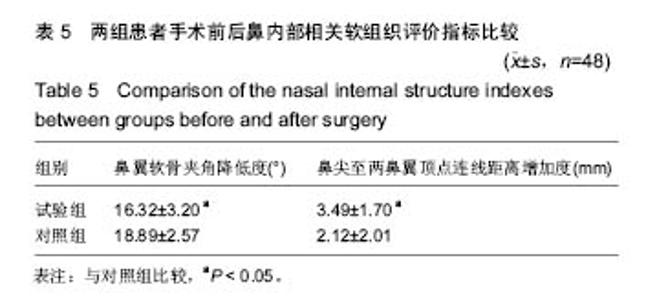
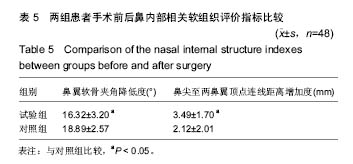
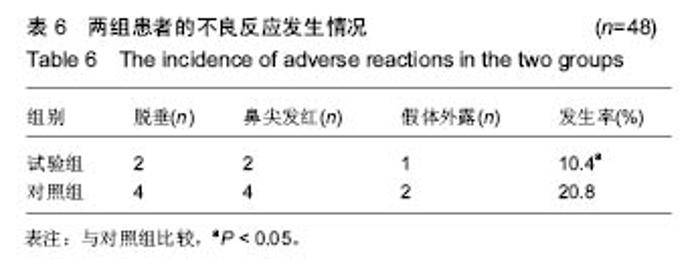
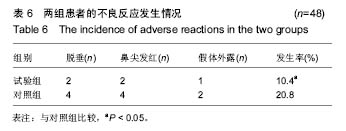
| [1] |
Jing Jinpeng, Zhang Yue, Liu Xiaomin, Liu Yi.
Traditional Chinese medicine injection for promoting blood circulation in prevention of deep vein thrombosis after orthopedic surgery: network meta-analysis
[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1467-1476.
|
| [2] |
Liu Gang, Ma Chao, Wang Le, Zeng Jie, Jiao Yong, Zhao Yi, Ren Jingpei, Hu Chuanyu, Xu Lin, Mu Xiaohong.
Ankle-foot orthoses improve motor function of children with cerebral palsy: a Meta-analysis based on 12 randomized controlled trials
[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1299-1304.
|
| [3] |
Wei Zhoudan, Li Wenjin, Zhu Li, Wang Yu, Zhao Jiaoyang, Chen Yanan, Guo Dong, Hao Min.
Platelet-rich fibrin as a material for alveolar ridge preservation significantly reduces the resorption of alveolar bone height and width after tooth extraction: a meta-analysis
[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 643-648.
|
| [4] |
Zhao Tianyu, Jin Song, Zhang Di, Liu Xiaoxiao, Ma Jiang, Wang Ju.
Baduanjin training for patellar tendinopathy in a randomized controlled trial: improving pain, muscle flexibility and lower limb balance stability
[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(11): 1662-1668.
|
| [5] |
Zhan Fangbiao, Cheng Jun, Zou Xinsen, Long Jie, Xie Lizhong, Deng Qianrong.
Intraoperative intravenous application of tranexamic acid reduces perioperative bleeding in multilevel posterior spinal surgery: a meta-analysis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 977-984.
|
| [6] |
Wan Dadi, Duan Xiangrui, Fan Xinchao, Yuan Ye, Huang Teng, Pan Dikang, Liu Jingyan, Li Xicheng.
Efficacy of posterior cruciate ligament retaining versus posterior stabilized prostheses in total knee arthroplasty: a systematic review and a meta-analysis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(36): 5897-5904.
|
| [7] |
Zhang Feng.
Long-term effects and adverse events of polyetheretherketone versus titanium mesh materials in repairing skull defects: a prospective, single-center, non-randomized controlled, 2-year follow-up clinical trial protocol
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(34): 5501-5505.
|
| [8] |
Jia Hongsheng, Li Xianlin, Cai Lei, Zhang Chongfeng, Chen Zuchuang, Zhang Ye.
Network meta-analysis of different drugs for the treatment of ankylosing spondylitis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(33): 5404-5412.
|
| [9] |
Bai Fuyu, Wang Mengqi, Xue Feng, Li Zhenrui, Yan Qinghao, Zhang Zhiyi, Wang Feng.
Characteristics of randomized controlled trials on acupuncture for stress urinary incontinence in women
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(32): 5197-5203.
|
| [10] |
Huang Cihui, Liu Jiayue, Huang Yingjie, Zhuang Zeqin, Lin Yunxin, Li Dan, Zheng Liang.
Clinical efficacy of small needle knife combined with traditional Chinese medicine in the treatment of knee osteoarthritis: a network Meta-analysis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(32): 5240-5248.
|
| [11] |
Yuan Jiaqin, Luan Fujun, Chen Yangfan, Li Bo.
Efficacy of anterograde and retrograde intramedullary nails in the treatment of distal femoral extraarticular fracture: a meta-analysis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(30): 4915-4920.
|
| [12] |
Li Yanle, Yue Xiaohua, Wang Pei, Nie Weizhi, Zhang Junwei, Tan Yonghai, Jiang Hongjiang.
Intramedullary nail fixation versus plate fixation in the treatment of displaced midshaft clavicular fractures in adults: a meta-analysis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 471-476.
|
| [13] |
Peng Yifan, Wang Chong, Shan Yuanfei, Lu Wenli.
Efficacy of absorbable collagen suture for type I and II surgical incisions: a meta-analysis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(28): 4587-4592.
|
| [14] |
Lü Hui, Huang Denghua, Zou Longfei, Xue Hao, Xie Zonghui, Yu Peigen, Tan Meiyun.
Total hip arthroplasty versus artificial femoral head replacement for the effect of displaced femoral neck fracture: a meta-analysis of 14 randomized controlled trials
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(27): 4421-4428.
|
| [15] |
Zhong Liqing, Zhong Shanshan, Han Weichao, Hu Runkai, He Shufen, Ding Shaobo.
Systematic review of the efficacy and safety of umbilical cord-derived cell transplantation for patients with cerebral palsy
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 4095-4100.
|