Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (3): 450-455.doi: 10.3969/j.issn.2095-4344.2017.03.023
Previous Articles Next Articles
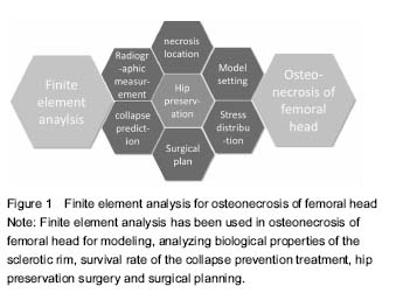
Finite element analysis applied to the diagnosis andtreatment of osteonecrosis of femoral head: latest progress
Hong Guo-ju1, 2, 3, Han Xiao-rui4, Fang Bin1, 2, Zhou Guang-quan1, He Wei1, 2, Chen Lei-lei1, 2
- 1The National Key Discipline and the Orthopedic Laboratory, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 2Orthopedic Department, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 3School of Pathology and Laboratory Medicine, The University of Western Australia, Nedlands WA 6009, Australia; 4Radiographic Department, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China
-
Online:2017-01-28Published:2017-03-14 -
Contact:Chen Lei-lei, M.D., Attending physician, The National Key Discipline and the Orthopedic Laboratory, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; Orthopedic Department, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
About author:Hong Guo-ju, Studying for master’s degree, The National Key Discipline and the Orthopedic Laboratory, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; Orthopedic Department, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; School of Pathology and Laboratory Medicine, The University of Western Australia, Nedlands WA 6009, Australia -
Supported by:the National Natural Science Foundation of China, No. 81673999, 81302990; the Outstanding Youth Science Foundation of Guangdong Province, No. 2015A030306037; the Discipline and Specialty Construction Foundation of Higher Education Schools in Guangdong Province, No. 2013LYM0010
CLC Number:
Cite this article
Hong Guo-ju, Han Xiao-rui, Fang Bin, Zhou Guang-quan, He Wei, Chen Lei-lei. Finite element analysis applied to the diagnosis andtreatment of osteonecrosis of femoral head: latest progress[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(3): 450-455.
share this article
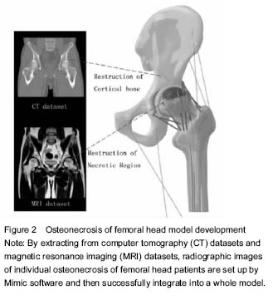
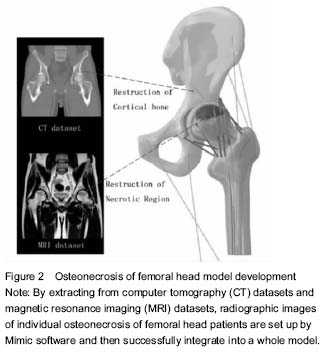
ONFH and finite element model There were many ONFH finite element models exiting, such as cone model, spherical model and pathological models. However, with further development of the technology and the needs from clinical practices, model design and building experiments were required to be invented on the basis of previous modeling application by using the original CT data from ONFH patients[12]. Li et al.[13] used the 142-layer of 0.625-mm-thick consecutive CT Dicom format images to scan the ONFH, and then define the threshold of bone tissue, extract each outline, partition each edges, edit and repair by hole processing. Finally, they created the three-dimensional geometry model and meshed body line, assigned, and generate a model. The author believes that this method can quickly establish realistic femoral head in computer and calculate the precise three-dimensional finite element model of ONFH. Undeniably, some limitations exist in modeling by CT, due to its poor ability to detect cartilage and other tissues, which reduces credibility of the biomechanical analysis. Innovative application was used by Pang et al. to register several radiologic images and fuse them into a three-dimensional reconstruction of femoral head[14]. By combining X-ray images, CT and MRI image model of individual ONFH patients after images conversion, the radiologic images are registered by Mimic software and then successfully integrated into a model. This multi-images registration technique allows the model to cover cortical bone, cancellous bone, cartilage, necrosis areas and trabecular bone (Figure 2)."

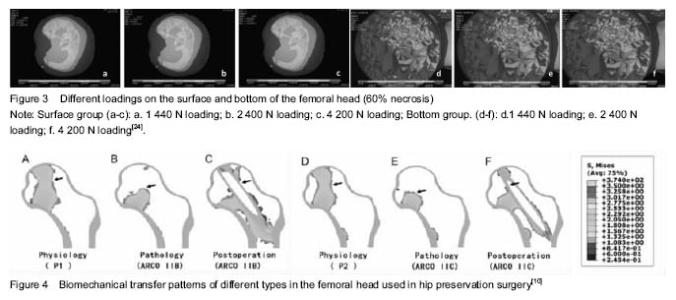
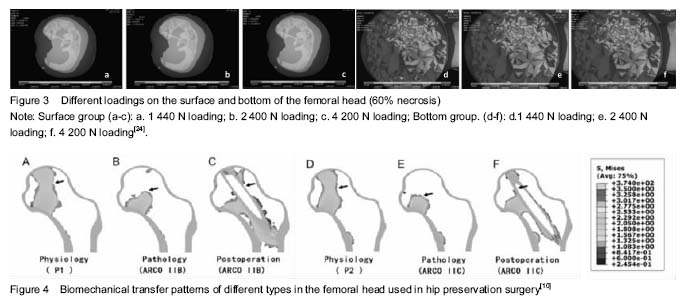
Stress analysis in femoral head necrotic area Biomechanical effect of sclerosis rim: Sclerosis band is a common pathologic feature in ONFH around the necrosis area, although its biomechanical effect is seldom to be paid attention[15]. Sclerosis band is a specific mechanical product after ONFH progress with the formation of necrosis, but also the marker of necrosis healing[16]. Natural history of sclerosis formation is less studied. Nevertheless, it can be regarded as a biomechanical support to postpone or prevent ONFH from malformation[17]. Protection of sclerosis band keeps further damage away, including collapse. Yu et al.[18] use finite element analysis to evaluate the stress of sclerosis band at the proximal femur. The study simulated different proportions of sclerosis band in the proximal edge and added loads on it. Series of indicators are ultimately evaluated, like total deformation value, maximum stress, minor stress and the contacted pressure. The results showed sclerosis rim increased gradually with the total value of the femoral head deformation and maximum stress decreasing in turn. Therefore, the authors believe that the proximal edge of the sclerosis band can provide effective mechanical support for the femoral head. Chen et al.[19] hold the same views for sclerosis band. They simulated the stress distribution after bone remodeling and calculated the maximum Von-Mises stress of bone. The results suggest that sclerosis band is an adaptive mechanical product to compensate the elastic modulus of the femoral head. Sclerosis band is an alternative mechanical reinforcing structure, which can significantly reduce the pressure loading in the subchondral bone, but only when the necrosis is small. However, Karasuyama et al.[20] made use of the finite element model to assess stress distribution in fracture zones of ONFH. They found that both necrosis healing and fracture appearing are resulted from the change of sclerosis band. Stress distribution and collapse Range of necrosis area and stress distribution have a significant impact on the collapse occurring[21]. Stress levels in necrosis area decrease and stress distribution concentrate around the necrotic bone, both result in trabecular fracture and interruption of blood vessel, which will inevitably lead to biomechanical instability in the femoral head[22-23]. Currently, focus of the researches has breakthrough the original base in gross stress analysis, and inclined to study each supporting column in the femoral head. Such kinds of methods in simulating every portion are the innovational tendency to investigate the collapse stress. Fang et al.[24] established different volumes of necrotic tissue in ONFH by a finite element model, and put on and increase the load on the model (Figure 3). It was found that the range of necrosis area would confirmedly influence stress distribution in femoral head. The stress in the surface is higher than the one in bottom of the necrotic area. The pathologic mechanism can be described that cancellous bone fracture occurs under the subchondral region when the peak stress exceeds a critical value, which is the direct cause of the collapse of the femoral head. Karasuyama et al.[20] assessed the stress distribution and evaluate the subchondral fracture zone by measuring Von Mises value. In the meantime, the results are combined with the detection of osteoclasts count in necrosis tissue by pathologic methods. The authors finally concluded that the shear stress and shear tension force tend to be concentrated on trabecular site near the thicken border and osteoclast activity is reason to enhance the rate of collapse. Finite element analysis for therapy Finite element analysis and kinds of hip preservation surgeries Core decompression with multiple drilling, a kind of mature hip preservation surgery, aims to reduce intramedullary pressure, prevent the development of ONFH progress and recover the microcirculation through the hole and tunnel[25-26]. This surgery is relatively simple and easy to operate, even if there are still some technical difficulties[27-28]. For example, a lack of mechanical support, selection of the optimal entry for grafting, suitable number of the drilling holes and so on. Bae et al.[28] studied drilling technique by finite element analysis and investigated the clinical effect of the quantity and entry location of drilling holes. They calculated femoral stress and strain state when the surgeons took the drilling technique. They also analyzed cadavers to assess the best number of the drilling, related trabecular bone density near the entrance according to Von-Mises stress. The results supported that the treatment with multiple drilling is a stable operation. Tran et al. [29] had a similar study in simulating and analyzing core decompression. The results showed that the use of standard drilling core decompression combined with graft implanted will prevent patients from a risk of hip fracture in daily activities. Only when the entry point is at the distal femur, the risk of hip fracture increases. The optimal entry point should be placed at a proximal end of the rotor. Thilo et al.[27] studied core decompression with tantalum rod implant. Moreover, they concluded that core decompression using small drill holes seems to be better compared to the tantalum implant and to conventional core decompression for its low risk of fracture. The results also suggest that a superior performance for core decompression presumably due to the lack of complete bone ingrowth of the tantalum implant. Shi et al.[31] compared the collapse level and collapse ranges under different weights after the patients accepted tantalum rod implant, fibula implant or core decompression. The results showed that smaller necrosis range (60°) is, less different the three operations effect on collapse level. When the necrotic area is in a wide range (120°), all the operations can reduce this level. Lutz et al.[32] compared three kinds of operations by simulating models in finite element analysis, including core decompression, minimal invasive drilling and tantalum rod implant, and then predict their long-term efficacy. Zhou et al.[10] found that the finite element can reconstruct the biomechanical transfer path of the femoral head and reduce the risk of a collapse in the cortical bone (Figure 4). Hip resurfacing arthroplasty Hip resurfacing arthroplasty is a mature surgical treatment for ONFH[33]. Biomechanical changes surrounding the hip resurfacing arthroplasty, however, have not been completely studied[34]. The studies are focused on individual necrosis range and different insertion angles of the implant. Tai et al.[35] investigated that ONFH patients who accepted hip resurfacing arthroplasty were usually to have fracture near femoral neck in long-term follow-up. This phenomenon is caused by stress shielding. The authors simulated four different degrees of necrotic area (0°, 60°, 100°, 115°) and three different insertion angles of implant (10° vara, neutral hip, 10° valgus) in the finite element model. It was noted that the stress distribution after prosthesis of hip resurfacing arthroplasty occurs in the femoral neck. Sakagoshi et al.[36] evaluated the biomechanical changes of HRA in various degrees of necrosis area and implantation angles. Similarly, the authors established three modes of necrosis range of femoral head surface and designed five hip resurfacing arthroplasty handle angles. The results show that the maximum stress distribution on the implant was decreased with stem angles ranging from 130° to 140°. In addition, the peak stress of cement increased with the stem angle turns to vara. Thus, it is required to be mentioned that a large area of necrosis, valgus or varus prosthesis are likely to worsen the biomechanical environment, even resulting in revision arthroplasty. Other operations and surgical planning Besides the treatment as described above, there are many other kinds of innovative affiliated technologies used in reducing head stress or supporting collapse in ONFH[37]. Surgeons can also utilize finite element analysis to demonstrate their feasibility. Xiao et al.[11] analyzed a support device for collapse femoral head. According to finite element analysis of the femoral head surface load stress and strain, author reported that stress concentration of normal femoral head model is in the distance of the femur. The model of the implant stress concentration should focus on the lower part of the lesser trochanter of the femur and the displacement of the stress point is smaller than normal. The authors believed that the device can effectively maintain the biomechanical properties of the femoral neck. Zhou et al.[38] use finite element analysis to modulate the process of percutaneous balloon filling with bone cement femoral head necrosis. The steps include simulating the walking by a single leg and hip peak stress; simulating the avascular necrosis model and balloon filling with bone cement model respectively; calculating Von-Mises values at the top of the femoral head, femoral neck and surrounding area. Fresh human avascular necrosis of femoral head specimens for biomechanical analysis showed that percutaneous balloon filling with bone cement can indeed prevent collapse. Pang et al.[39] used finite element analysis to have a quantitative research in hip preservation surgery based on surgical indications and optimized surgical plan the finite element analysis provided. Finite element analysis can calculate the necrotic area, location, size, volume, weight loading area, percentage of necrosis area in three-dimensional visualization and simulate the procedures of core decompression surgery, bone graft supporting surgery, fibula supporting surgery so as to determine and optimize various indexes in the hip preservation program. "
| [1] Moya-Angeler J, Gianakos AL, Villa JC, et al. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015;6(8):590-601. [2] Motomura G, Yamamoto T, Yamaguchi R, et al. Morphological analysis of collapsed regions in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2011;93(2):184-187. [3] Gou WL, Lu Q, Wang X, et al. Key pathway to prevent the collapse of femoral head in osteonecrosis. Eur Rev Med Pharmacol Sci. 2015;19(15):2766-2774. [4] Zhou GQ, Pang ZH, Chen QQ, et al. Reconstruction of the biomechanical transfer path of femoral head necrosis: a subject-specific finite element investigation. Comput Biol Med. 2014;52(3):96-101. [5] Zhou G, Ying Z, Zeng L, et al. Should thorough Debridement be used in Fibular Allograft with impaction bone grafting to treat Femoral Head Necrosis: a biomechanical evaluation. Bmc Musculoskeletal Disorder. 2014;16(1):1-9. [6] Ueo T, Tsutsumi S, Yamamuro T, et al. Biomechanical aspects of the development of aseptic necrosis of the femoral head. Arch Orthop Trauma Surg. 1985;104(3):145-149. [7] Yang JW, Koo KH, Lee MC, et al. Mechanics of femoral head osteonecrosis using three-dimensional finite element method. Arch Orthop Trauma Surg. 2002;122(2):88-92. [8] Yu T. Research Progress on role of sclerosis band in the collapse of osteonecrosis of the femoral head. Medical Recapitulate. 2015;21(1):84-86.[9] Shi J, Chen J, Wu J, et al. Evaluation of the 3D finite element method using a tantalum rod for osteonecrosis of the femoral head. Med Sci Monit. 2013;20(4):2556-2564. [10] Penix AR, Cook SD, Skinner HB, et al. Femoral head stresses following cortical bone grafting for aseptic necrosis. A finite element study. Clin Orthop Relat Res. 1983;(173): 159-165. [11] Xiao D, Ye M, Li X et al. Development of femoral head interior supporting device and 3d finite element analysis of its application in the treatment of femoral head avascular necrosis. Med Sci Monit. 2015;21(7):1520-1526. [12] Volokh KY, Yoshida H, Leali A, et al. Prediction of femoral head collapse in osteonecrosis. J Biomech Eng. 2006; 128(3): 467-470. [13] Li XL, Lv ZP and Liu YJ. Constructing a finite element model of osteonecrosis of the femoral head. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;30: 5522-5525.[14] Pang ZH, Wei QS, Zhou GQ, et al. Establishment and application of subject-specific three-dimensional finite element mesh model for osteonecrosis of femoral head. Shengwu Yixue Gongchengxue Zazhi. 2012;2:251-255. [15] Liu ZH, Li ZR, Sun W, et al. Risk factors for collapse inpatients with osteonecrosis of bilateral femoral heads: Retrospectiveanalysis based on MRI and CT. J Clin Rehabil Tissue Eng Res. 2008;22:4249-4252.[16] Hofstaetter JG, Wang J, Yan J, et al. Changes in bonemicroarchitecture and bone mineral density following experimental osteonecrosis of the hip in rabbits. Cells Tissues Organs. 2006;184(3-4):138-147. [17] Yu T, Xie L, Zhang Z, et al. Prediction of osteonecrosis collapse of the femoral head based on the proportion of the proximal sclerotic rim. Int Orthop. 2015;39(3):1045-1050. [18] Yu T, Xie L, Chu F. A sclerotic rim provides mechanical support for the femoral head in osteonecrosis. Orthopedics. 2015;38(5):e374-e379.[19] Chen ZP, Xu Y, Qi XQ. The formation and function of the sclerosis rim in the femoral head: a biomechanical point of view. Med Eng Phys. 2015;(15):1-8. [20] Karasuyama K, Yamamoto T, Motomura G, et al. The role of sclerotic changes in the starting mechanisms of collapse: a histomorphometric and FEM study on the femoral head of osteonecrosis. Bone. 2015:644-648. [21] Mont MA, Zywiel MG, Marker DR, et al. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010;92(12):2165-2170. [22] Sakagoshi D, Kabata T, Umemoto Y, et al. A mechanical analysis of femoral resurfacing implantation for osteonecrosis of the femoral head. J Arthrop. 2010;25(8): 1282-1289. [23] Aruwajoye OO, Kim HKW, Aswath PB. Bone Apatite Composition of Necrotic Trabecular Bone in the Femoral Head of Immature Piglets. Calcif Tissue Int. 2015;96(4): 324-334. [24] Fang B, He W, Zhan L, et al. Finite element analysis of stress distribution over femoral head necrosis zones in different necrosis areas. Zhong Yi Zheng Gu. 2012;10: 10-15. [25] Omran AA. Multiple drilling compared with standard core decompression for avascular necrosis of the femoral head in sickle cell disease patients. Arch Orthop Trauma Surg. 2013; 133(5):609-613. [26] Kang P, Pei F, Shen B, et al. Are the results of multiple drilling and alendronate for osteonecrosis of the femoral head better than those of multiple drilling? A pilot study. Joint Bone Spine. 2012;79(1):67-72. [27] Floerkemeier T, Lutz A, Nackenhorst U, et al. Core decompression and osteonecrosis intervention rod in osteonecrosis of the femoral head: clinical outcome and finite element analysis. Int Orthop. 2010;35(10):1461-1466. [28] Marker DR, Seyler TM, Ulrich SD, et al. Do Modern techniques improve core decompression outcomes for hip osteonecrosis? Clin Orthop Relat Res. 2008;466(5): 1093-1103. [29] Bae JY, Kwak DS, Park KS, et al. Finite element analysis of the multiple drilling technique for early osteonecrosis of the femoral head. Ann Biomed Eng. 2013;41(12):2528-2537. [30] Tran TN, Warwas S, Haversath M, et al. Experimental and computational studies on the femoral fracture risk for advanced core decompression. Clin Biomech. 2014;29(4): 412-417. [31] Shi J, Chen J, Wu J, et al. Evaluation of the 3D finite element method using a tantalum rod for osteonecrosis of the femoral head. Med Sci Monit. 2014;20:2556-2564. [32] Lutz A, Nackenhorst U, von Lewinski G, et al. Numerical studies on alternative therapies for femoral head necrosis. Biomech Model Mechanobiol. 2011;10(5):627-640. [33] Sardana V, Philippon MJ, de Sa D, et al. Revision hip arthroscopy indications and outcomes: a systematic review. Arthroscopy. 2015;31(10):2047-2055. [34] Marshall DA, Pykerman K, Werle J, et al. Hip resurfacing versus total hip arthroplasty: a systematic review comparing standardized outcomes. Clin Orthop Relate Res. 2014; 472(7):2217-2230. [35] Marshall DA, Pykerman K, Werle J, et al. Hip resurfacing versus total hip arthroplasty: a systematic review comparing standardized outcomes. Clin Orthop Relate Res. 2014; 472(7):2217-2230. [36] Tai CL, Chen YC, Hsieh PH. The effects of necrotic lesion size and orientation of the femoral component on stress alterations in the proximal femur in hip resurfacing-a finite element simulation. BMC Musculoskel Disord. 2014;15(10): 1092-1096. [37] Sakagoshi D, Kabata T, Umemoto Y, et al. A Mechanical Analysis of Femoral Resurfacing Implantation for Osteonecrosis of the Femoral Head. J Arthrop. 2010;25(8): 1282-1289. [38] Zhou JW, Wu JG, Zhang MC, et al. Balloon inflating and cement filling for treatment of avascular necrosis of the femoral head: a three-dimensional infinite-element study. Nan Fang Yi Ke Da Xue Xue Bao. 2011;10:1724-1728. [39] Pang ZH, He Wei. Clinical application of a new technique based on three-dimensional reconstruction and finite element analysis for osteonecrosis of femoral head in peri-collapse stage. Chin J Joint Surg (Electronic Edition). 2013;3:301-308. [40] Moya-Angeler J, Gianakos AL, Villa JC, et al. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6(8):590-601. |
| [1] | Xue Yadong, Zhou Xinshe, Pei Lijia, Meng Fanyu, Li Jian, Wang Jinzi . Reconstruction of Paprosky III type acetabular defect by autogenous iliac bone block combined with titanium plate: providing a strong initial fixation for the prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1424-1428. |
| [2] | Zhuang Zhikun, Wu Rongkai, Lin Hanghui, Gong Zhibing, Zhang Qianjin, Wei Qiushi, Zhang Qingwen, Wu Zhaoke. Application of stable and enhanced lined hip joint system in total hip arthroplasty in elderly patients with femoral neck fractures complicated with hemiplegia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1429-1433. |
| [3] | Xu Kuishuai, Zhang Liang, Chen Jinli, Ren Zhongkai, Zhao Xia, Li Tianyu, Yu Tengbo. Effect of force line changes on lower limb joints after medial open wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 821-826. |
| [4] | Shao Yangyang, Zhang Junxia, Jiang Meijiao, Liu Zelong, Gao Kun, Yu Shuhan. Kinematics characteristics of lower limb joints of young men running wearing knee pads [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 832-837. |
| [5] | Wang Shaoling, Wang Yanxue, Zheng Yaochao, Yu Shaojun, Ma Chao, Wu Shaoling. Feasibility of ultrasound-guided intra-articular injection in rabbit hip joint [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 657-662. |
| [6] | Zhao Tianyu, Jin Song, Zhang Di, Liu Xiaoxiao, Ma Jiang, Wang Ju. Baduanjin training for patellar tendinopathy in a randomized controlled trial: improving pain, muscle flexibility and lower limb balance stability [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(11): 1662-1668. |
| [7] | Chen Junming, Yue Chen, He Peilin, Zhang Juntao, Sun Moyuan, Liu Youwen. Hip arthroplasty versus proximal femoral nail antirotation for intertrochanteric fractures in older adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1452-1457. |
| [8] | Song Yuanzheng, Ma Hongli, Li Wei, Man Jia, Lin Feng, Zhang Yudong. Application of preoperative virtual simulation plan based on three-dimensional printing technology for complex acetabular fractures: a prospective randomized controlled study [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(36): 5747-5752. |
| [9] | Tang Shuo, Hou Decai. Correlation between traditional Chinese medicine syndrome types of femoral head necrosis and hip joint morphology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(36): 5867-5871. |
| [10] | Cheng Chongjie, Yan Yan, Zhang Qidong, Guo Wanshou. Diagnostic value and accuracy of D-dimer in periprosthetic joint infection: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3921-3928. |
| [11] | Fu Panfeng, Shang Wei, Kang Zhe, Deng Yu, Zhu Shaobo. Efficacy of anterolateral minimally invasive approach versus traditional posterolateral approach in total hip arthroplasty: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(21): 3409-3415. |
| [12] | Yang Kun, Fei Chen, Wang Pengfei, Zhang Binfei, Yang Na, Tian Ding, Zhuang Yan, Zhang Kun . Meta-analysis of the efficacy of robot-assisted and traditional manual implantation of cannulated screws in the treatment of femoral neck fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(18): 2938-2944. |
| [13] | Liu Yongyu, Xu Jingli, Lin Tianye, Wu Feng, Shen Chulong, Xiong Binglang, Zou Qizhao, Lai Qizhong, Zhang Qingwen . Medium-and long-term follow-up of Salter pelvic osteotomy and implant fixation for children with developmental hip dislocation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(15): 2380-2384. |
| [14] | Zhang Haifeng, Zhao Can, Liu Meixiao, Song Cuirong, Pang Yin. Analysis of various degrees of freedom of the hip movement based on motion capture technology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(12): 1815-1819. |
| [15] | Zhang Nianjun, Liu Xiaofang, Zhou Guanming, Su Yao, Hong Shi. Three-dimensional-printing assisted total hip arthroplasty for individualized treatment of adult developmental dysplasia of the hip [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(12): 1820-1825. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||