化,促进软组织的修复[17],以及促进体外培养的成骨细胞及成骨样细胞增殖[18]。研究表明,富血小板血浆有很强的刺激毛细血管再生作用,对于促进骨创的早期愈合效果十分显著[19]。富血小板血浆因其来自自体,回输可避免免疫原性,富含生长因子,取材与制备简便,因而广泛在组织工程中研究及应用。在组织工程和再生医学中应用富血小板血浆,有可能帮助开发新的生物产品和技术,以利用患者自身的物质改善骨的愈合、再生和形成。 2.1.2 富血小板血浆主要分类及特点 根据其白细胞和纤维蛋白含量,富血小板血浆主要有4种一般类型:Leukocyte reduced PRP (P-PRP;leukocyte reduced or pure PRP)、Leukocyte-rich PRP (L-PRP)、Leukocyte platelet-rich fibrin(L-PRF)和Pure platelet- rich fibrin(P-PRF)[20],在后续研究和应用过程中进一步衍生出了多种形式,比如凝胶富血小板血浆、3 D结构的富血小板血浆、冻干富血小板血浆(freeze-dried PRP,FD-PRP)等。富血小板血浆的制备和激活方法不同可能导致其所含细胞、细胞因子等有所差异,从而具有不同的特性和发挥不同的效果。因此,有研究评估了富血小板血浆在不同的制备和激活方式下细胞因子释放情况,该研究以SS(Single-spin,SS法: 900×g 5 min)及DS (Double-spin,DS: 900×g 5 min,后1 500×g 15 min)两种方案进行制备富血小板血浆,结果表明SS方法制备的富血小板血浆含有低浓度的血小板和白细胞,而DS法制备的富血小板血浆的血小板和白细胞浓度则较高,而且以不同浓度的钙离子或凝血酶激活时,不同的激活剂剂量也会对细胞因子的释放和维持产生较大的影响,总体来说低剂量钙离子或凝血酶激活有利于提高细胞因子的含量[21]。下面主要描述去白细胞富血小板血浆(P-PRP)与富白细胞富血小板血浆(L-PRP)的制备和特点,见表1,见图2。"

在Filardo等[24]的研究中,与白细胞浓度较低的去白细胞富血小板血浆相比,尽管富白细胞富血小板血浆由于富含白细胞而具有一定的抗感染功效,然而在骨损伤部位同时也释放较高浓度的促炎细胞因子,导致大量金属蛋白酶产生,抑制细胞外基质的形成和促进其降 解[25],以及通过激活核因子κB信号通路诱导炎症递质的大量产生加剧炎症反应[26],从而不利于组织愈合。此外,富白细胞富血小板血浆对细胞和组织具有分解代谢作用,且在马腓肠肌腱移植体中诱导了最高水平的白细胞介素1β和肿瘤坏死因子α,增加了人软骨细胞的白细胞介素1β和白细胞介素6的水平[23]。相较于富白细胞富血小板血浆中的高水平促炎细胞因子白细胞介素1β和肿瘤坏死因子α激活核因子κB途径来抵消生长因子对骨再生的有益作用,去白细胞富血小板血浆对软骨再生的治疗作用可以通过去除白细胞以避免核因子κB通路的过度激活,而且去白细胞富血小板血浆可能主要发挥增强合成代谢的作用[27]。因此,含有少量浓缩白细胞的去白细胞富血小板血浆可能更适合于治疗骨组织损伤[28]。此外,在老鼠后外侧融合模型中冻干富血小板血浆支持早期骨愈合,冻干富血小板血浆处理组中与对照组(新鲜的富血小板血浆组及骨形态发生蛋白组)相比增加了骨形成和小梁骨重塑的数量,并证实了具有更多的小梁分支和生物力学刚性[29]。 2.2 富血小板血浆中主要血小板因子及其骨愈合作用 富血小板血浆中含有许多可溶性因子,其中包括促炎性因子、抗炎性因子和生长因子[20,23,30],如抗炎细胞因子白细胞介素4和α干扰素,促炎细胞因子肿瘤坏死因子α等在富血小板血浆二次离心后是增加的[31],这些可溶性因子能够刺激细胞趋化和成熟,是炎症反应和组织愈合过程的重要介质。 2.2.1 促炎因子 许多炎性因子,如白细胞介素1β、白细胞介素6、肿瘤坏死因子α等,在受伤数小时至数日都参与了骨缺损部位的炎症反应,它们和骨组织的愈合过程紧密相联[32]。 白细胞介素1β主要由巨噬细胞、中性粒细胞等分泌,是炎性反应主要的调控因子之一[33]。在小鼠骨折模型中,白细胞介素1β的分泌已被证实在24 h内达到高峰,它一方面促进血管和软骨组织的再生,另一方面还能触发级联反应,刺激下游的白细胞介素6等其他炎症因子的大量生成,抑制新骨形成[34]。另有研究表明,白细胞介素1β在高质量浓度(500 ng/L)时明显促进形成直径更小、密度更大、孔隙度更低的纤维凝块,该致密的凝块对骨缺损的愈合可能产生较大影响[35]。白细胞介素1β还能刺激滑膜细胞和软骨细胞合成过量金属蛋白酶,破坏软骨基质[36]。有研究显示富血小板血浆能够抑制白细胞介素1β诱导的软骨细胞凋亡,并促进胞外基质的合成代谢,表明富血小板血浆可能保护软骨细胞免于凋亡,可能成为治疗关节炎的潜在治疗策略[37]。 白细胞介素6由多种细胞如巨噬细胞、内皮细胞、T/B细胞等分泌,具有多种生物学功能,在机体内复杂的细胞因子调控网络中起着关键的作用[38],包括激活淋巴细胞(B/T细胞)、合成分泌急性期Ⅺ蛋白,介导炎症反应细胞信号通路等[39]。研究表明,将白细胞介素6直接添加50 μg/L RANKL诱导RAW264.7细胞分化的破骨细胞中,能够抑制成熟的破骨细胞分化,明显降低了骨溶解作用,当白细胞介素6联合骨保护素治疗时,能更有效地治疗骨质疏松症[40]。此外,在小鼠模型中,白细胞介素6的血清水平在骨折后是增高的,通过使用受体抗体MR16-1(rat anti-mouse IL-6 receptor antibody,MR16-1)来阻断白细胞介素6受体后,成骨细胞特异性基因mRNA表达上调,说明白细胞介素6对骨愈合作用的影响可能与调控成骨细胞特异性基因的表达相关[41]。白细胞介素6对成骨/破骨细胞有独特的双重调节作用。 肿瘤坏死因子α是由巨噬细胞、单核细胞等多种细胞分泌,在骨组织受损后短时间内高水平表达,通过趋化作用招募中性粒细胞和周围的细胞,并通过刺激CCL2产生促进单核细胞的招募,从而影响骨再生[42-43],以及刺激成纤维细胞生长和塑形的作用[44]。当骨折发生后,随着体内肿瘤坏死因子α分泌增加[41],成骨细胞的存活率降低[45]。此外,研究发现骨折后局部释放的高浓度肿瘤坏死因子α会抑制骨愈合,但是低剂量的肿瘤坏死因子α会通过上调固有免疫应答加强正常的和骨质疏松性骨愈合[46]。 2.2.2 抗炎性细胞因子 富血小板血浆在人骨关节炎的软骨和滑膜共培养系统中发挥了显著的抗炎作用,明显降低了组织中炎症标志物如ADAMTS-5、组织基质金属蛋白酶抑制剂 1的表达[47],可能与抗炎因子白细胞介素4、转化生长因子β1、白细胞介素1Ra和白细胞介素10的释放相关,这4种抗炎细胞因子均具有促进成骨细胞形成,促进骨愈合的效用。 白细胞介素4主要由活化的Th2细胞、肥大细胞产生,研究认为白细胞介素4能阻滞抗体依赖的细胞毒作用,负调控干扰素-γ(Interferon-γ,INF-γ)的产生[48]。鼠模型研究表明,体内骨量变化与白细胞介素4的表达显著相关[49]。此外,白细胞介素4还能增加B细胞和T细胞之间的相互作用,从而促进体液免疫[50],以减轻炎症反应的不良反应。白细胞介素4作为重要的Th2型细胞因子,可以抑制RANKL信号,导致破骨细胞生成,影响骨再生过程[51]。 转化生长因子β1是一种多功能蛋白质,具有调控多种细胞的生长、分化、凋亡及免疫调节等功能。体外研究表明,转化生长因子β1预处理可通过增加黏附力、牵引力和迁移来加快伤口的闭合[52]。在兔胫骨骨折模型中,连续应用剂量为1 µg/d和10 µg/d的转化生长因子β达6周,显示其增加了最大弯曲强度和结痂形成,表明局部应用外源性转化生长因子β有助于骨折愈合[53]。 白细胞介素1ra是抑制白细胞介素1的活性物质,与白细胞介素1受体结合后,本身无激动作用,可消除或减轻白细胞介素1的生物学效应,从而影响机体的病理生理过程。早期分化的巨噬细胞中释放较多的白细胞介素1Ra,而在晚期分化的巨噬细胞中释放较少,释放量少时可能与骨折延迟愈合相关[54]。 白细胞介素10是重要的抗炎细胞因子,由NK细胞、T/B细胞等分泌,可抑制Th1细胞产生INF-γ、肿瘤坏死因子α[55],可能参与类风湿性关节炎疾病的活动[56]。白细胞介素10还可以调控骨骼的吸收,白细胞介素10敲除小鼠显示出骨质疏松,脆弱的骨骼机械性,以及骨形成的缺陷[57]。 2.2.3 生长因子释放、黏附和成骨作用 富血小板血浆对骨骼的愈合有良好的效果,可能归因于其所释放的生长因子的调节[58]。CaCl2和凝血酶是制备富血小板血浆常用激活剂,富血小板血浆被激活后血小板中的α颗粒可通过胞吐作用释放多种生长因子,如血管内皮生长因子、血小板源性生长因子、转化生长因子β、胰岛素样生长因子、表皮生长因子等[59],其中血小板源性生长因子和转化生长因子β比例较大[60],这些因子都是已知的有利于成骨作用的[61]。 血管内皮生长因子在血管生成和骨生成中发挥重要作用[58],可通过调控血管内皮细胞的迁移、增殖和分化,从而支持、协调血管等结构的早期形成[62],是最有效的促血管生长因子,血管生成可能是富血小板血浆加速伤口愈合的途径[63]。此外,可刺激成骨细胞分化,是骨修复过程中关键的生长因子[64]。 血小板源性生长因子可由血小板、巨噬细胞、单核细胞、骨基质产生,包括3种形式:血小板源性生长因子AA、血小板源性生长因子AB、血小板源性生长因子BB[65]。研究显示富血小板血浆通过ERK1/2、PI3K/Akt和JNK信号通路促进人脂肪干细胞的增殖,而血小板源性生长因子BB在其中起着重要作用[66]。血小板源性生长因子是一种重要的促有丝分裂因子,具有刺激特定细胞群分裂增殖的能力[67],可作为激动因子刺激成骨细胞的趋化[68],促进骨再生作用,能调控干细胞迁移和增殖,在瘢痕形成、组织重塑过程中发挥重要作用[69-70]。血小板源性生长因子也可诱导间充质干细胞增殖并抑制白细胞介素1β诱导的软骨细胞凋亡和炎症[14]。 转化生长因子β来源于血小板、胞外骨基质、软骨基质等,有转化生长因子β1、转化生长因子β2、转化生长因子β3三种类型。转化生长因子β是重要的转化因子,与Ras/mitog活化蛋白激酶(Ras/mitogen-activated protein kinase,MAPK)、细胞外信号调节蛋白激酶和Rho/Rho关联的螺旋激酶途径相互作用,通过结合受体1和受体2,触发细胞内信号通路调节靶细胞反应,从而细胞外基质合成,影响瘢痕形成[71]。可诱导纤维母细胞和成骨细胞趋化和有丝分裂,调节细胞生长和成骨细胞增殖,诱导骨基质沉积[65],以及促进胶原蛋白合成[72],刺激成纤维细胞、成骨细胞合成和释放Ⅰ型胶原蛋白及纤维粘连蛋白[73],使胞外基质进行重塑,从而促进骨愈合[74]。转化生长因子β的减少可能与骨延迟愈合或骨不连相关[75]。 胰岛素样生长因子可由血小板、骨基质等产生,分为胰岛素样生长因子1和胰岛素样生长因子2。胰岛素样生长因子是影响细胞生长代谢的关键因子[76],在骨折愈合中是重要的局部调控者,尤其胰岛素样生长因子1,它的缺陷可能导致老年群体骨折延迟愈合或骨不愈合的风险增加;参与间充质细胞、骨膜细胞、成骨细胞、破骨细胞、软骨细胞的增殖和分化,调控骨基质形成;刺激成骨细胞和软骨细胞分泌血管内皮生长因子促进血管生成[65,77]。 2.3 富血小板血浆的临床应用 在口腔科、矫形外科等领域的骨缺损治疗中,富血小板血浆已被广泛运用。研究表明富血小板血浆具有成骨诱导作用,能显著促进损伤软组织的愈合及骨组织的再生,并且提高骨缺损的再生速度,缩短愈合时长,在临床治疗中具有广阔的应用前景。 2.3.1 在动物模型中的研究应用 在动物模型研究中显示,自体骨、自体骨髓或基质细胞与富血小板血浆相结合进行移植,可以加速骨骼的愈合和在长骨骨折后的重塑。但也出现了矛盾的研究结果,即在其他动物研究中发现单独使用富血小板血浆或者与自体骨联合移植的效果并不比单独使用自体骨移植的好,这也许是由于富血小板血浆的制备方法不同而导致所含成分的差异所造成[78-79]。在兔骨干骨缺损的模型研究中表明富血小板血浆和高压氧治疗对血管生成和骨再生具有协同作用,提高了两种方法单一应用的骨诱导能力,这种协同作用可能为改善骨骼再生产生一个有价值的选择,但潜在的机制还需进一步的研究[80]。此外,He等[81]研究报道富血小板血浆在结合具有单向性层状孔结构的聚乳酸-羟基乙酸共聚物/磷酸钙骨水泥(poly lactic-coglycolic acid/ Calcium phosphate cement,PLGA/CPC)支架后能够明显改善体外细胞的播种、附着、增殖和分化,改善了兔股骨和桡骨缺损的骨修复。在兔股骨头骨折模型中,富血小板血浆能够改善股骨头骨折后的血液流变学指标,修复局部血管,促进与成骨相关和血管生成相关因子的表达,对修复股骨头缺血性坏死的效果非常好[82]。在Beagle犬拔除了下颌第三前磨牙外的所有下颌前磨牙后的种植体周围骨缺损区模型的研究中,分别种植了PRP/OAM(osteoinduction active material,OAM)、富血小板血浆/磷酸三钙和单纯磷酸三钙,结果显示相比其他两组,PRP/OAM组新骨形成更明显,新骨成熟度更高,促进骨缺损修复的效果更好[83]。 2.3.2 富血小板血浆联合间充质干细胞的应用 间充质干细胞被用于修复骨缺损及增强软骨再生的研究[84]。研究表明富血小板血浆能够对骨髓间质干细胞的发育能力、生存和增殖能力产生积极的影响,这表明它可以在体外细胞管理中代表一种好的血清替代品,用于细胞治疗,同时也可能对骨髓间质干细胞有潜在的益处。富血小板血浆促进肌原细胞增殖和分化,表明它有可能影响局部肌肉干细胞的命运,从而增加了骨骼肌修复/再生的内生机制。在肌细胞增殖和分化方面,富血小板血浆/骨髓间质干细胞的联合治疗比富血小板血浆更有效,富血小板血浆/骨髓间质干细胞联合疗法可能作为一种潜在的治疗工具,用于改善骨骼肌的修复/再生和骨髓间质干细胞的功能[85]。此外,在体外共培养脱钙骨基质和间质干细胞体系中添加富血小板血浆可以成功诱导成骨发生[86]。富血小板血浆联合间质干细胞应用于牙槽骨增高术并获得了较好的治疗骨缺损效果[87]。 2.3.3 富血小板血浆联合生物材料的临床应用 通过与骨矿物质联合移植能进一步增强骨组织的修复能力[88]。Kassolis等[89]将富血小板血浆联合冻干骨植入15例牙槽嵴增高术和上颌窦提升术的患者,取得了良好的治疗效果。富血小板血浆和β-磷酸三钙的联合应用,能增加β-磷酸三钙的骨诱导性、骨传导性、骨生成和血管生成的功能[90]。文献表明将富血小板血浆与多孔羟基磷灰石、脱矿冻干骨同种异体移植物、牛来源的异种移植物联合用于人牙周骨内缺损的治疗,皆可获得满意的疗效[91]。 2.3.4 富血小板血浆作为治疗/辅助物的应用 研究表明使用富血小板血浆和透明质酸治疗骨关节炎后,在24周内,疼痛和功能显著改善,且富血小板血浆的效果优于透明质酸,通过对炎性因子白细胞介素1β和肿瘤坏死因子a的检测显示富血小板血浆治疗组明显低于透明质酸治疗组,表明富血小板血浆可能通过发挥抗炎作用机制有助于改善骨关节炎症状[92-94]。在关节镜显微骨折手术中,应用富血小板血浆作为该手术的辅助治疗物,比较了单纯的骨折手术的效果及该手术与富血小板血浆结合的效果,在这项研究中发现,与单纯的关节镜显微骨折手术相比,富血小板血浆似乎能提供更好恢复功能的可能性,它与关节镜显微骨折手术的共同作用可以发挥更好的功能效果[95]。乔永平等[96]分析了Ilizarov重建外固定技术联合富血小板血浆注射免植骨治疗超大段胫骨缺损的临床效果表明两种方式的联合使用能有效的加快修复超大段骨缺损。在蒋李青等[97]的研究中发现富血小板血浆联合人工骨植骨在骨折不愈合合并骨缺损的治疗中对骨折的愈合及患肢功能的恢复具有良好的效果。 目前富血小板血浆用于软骨、肌腱、韧带和骨骼几种骨科疾病,富血小板血浆可能成为一种保守的替代疗法,也可能成为标准外科治疗的辅助剂,无论用于哪一种治疗,富血小板血浆的功能是刺激“低的-固有的-愈合潜能的组织”的再生[98],抑或是通过所释放的细胞因子和生长因子调控修复的微环境,增加修复区域细胞的趋化性、活性,刺激修复区组织的再生和血管化[97]。"

| [1] O'Keefe RJ. Fibrinolysis as a Target to Enhance Fracture Healing. N Engl J Med. 2015;373(18):1776-1778. [2] Sinder BP, Salemi JD, Ominsky MS, et al. Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone. 2015;71:115-123. [3] Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55(12): 1613-1629. [4] Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45-54. [5] Gomez-Barrena E, Rosset P, Lozano D, et al. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93-101. [6] Zhou L, Hu C, Chen Y, et al. Investigations of silk fiber/calcium phosphate cement biocomposite for radial bone defect repair in rabbits. J Orthop Surg Res. 2017;12(1):32. [7] Castro AB, Meschi N, Temmerman A, et al. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J Clin Periodontol. 2017;44(2):225-234. [8] Castro AB, Meschi N, Temmerman A, et al. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol. 2017;44(1):67-82. [9] Harke H, Tanger D, Furst-Denzer S, et al. Effect of a preoperative separation of platelets on the postoperative blood loss subsequent to extracorporeal circulation in open heart surgery (author's transl). Anaesthesist. 1977;26(2): 64-71. [10] Nagata MJ, Messora MR, Furlaneto FA, et al. Effectiveness of two methods for preparation of autologous platelet-rich plasma: an experimental study in rabbits. Eur J Dent. 2010; 4(4):395-402. [11] Yuan T, Zhang C, Zeng B. Treatment of chronic femoral osteomyelitis with platelet-rich plasma (PRP): a case report. Transfus Apher Sci. 2008;38(2):167-173. [12] Pietramaggiori G, Scherer SS, Mathews JC, et al. Healing modulation induced by freeze-dried platelet-rich plasma and micronized allogenic dermis in a diabetic wound model. Wound Repair Regen. 2008;16(2):218-225. [13] Bajaj P, Pradeep AR, Agarwal E, et al. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: a randomized controlled clinical trial. J Periodontal Res. 2013;48(5):573-581. [14] Cassano JM, Kennedy JG, Ross KA, et al. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc. 2018;26(1):333-342. [15] Jeon YR, Jung BK, Roh TS, et al. Comparing the Effect of Nonactivated Platelet-Rich Plasma, Activated Platelet-Rich Plasma, and Bone Morphogenetic Protein-2 on Calvarial Bone Regeneration. J Craniofac Surg. 2016;27(2):317-321. [16] Dimauro I, Grasso L, Fittipaldi S, et al. Platelet-rich plasma and skeletal muscle healing: a molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS One. 2014;9(7):e102993. [17] Kim SJ, Kim SY, Kwon CH, et al. Differential effect of FGF and PDGF on cell proliferation and migration in osteoblastic cells. Growth Factors. 2007;25(2):77-86. [18] Weibrich G, Gnoth SH, Otto M, et al. Growth stimulation of human osteoblast-like cells by thrombocyte concentrates in vitro. Mund Kiefer Gesichtschir. 2002;6(3):168-174. [19] Lindeboom JA, Mathura KR, Aartman IH, et al. Influence of the application of platelet-enriched plasma in oral mucosal wound healing. Clin Oral Implants Res. 2007;18(1):133-139.[20] Pavlovic V, Ciric M, Jovanovic V, et al. Platelet Rich Plasma: a short overview of certain bioactive components. Open Med (Wars). 2016;11(1):242-247. [21] Roh YH, Kim W, Park KU, et al. Cytokine-release kinetics of platelet-rich plasma according to various activation protocols. Bone Joint Res. 2016;5(2):37-45. [22] Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. [23] Zhou Y, Zhang J, Wu H, et al. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173. [24] Filardo G, Kon E, Pereira Ruiz MT, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2082-2091. [25] Riboh JC, Saltzman BM, Yanke AB, et al. Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Am J Sports Med. 2016; 44(3):792-800. [26] Liacini A, Sylvester J, Li WQ, et al. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21(3): 251-262. [27] Cavallo C, Filardo G, Mariani E, et al. Comparison of platelet-rich plasma formulations for cartilage healing: an in vitro study. J Bone Joint Surg Am. 2014;96(5):423-429. [28] Yin W, Xu H, Sheng J, et al. Comparative evaluation of the effects of plateletrich plasma formulations on extracellular matrix formation and the NFkappaB signaling pathway in human articular chondrocytes. Mol Med Rep. 2017;15(5): 2940-2948. [29] Shiga Y, Orita S, Kubota G, et al. Freeze-Dried Platelet-Rich Plasma Accelerates Bone Union with Adequate Rigidity in Posterolateral Lumbar Fusion Surgery Model in Rats. Sci Rep. 2016;6:36715. [30] Fernandes G, Yang S. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 2016;4:16036. [31] Mussano F, Genova T, Munaron L, et al. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets. 2016;27(5):467-471. [32] Balogh ZJ, Reumann MK, Gruen RL, et al. Advances and future directions for management of trauma patients with musculoskeletal injuries. Lancet. 2012;380(9847): 1109-1119. [33] Liu M, Zhong S, Kong R, et al. Paeonol alleviates interleukin-1beta-induced inflammatory responses in chondrocytes during osteoarthritis. Biomed Pharmacother. 2017;95:914-921. [34] Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17(3):513-520. [35] Wang X, Luo Y, Masci PP, et al. Influence of Interleukin-1 Beta on Platelet-Poor Plasma Clot Formation: A Potential Impact on Early Bone Healing. PLoS One. 2016;11(2): e0149775. [36] DiBattista JA, Martel-Pelletier J, Wosu LO, et al. Glucocorticoid receptor mediated inhibition of interleukin-1 stimulated neutral metalloprotease synthesis in normal human chondrocytes. J Clin Endocrinol Metab. 1991;72(2): 316-326. [37] Yang J, Lu Y, Guo A. Platelet-rich plasma protects rat chondrocytes from interleukin-1beta-induced apoptosis. Mol Med Rep. 2016;14(5):4075-4082. [38] Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110(9):1911-1928. [39] Flower L, Gray R, Pinkney J, et al. Stimulation of interleukin-6 release by interleukin-1beta from isolated human adipocytes. Cytokine. 2003;21(1):32-37. [40] Wang X, Luo Y, Liao WB, et al. Effect of osteoprotegerin in combination with interleukin-6 on inhibition of osteoclast differentiation. Chin J Traumatol. 2013;16(5):277-280. [41] Huang L, Liu S, Song T, et al. Blockade of Interleukin 6 by Rat Anti-mouse Interleukin 6 Receptor Antibody Promotes Fracture Healing. Biochemistry (Mosc). 2017;82(10): 1193-1199. [42] Loi F, Cordova LA, Pajarinen J, et al. Inflammation, fracture and bone repair. Bone. 2016;86:119-130. [43] Edderkaoui B. Potential Role of Chemokines in Fracture Repair. Front Endocrinol (Lausanne). 2017;8:39. [44] Liu KG, He QH, Tan JW, et al. Expression of TNF-alpha, VEGF, and MMP-3 mRNAs in synovial tissues and their roles in fibroblast-mediated osteogenesis in ankylosing spondylitis. Genet Mol Res. 2015;14(2):6852-6858. [45] Sun M, Yang J, Wang J, et al. TNF-alpha is upregulated in T2DM patients with fracture and promotes the apoptosis of osteoblast cells in vitro in the presence of high glucose. Cytokine. 2016;80:35-42. [46] Chan JK, Glass GE, Ersek A, et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol Med. 2015;7(5):547-561. [47] Osterman C, McCarthy MB, Cote MP, et al. Platelet-Rich Plasma Increases Anti-inflammatory Markers in a Human Coculture Model for Osteoarthritis. Am J Sports Med. 2015;43(6):1474-1484. [48] Minciullo PL, Catalano A, Mandraffino G, et al. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch Immunol Ther Exp (Warsz). 2016;64(2): 111-126. [49] Ribeiro FV, Pino DS, Franck FC, et al. Resveratrol Inhibits Periodontitis-Related Bone Loss in Rats Subjected to Cigarette Smoke Inhalation. J Periodontol. 2017;88(8):788-798. [50] Cornell CN, Lane JM. Newest factors in fracture healing. Clin Orthop Relat Res. 1992(277):297-311. [51] Kolar P, Schmidt-Bleek K, Schell H, et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010;16(4):427-434. [52] Ghosh D, McGrail DJ, Dawson MR. TGF-beta1 Pretreatment Improves the Function of Mesenchymal Stem Cells in the Wound Bed. Front Cell Dev Biol. 2017;5:28. [53] Lind M, Schumacker B, Soballe K, et al. Transforming growth factor-beta enhances fracture healing in rabbit tibiae. Acta Orthop Scand. 1993;64(5):553-556. [54] Gibon E, Loi F, Cordova LA, et al. Aging Affects Bone Marrow Macrophage Polarization: Relevance to Bone Healing. Regen Eng Transl Med. 2016;2(2):98-104. [55] Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6): 2081-2095. [56] Saxena A, Khosraviani S, Noel S, et al. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74(1): 27-34. [57] Toben D, Schroeder I, El Khassawna T, et al. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res. 2011;26(1):113-124. [58] Seyhan N, Keskin S, Aktan M, et al. Comparison of the Effect of Platelet-Rich Plasma and Simvastatin on Healing of Critical-Size Calvarial Bone Defects. J Craniofac Surg. 2016; 27(5):1367-1370. [59] Magalon J, Bausset O, Serratrice N, et al. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy. 2014;30(5):629-638. [60] Okuda K, Kawase T, Momose M, et al. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74(6):849-857. [61] Yin W, Qi X, Zhang Y, et al. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects. J Transl Med. 2016;14:73. [62] Harry LE, Paleolog EM. From the cradle to the clinic: VEGF in developmental, physiological, and pathological angiogenesis. Birth Defects Res C Embryo Today. 2003;69(4):363-374. [63] Alves R, Grimalt R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018;4(1):18-24. [64] Sarahrudi K, Thomas A, Braunsteiner T, et al. VEGF serum concentrations in patients with long bone fractures: a comparison between impaired and normal fracture healing. J Orthop Res. 2009;27(10):1293-1297. [65] Oryan A, Alidadi S, Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther. 2016;16(2): 213-232. [66] Lai F, Kakudo N, Morimoto N, et al. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res Ther. 2018;9(1):107. [67] Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79(4): 1283-1316. [68] Kaigler D, Avila G, Wisner-Lynch L, et al. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin Biol Ther. 2011;11(3): 375-385. [69] Younesi M, Knapik DM, Cumsky J, et al. Effects of PDGF-BB delivery from heparinized collagen sutures on the healing of lacerated chicken flexor tendon in vivo. Acta Biomater. 2017; 63:200-209. [70] Huang XD, Zhang H, He MX. A PDGF/VEGF homologue provides new insights into the nucleus grafting operation and immune response in the pearl oyster Pinctada fucata. Gene. 2017;637:1-8. [71] Min Nam S, Bae Kim Y. The effects of platelet-rich plasma on hypertrophic scars fibroblasts. Int Wound J. 2018. [72] Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685-700. [73] Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331(19):1286-1292. [74] Balooch G, Balooch M, Nalla RK, et al. TGF-beta regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci U S A. 2005;102(52):18813-18818. [75] Gutierrez-Fernandez A, Inada M, Balbin M, et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). Faseb j. 2007;21(10):2580-2591. [76] Danielpour D, Song K. Cross-talk between IGF-I and TGF-beta signaling pathways. Cytokine Growth Factor Rev. 2006;17(1-2):59-74. [77] Wang T, Wang Y, Menendez A, et al. Osteoblast-Specific Loss of IGF1R Signaling Results in Impaired Endochondral Bone Formation During Fracture Healing. J Bone Miner Res. 2015; 30(9):1572-1584. [78] Griffin XL, Smith CM, Costa ML. The clinical use of platelet-rich plasma in the promotion of bone healing: a systematic review. Injury. 2009;40(2):158-162. [79] 张长青,袁霆.富血小板血浆在临床应用中的争议与研究进展[J].中华关节外科杂志(电子版),2016,10(6):588-591.[80] Schneppendahl J, Jungbluth P, Sager M, et al. Synergistic effects of HBO and PRP improve bone regeneration with autologous bone grafting. Injury. 2016;47(12):2718-2725. [81] He F, Chen Y, Li J, et al. Improving bone repair of femoral and radial defects in rabbit by incorporating PRP into PLGA/CPC composite scaffold with unidirectional pore structure. J Biomed Mater Res A. 2015;103(4):1312-1324. [82] Zhang XL, Shi KQ, Jia PT, et al. Effects of platelet-rich plasma on angiogenesis and osteogenesis-associated factors in rabbits with avascular necrosis of the femoral head. Eur Rev Med Pharmacol Sci. 2018;22(7):2143-2152. [83] 王毅,刘杨,杜军,等.PRP及PRP/OAM对种植体周围骨缺损修复的实验研究[J].口腔医学,2017,37(4):302-306.[84] Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12(12):3405-3416.[85] Sassoli C, Vallone L, Tani A, et al. Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: new therapeutic perspectives for skeletal muscle repair/regeneration. Cell Tissue Res. 2018;372(3):549-570. [86] Souza TF, Sakamoto SS, Ferreira GT, et al. Osteogenic potential of mesenchymal cells derived from canine umbilical cord matrix co-cultured with platelet-rich plasma and demineralized bone matrix. J Vet Sci. 2015;16(3):381-384. [87] Pieri F, Lucarelli E, Corinaldesi G, et al. Effect of mesenchymal stem cells and platelet-rich plasma on the healing of standardized bone defects in the alveolar ridge: a comparative histomorphometric study in minipigs. J Oral Maxillofac Surg. 2009;67(2):265-272. [88] Rodrigues SV, Acharya AB, Thakur SL. An evaluation of platelet-rich plasma without thrombin activation with or without anorganic bone mineral in the treatment of human periodontal intrabony defects. Platelets. 2011;22(5):353-360. [89] Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000;71(10):1654-1661. [90] Zhong D, Wang CG, Yin K, et al. In vivo ossification of a scaffold combining beta-tricalcium phosphate and platelet-rich plasma. Exp Ther Med. 2014;8(5):1381-1388. [91] Markou N, Pepelassi E, Vavouraki H, et al. Treatment of periodontal endosseous defects with platelet-rich plasma alone or in combination with demineralized freeze-dried bone allograft: a comparative clinical trial. J Periodontol. 2009; 80(12):1911-1919. [92] Cole BJ, Karas V, Hussey K, et al. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am J Sports Med. 2017;45(2):339-346. [93] 侯晓明,李丽,臧静一,等.富血小板血浆(PRP)用于膝关节骨性关节炎关节软骨修复作用研究[J].临床医药文献电子杂志, 2017, 4(92):18036-18037.[94] 刘步云,孙育良,何本祥,等.关节腔注射富血小板血浆与玻璃酸钠治疗膝关节骨关节炎的疗效比较[J].实用骨科杂志, 2017,23(1): 71-73.[95] Guney A, Akar M, Karaman I, et al. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. [96] 乔永平,原巧玲,丛培军,等.Ilizarov重建外固定技术联合PRP免植骨治疗超大段胫骨缺损[J].中国骨与关节损伤杂志, 2016, 31(12):1323-1325.[97] 蒋李青,方炳木,赵冬梅,等.富血小板血浆复合人工骨植骨治疗骨折不愈合合并骨缺损[J].中医正骨,2016,28(12):58-60.[98] Gianakos A, Zambrana L, Savage-Elliott I, et al. Platelet-Rich Plasma in the Animal Long-Bone Model: An Analysis of Basic Science Evidence. Orthopedics. 2015;38(12):e1079-1090. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Jiang Hongying, Zhu Liang, Yu Xi, Huang Jing, Xiang Xiaona, Lan Zhengyan, He Hongchen. Effect of platelet-rich plasma on pressure ulcers after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1149-1153. |
| [3] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [4] | He Xiangzhong, Chen Haiyun, Liu Jun, Lü Yang, Pan Jianke, Yang Wenbin, He Jingwen, Huang Junhan. Platelet-rich plasma combined with microfracture versus microfracture in the treatment of knee cartilage lesions: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 964-969. |
| [5] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [6] | Wang Yujiao, Liu Dan, Sun Song, Sun Yong. Biphasic calcium phosphate loaded with advanced platelet rich fibrin can promote the activity of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 504-509. |
| [7] | Liu Jiangfeng. Nano-hydroxyapatite/polyamide 66 composite filling combined with locking plate in the treatment of fibrous dysplasia of femoral bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 542-547. |
| [8] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [9] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [10] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [11] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [12] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [13] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [14] | Shi Qin, Sun Baolan, Yang Xiaoqing, Zhang Yuquan . Mesenchymal stem cell-derived exosomes carrying miRNAs in tissue repair and treatment of related diseases: application and advantages [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 5040-5045. |
| [15] | Wang Xinwei, Zhao Yingjie, Chang Yan, Wei Wei. Mesenchymal stem cells in the treatment of cartilage damage in osteoarthritis: role, application, and problem [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 5053-5058. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||