Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (34): 5552-5557.doi: 10.3969/j.issn.2095-4344.2017.34.023
Previous Articles Next Articles
Novel bio-mimetic receptors for early detection of Alzheimer’s disease biomarkers
- Qingdao University of Science and Technology, Qingdao 266000, Shandong Province, China
-
Received:2017-07-10Online:2017-12-08Published:2018-01-04 -
Contact:Su Feng, Qingdao University of Science and Technology, Qingdao 266000, Shandong Province, China -
About author:Su Feng, M.D., Associate professor, Master’s supervisor, Qingdao University of Science and Technology, Qingdao 266000, Shandong Province, China -
Supported by:the Science and Technology Benefiting Policy of Qingdao, No. 16-6-2-17-nsh; College Students Innovation and Entrepreneurship Training Program in Qingdao University of Science and Technology, No. 201501004, 201601007
CLC Number:
Cite this article
Su Feng, Yun Peng, Liu Xue, Shen Xin, Li Cheng-long, Li Rong-ye.
share this article
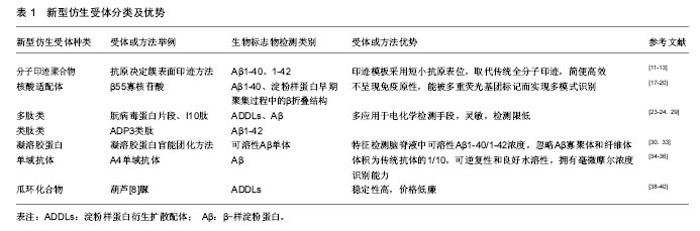
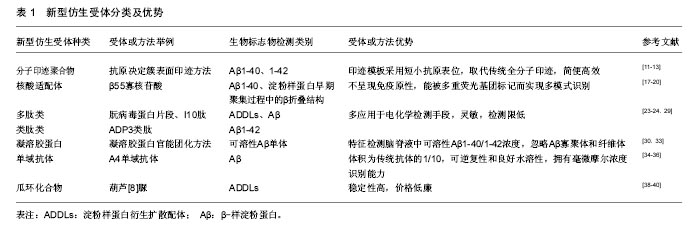
2.1 分子印迹聚合物 人工合成能与生物大分子(如蛋白质)结合的分子印迹聚合物技术是大家关注的热点,近几年已被运用到生物检测领域[10]。最新采用的“抗原决定簇表面印迹”方式[11],与之前的印迹方式不同,是对蛋白质结构表面短小的抗原表位(≥ 9 aa)进行的小巧高效印迹方法,取代了传统的全分子印迹方法。首先采用合成多肽来模拟抗原表位,再使用特定的功能单体和交联剂通过多种方法合成分子印迹聚合物[12]。 因此,可应用抗原决定簇表面印迹方法,将Aβ1-40和Aβ1-42肽段的羧基末端作为模板分子进行印迹,模板分子遵循的氨基酸序列与Aβ33-40和Aβ33-42序列保持一致,使用合成多肽模拟模板分子合成相应的分子印迹聚合物,并对印迹分子与其分子印迹聚合物之间的结合能力进行评价[13]。将分子印迹聚合物固定在固相柱上,对含有标准浓度合成多肽模板分子和Aβ1-40全长多肽分子的血清样品进行分离,洗脱片段经高效液相色谱和十二烷基磺酸钠聚丙烯酰胺凝胶电泳分析,结果表明分子印迹聚合物对合成多肽模板分子和全长多肽分子表现出相近亲和力[14]。根据阶段研究成果,分子印迹聚合物将是极具发展前景的新型仿生受体,在多肽聚集性基础研究和阿尔茨海默病诊断研究中均具有应用价值[15]。 2.2 核酸适配体 核酸适配体是通过体外指数富集的配体系统进化技术,从核酸分子文库中筛选得到的寡核苷酸片段[16]。研究者将其作为探针,应用于电化学检测传感器中,与以往的抗体探针相比,核酸适配体不呈现免疫原性,具有更小的尺寸,操作简单成本低,且能被多重荧光基团标记而实现多模式识别[17]。20世纪90年代以来,用于治疗或诊断的大量核酸适配体经过筛选获得,但仅有少数核酸适配体表现出对阿尔茨海默病生物标志物的特异选择性。Ylera团队发现了一种能特异结合Aβ1-40的核酸适配 体——β55寡核苷酸,并通过亲和色谱法将其分离,其只与淀粉样蛋白纤维状态发生相互作用[18],结合是由于目标蛋白在聚集过程中产生的β折叠区域与核酸适配体发生特异性相互作用。此外,核酸适配体表现出对几种淀粉样蛋白纤维的亲和性,纤维由Aβ多肽组成或由其它淀粉样蛋白分子组成,比如溶解酵素和朊病毒蛋白[19]。 尽管核酸适配体对阿尔茨海默病生物标志物特异性结合效果不显著,但可用其代替淀粉样蛋白检测标识物(硫代黄素)来检测淀粉样蛋白早期聚集过程中形成的β折叠结构,已有研究完成体外β55核酸适配体在天然淀粉样蛋白斑块中的相关实验[20]。通过插入荧光核苷酸对核酸适配体进行标记,比抗体标记过程更简单更高效,同时核酸适配体更易引入活细胞。目前还没有与tau蛋白存在特异结合能力的核酸适配体被确认,但通过Flu-Mag SELEX筛选出与tau蛋白氨基酸序列226-240段相似的一段多肽所匹配的核酸适配体,并完成该核酸适配体的分离工作[21]。 2.3 多肽类及类肽类 多肽及类肽受体多应用于电化学检测手段,包括阻抗法(电化学阻抗谱和伏安法)和微分脉冲伏安法[22]。在合成多肽中,近几年朊病毒蛋白片段和I10肽成为非常有发展前景的合成受体,可用于生物检定ADDLs和Aβ[23-24]。细胞型(正常型)朊病毒蛋白已被证实对Aβ寡聚物有高亲和力,有研究指出朊病毒蛋白内负责与Aβ进行相互作用的核心区域是朊病毒蛋白片段(95-110,THSQWNKPSKPKTNMK),位于朊病毒蛋白的氨基端非结构化区域。并且发现只需相对于Aβ化学计量1/20的朊病毒蛋白就可有效结合Aβ寡聚物,抑制淀粉样纤维形成,通过电子显微镜可观察出朊病毒蛋白能使Aβ停留于低聚体状态[25]。Rushworth[26]和同事们通过生物素/亲和素相互作用对朊病毒蛋白片段多肽(95-110氨基酸位点)进行固定化,采取阻抗法检测ADDLs浓度。将朊病毒蛋白片段多肽固定在传感器表面,增加ADDLs浓度,观察到电荷转移和电阻数值降低。Liu[27]开发了一种利用朊病毒蛋白作为受体的双抗体夹心检测法,朊病毒蛋白既用来捕捉ADDLs,并且还可通过电化学反应对ADDLs进行定量检测。将含有半胱氨酸的朊病毒蛋白片段多肽固定于金电极上来捕获Aβ低聚物,同时将朊病毒蛋白片段(95-110)与碱性磷酸酯酶共轭后用于识别被捕获的分析物并且产生电活性物质,该方法检测限度可低至3 pM[27]。 类肽或聚-N-取代甘氨酸是一类拟肽化合物,相当于人工合成的氨基末端被取代的寡聚甘氨酸,氨基末端的取代可防止主链内部氢键结合[28]。关于在血清样品中通过SPR生物传感器技术来进行Aβ1-42检测的研究中利用了ADP3类肽,该类肽能与Aβ1-42特异结合。ADP3是从类肽库中经对比筛选获得,发现其具有区分正常组和阿尔茨海默病组血清的特性,可和阿尔茨海默病组血清中的生物信号分子(Aβ1-42)发生相互作用[29]。 2.4 凝溶胶蛋白 凝溶胶蛋白是一种分泌性蛋白,能在适当浓度下选择性结合可溶性Aβ单体(Aβ1-40和Aβ1-42),但是不能与Aβ的低聚态和纤维态发生结合。因此利用凝溶胶蛋白可检测脑脊液中可溶性Aβ1-40/1-42的浓度,但无法区分Aβ1-40和Aβ1-42[30]。研究中使用丝网印刷碳电极作为捕捉电极,向电极表面引入多壁碳纳米管和金纳米粒子进行修饰,目的是促进电极表面的电子传递。然后通过氨基耦合将凝溶胶蛋白固定在电极上,剩余表面通过小牛血清白蛋白进行填补。最后,在感受器表面添加Aβ1-40/1-42进行分析。同时为了实现夹心法试验,用硫堇对凝溶胶蛋白进行官能团化,或者与山葵过氧化物酶官能团化,将其加入到已经和电极结合的Aβ1-40/1-42分子中[31-32]。因为凝溶胶蛋白可识别Aβ1-40/1-42多肽的多个识别位点,所以能发生第二次识别结合。通过监测硫堇信号改变,从而检测Aβ单体浓度,该方法检测极限为50 pM。采用山葵过氧化物酶官能团化之后,通过酶促反应可使检测敏感度改善至28 pM。在健康大鼠和阿尔茨海默病大鼠脑脊液检测的对比中,虽然检测基质中存在Aβ寡聚体、纤维体、原纤维体等干扰因素,但凝溶胶蛋白对Aβ单体仍然表现出高选择性[33]。 2.5 单域抗体及瓜环化合物 单域抗体也称纳米抗体,与传统抗体不同的是,单域抗体仅由重链构成,其体积约为传统抗体的1/10。其具有极小的尺寸、很高的稳定性、可逆的复性及在水溶液中良好的溶解性,同时还拥有在毫微摩尔浓度下识别特异性抗原决定簇的能力[34-35]。单域抗体可成为阿尔茨海默病诊断中更有效的生物感受器,已发现能特异性识别Aβ的A4纳米抗体,将其固定于印刷电路上,再通过电化学阻抗谱法检测Aβ浓度[36]。此外,还发现D5型单域抗体能特异性识别α-突触核蛋白(帕金森的生物标志物)[37]。这两种纳米抗体在针对阿尔茨海默病患者、帕金森患者及健康对照组的脑脊液对比检测中呈现出一致准确的区分结果。 另外,葫芦[n]脲(瓜环化合物)也是得到成功应用的仿生受体,葫芦脲是由甘脲单体之间的亚甲基桥连接而成的大环化合物,其中的环状空腔高度对称,两端有两个相同的尺寸可变的开口,开口大小取决于n的数目[38]。葫芦脲最早作为药物载体出现,随着对其功能化的研究进展,发现可用为生物感受器。研究者将作为捕捉受体的I10蛋白结合肽与作为信号传达器的葫芦[8]脲相结合,用于ADDLs和肿瘤坏死因子的检测[39-40]。当一部分分析物被多肽捕捉之后,其它自由的分子能够与葫芦脲及相应的电化学传感器发生相互作用,因此被分析物浓度和信号强度之间存在比例关系。应用该方法可以检测分析ADDLs浓度,检测限为48 pM。 "
| [1]Heneka MT,Golenbock DT,Latz E.Innate immunity in Alzheimer's disease.Nat Immunol.2015;16(3): 229-236.[2]Walji AM,Hostetler ED,Selnick H,et al.Discovery of 6-(Fluoro-18 F)-3-(1 H-pyrrolo [2, 3-c] pyridin-1-yl) isoquinolin-5-amine ([18F]-MK-6240): A Positron Emission Tomography (PET) Imaging Agent for Quantification of Neurofibrillary Tangles(NFTs).J Med Chem. 2016;59(10): 4778-4789.[3]Barage SH,Sonawane KD.Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer's disease.Neuropeptides.2015;52:1-18.[4]Hansson O,Zetterberg H,Buchhave P,et al.Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006; 5(3):228-234.[5]Shi XD,Sun K,Hu R,et al. Blocking the Interaction between EphB2 and ADDLs by a Small Peptide Rescues Impaired Synaptic Plasticity and Memory Deficits in a Mouse Model of Alzheimer's Disease.J Neurosci. 2016;36(47): 11959-11973.[6]Chen Y,Kong D,Liu L,et al.Development of an enzyme-linked immunosorbent assay (ELISA) for natamycin residues in foods based on a specific monoclonal antibody. Anal Methods-UK.2015;7(8):3559-3565.[7]Gupta V,Davancaze T,Good J,et al.Bioanalytical qualification of clinical biomarker assays in plasma using a novel multi-analyte Simple Plex™ platform.Bioanalysis.2016;8(23):2415-2428.[8]Chiasserini D,Biscetti L,Farotti L,et al.Performance Evaluation of an Automated ELISA System for Alzheimer’s Disease Detection in Clinical Routine.J Alzheimers Dis. 2016;54(1): 55-67.[9]Cowen T,Karim K,Piletsky S.Computational approaches in the design of synthetic receptors–A review. Anal Chim Acta. 2016; 936:62-74.[10]Kunath S,Panagiotopoulou M,Maximilien J,et al.Cell and tissue imaging with molecularly imprinted polymers as plastic antibody mimics.Adv Healthc Mater.2015;4(9):1322-1326.[11]Li S,Yang K,Liu J,et al.Surface-Imprinted Nanoparticles Prepared with a His-Tag-Anchored Epitope as the Template. Anal Chem.2015;87(9):4617-4620.[12]Yang X,Dong X,Zhang K,et al.A molecularly imprinted polymer as an antibody mimic with affinity for lysine acetylated peptides.J Mater Chem B.2016;4(5):920-928.[13]Rossetti C,Abdel QA,Halvorsen TG,et al.Antibody-free biomarker determination: Exploring molecularly imprinted polymers for pro-gastrin releasing peptide.Anal Chem.2014; 86(24): 12291-12298.[14]Schirhagl R.Bioapplications for molecularly imprinted polymers. Anal Chem.2013;86(1): 250-261.[15]管习文,李欣怡,周琪,等.分子印迹聚合物的制备与应用进展[J].胶体与聚合物,2015,33(2):88-92.[16]Toh SY,Citartan M,Gopinath SCB,et al.Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay.Biosens Bioelectron.2015;64:392-403.[17]Scognamiglio V,Antonacci A,Lambreva MD,et al.Synthetic biology and biomimetic chemistry as converging technologies fostering a new generation of smart biosensors.Biosens Bioelectron.2015;74: 1076-1086.[18]Ylera F,Lurz R,Erdmann VA,et al.Selection of RNA aptamers to the Alzheimer's disease amyloid peptide.Biochem Bioph Res Co.2002;290(5):1583-1588.[19]Sarell CJ,Karamanos TK,White SJ,et al.Distinguishing closely related amyloid precursors using an RNA aptamer.J Biol Chem. 2014;289(39):26859-26871.[20]Tannenberg R,Lauridsen L,Kanwar J,et al.Nucleic acid aptamers as novel class of therapeutics to mitigate Alzheimer's disease pathology.Curr Alzheimer Res.2013;10(4):442-448.[21]Hamaguchi N,Ellington A,Stanton M.Aptamer beacons for the direct detection of proteins. Anal Biochem.2001;294(2):126-131.[22]Randviir EP,Banks CE.Electrochemical impedance spectroscopy: an overview of bioanalytical applications.Anal Methods-UK.2013;5(5):1098-1115.[23]Apostol MI,Perry K,Surewicz WK.Crystal structure of a human prion protein fragment reveals a motif for oligomer formation.J Am Chem Soc.2013;135(28):10202-10205.[24]尹文超,曹云鹏.朊病毒蛋白样作用的β淀粉样蛋白[J].国际神经病学神经外科学杂志,2016,43(2): 161-164.[25]Chen S,Yadav SP,Surewicz WK.Interaction between human prion protein and amyloid-β(aβ) oligomers role of N-terminal residues.J Biol Chem.2010;285(34):26377-26383.[26]Rushworth JV,Ahmed A,Griffiths HH,et al.A label-free electrical impedimetric biosensor for the specific detection of Alzheimer's amyloid-beta oligomers.Biosens Bioelectron.2014;56:83-90.[27]Liu L,Xia N,Jiang M,et al.Electrochemical detection of amyloid-β oligomer with the signal amplification of alkaline phosphatase plus electrochemical-chemical-chemical redox cycling.J Electroanal Chem.2015;754:40-45.[28]Sun J,Zuckermann RN.Peptoid polymers: a highly designable bioinspired material.ACS Nano.2013; 7(6):4715-4732.[29]Zhao Z,Zhu L,Bu X,et al.Label-free detection of Alzheimer’s disease through the ADP3 peptoid recognizing the serum amyloid-beta42 peptide.Chem Commun.2015;51(4):718-721.[30]Yu Y,Zhang L,Li C,et al.A Method for Evaluating the Level of Soluble β-Amyloid (1–40/1–42) in Alzheimer’s Disease Based on the Binding of Gelsolin to β-Amyloid Peptides.Angew Chem Int Edit. 2014;126(47):13046-13049.[31]Ganesh HVS,Chow AM,Kerman K.Recent advances in biosensors for neurodegenerative disease detection. Trac-Trend Anal Chem.2016;79:363-370.[32]Yu Y,Sun X,Tang D,et al.Gelsolin bound β-amyloid peptides (1-40/1-42): Electrochemical evaluation of levels of soluble peptide associated with Alzheimer's disease. Biosens Bioelectron. 2015; 68:115-121.[33]Ji L,Zhao X,Hua Z.Potential Therapeutic Implications of Gelsolin in Alzheimer's Disease. J Alzheimers Dis.2015;44(1):13-25.[34]Muyldermans S.Nanobodies: natural single-domain antibodies. Annu Rev Biochem.2013;82:775-797.[35]潘欣,潘伯驹,蔡家麟,等.单域抗体研究进展[J].生命科学,2012, 24(5):404-410.[36]Kasturirangan S,Li L,Emadi S,et al.Nanobody specific for oligomeric beta-amyloid stabilizes nontoxic form.Neurobiol Aging.2012;33(7):1320-1328.[37]Williams SM,Schulz P,Sierks MR.Oligomeric α-synuclein and β-amyloid variants as potential biomarkers for Parkinson's and Alzheimer's diseases.Eur J Neurosci.2016;43(1):3-16.[38]Isaacs L.Cucurbit[n]urils: from mechanism to structure and function.Chem Commun.2009;(6): 619-629.[39]Li H,Xie H,Cao Y,et al.A general way to assay protein by coupling peptide with signal reporter via supermolecule formation.Anal Chem.2012;85(2):1047-1052.[40]张宁强,黄晓玲,班琳哲,等.葫芦[n]脲应用研究进展[J].化学进展, 2014,27(2/3):192-211. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | Zheng Zhenquan, Rong Jiesheng. Sarcopenia: age-related muscle mass loss and functional declines [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 792-797. |
| [5] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [6] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [7] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [8] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [9] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [10] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [11] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [12] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [13] | Zhang Jian, Lin Jianping, Zhou Gang, Fang Yehan, Wang Benchao, Wu Yongchang. Semi-quantitative MRI evaluation of cartilage degeneration in early knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 425-429. |
| [14] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||