Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (37): 5617-5624.doi: 10.3969/j.issn.2095-4344.2016.37.021
Research progress of kinesin II family member 3A
Liu Yi, Cui Zhi-ming, Zhang Jin-long, Xue Peng-fei, Wang Song
- Department of Spine Surgery, the First People’s Hospital of Nantong & the Second Affiliated Hospital of Nantong University, Nantong 226001, Jiangsu Province, China
-
Online:2016-09-09Published:2016-09-09 -
About author:Liu Yi, Master, Attending physician, Department of Spine Surgery, the First People’s Hospital of Nantong & the Second Affiliated Hospital of Nantong University, Nantong 226001, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 81501076; the Major Program of Scientific and Technologic Innovation of Nantong, Clinical Research of Spine Surgery of Nantong, No. HS2014002; the Youth Foundation of Nantong Health Bureau, No. WQ2015016
CLC Number:
Cite this article
Liu Yi, Cui Zhi-ming, Zhang Jin-long, Xue Peng-fei, Wang Song. Research progress of kinesin II family member 3A[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(37): 5617-5624.
share this article
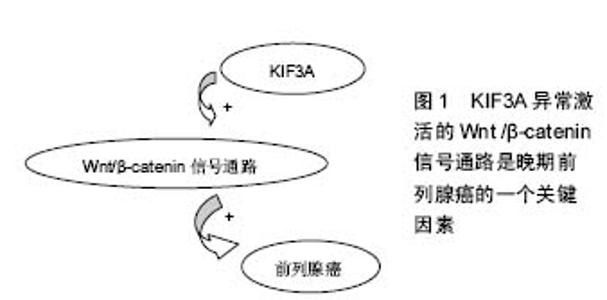
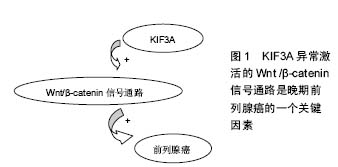
2.1 驱动蛋白的共性 细胞内某些细胞器、囊泡以及蛋白质等物质,在远距离运输的过程中需要依赖于微管运输的马达蛋白的协同作用。驱动蛋白则是基于微管的分子马达蛋白,主要参与细胞器、囊泡、mRNA以及蛋白质等的转运。驱动蛋白通过控制微管的动力学变化,进而调节细胞的动力学变化[2]。 Killesin超家族蛋白(KIFs)是一种动力蛋白,它们能利用ATP水解产生的能量,沿着微管把蛋白复合体、mRNA和细胞器运输到特定部位[3-5]。在有丝分裂和碱数分裂中,KIFs在染色体和纺锤体的运动中也发挥了重要作用[4-6]。KIFs在结构上分头部、颈部、主干和尾部。头部结构比较保守,是催化反应的核心,起ATPase的作用。颈部具有家族特异性,决足了分子的运动方向和对其他生物活动的调节作用。卷曲螺旋主干和尾部,能结合一些全酶和货物分子。KIFs可分为14个家族[7]。 驱动蛋白是真核细胞中以微管为轨道拖动负载做持续运动的分子马达。一个完整的驱动蛋白分子包括两个球状头部,一个颈部和一个扇状尾部[8]。头部上含有连接微管和结合催化ATP的位点,尾部结合货物。它在细胞生命活动中为一系列的运输过程提供动力。驱动蛋白沿微管的运动机制为“交替循环机制模型”[9]。驱动蛋白之间有一定的协同作用。驱动蛋白在细胞运输中起着重要的作用,其突变会是一些疾病产生的根源。KIF3A是Killesin超家族蛋白家族的成员之一,在增殖和迁移上发挥重要作用,且KIF3A参与 Wnt/β-catenin信号的调节,Wnt/β-catenin信号通路在细胞增殖、凋亡、细胞周期等方面发挥着重要的作用,因此,对于KIF3A的信号调节网络的研究具有十分重要的意义。 2.2 KIF3A的概述及作用 KIF3A是异三聚体驱动蛋白2的亚基,属于N-驱动蛋白,微管正端导向的马达蛋白[10]。异三聚体驱动蛋白2则由KIF3A 、KIF3B两个亚基和运载蛋白KAP3 组成[11]。 异三聚体微管马达驱动蛋白Ⅱ已被证明是运动的鞭毛和非运动感觉性纤毛的形成和维护所必须的。Whitehead等[12]报告了人类KIF3A的基因结构(HsKIF3A),并且描述KIF3A在人类和猴子视网膜的定位,还描述了驱动蛋白Ⅱ亚基KIF3A和KIF3B在非洲爪蟾视网膜的定位。有学者扩增了人视网膜cDNA的驱动蛋白Ⅱ亚基KIF3A的片段[12]。利用PCR产物和高度同源的小鼠KIF3A序列[13],在伯克利劳伦斯国家实验室(LBNL),研究者利用人类基因组计划产生的基因组序列,已经构建了KIF3A的完整的编码序列和基因组序列[14]。KIF3A的序列和小鼠KIF3A在氨基酸(99±4)%,核苷酸(87±9)%的水平都是高度相似的,这表明KIF3A基因全编码区是控制在选择性压力水平之下,可以和已知的最高度保守的蛋白质相媲美[15]。序列比较表明,KIF3A、KIF3B和KIF3c亚基相比其他kif3成员,更加紧密地相互关联,这表明异三聚体复合物的3个驱动蛋白很久以前就存在不同。Whitehead等[12]的免疫印迹结果表明,驱动蛋白Ⅱ在猴子、人类以及非洲爪蟾等脊椎动物视网膜中广泛的表达。 2.2.1 KIF3A与原生纤毛的关系 原生纤毛是在细胞分化和发育过程中起着信号中心的作用[16]。纤毛的装配和维修以及轴丝的生长,是由驱动马达蛋白为基础的转运过程所介导的,使粒子沿轴丝双向转运[17]。KIF3A是驱动蛋白Ⅱ复合体的一个组成部分,缺乏KIF3A,使原生纤毛的形成和维持均失败[18]。原生纤毛的关键作用已被初步的证明[18]。许多人类先天畸形综合征是由于蛋白质的纤毛和纤毛的基底部突变导致的[16]。 2.2.2 KIF3A在新生骨形成中的调节作用 原生纤毛存在于大多数哺乳动物细胞,包括成骨细胞、骨细胞[19-21],原生纤毛是动态的细胞器,其中驱动蛋白2调节鞭毛内运输筏向纤毛远端的顺行运输,以及动促进鞭毛内运输物质向纤毛基体的逆行运输[20-22]。驱动蛋白2是一个由两马达亚基组成的异三聚体复合物,驱动蛋白2家族蛋白A(KIF3A)和B(KIF3B)和非亚基,驱动蛋白相关蛋白(KAP3)[23]。 原生纤毛给很多信号通路提供场所,这些通道可能涉及到成骨细胞的发育和新生的成骨细胞功能[22,24-30],包括多囊蛋白1和多囊蛋白2,它能形成一个机械传感信号,定位于原生纤毛。最近已被证实KIF3A的C末端与PC2(也称为多囊蛋白2)的C-末端相绑定,决定了其在原生纤毛的定位和功能[31]。最近发现多囊蛋白1在成骨细胞的形成和骨骼加载的机械传感应答中起重要作用[21,32-33]。这表明,原生纤毛也具有调节成骨细胞的发育和功能的作用。 有强烈的证据表明,在胚胎时以及出生后的软骨内成骨形成期间,原生纤毛通过Hedgehog(Hh)信号调节胚胎软骨内骨形成。使用Prx1-CRE和Dermo1-CRE选择性的敲除小鼠的 IFT88或者KIF3A,Prx1-CRE和Dermo1-CRE可在肢体间充质中表达(包括软骨和软骨膜),因为胚胎软骨内成骨的改变,使得肢体骨变短[34-35]。 Ni等[23]选择性的敲除kif3anull/flox杂交突变小鼠成骨细胞中的KIF3A,会导致体内和体外成骨细胞功能缺陷和骨量减少,导致骨密度低于正常。此外,KIF3A缺乏的成骨细胞表现出响应体外流体剪应力的基因剂量依赖性的减少,破坏许多纤毛相关通路包括Hh和Wnt信号通路。 2.2.3 KIF3A引导MDCK细胞的微管动力学、细胞迁移和管腔形成 微管马达驱动蛋白2和它的亚基KIF3A是初级纤毛形成所必不可少的,牵涉到广泛的发育异常的细胞器。纤毛外,驱动蛋白介导的转运涉及囊泡和N-钙黏蛋白的运输,但是仍不明确纤毛外的KIF3A是否以及如何影响细胞的基本功能,如迁移或多细胞结构的形成。Christopher等[36]研究表明,MDCK细胞中四环素诱导的KIF3A缺乏减缓了上皮细胞迁移。KIF3A缺失细胞前缘的微管未能垂直性的长入前缘,并且KIF3A缺失细胞的微管动力学减弱。KIF3A的缺失阻碍了侧膜的形成,并且完全阻止胶原蛋白的三维结构的形成。这些数据揭示,KIF3A调节细胞外周的微管细胞骨架,表明纤毛外的KIF3A在形态发生中有着意想不到的功能[36]。 小鼠的KIF3A结构性缺失会导致无序的中胚层发育和中期胚胎致死,表明了Hedgehog信号缺陷[37]。纤毛中的少部分Hedgehog(HH)成分和细胞器在HH信号通路的激活中起着核心作用[37-38],然而,一些证据表明,驱动蛋白2在纤毛外介导转运:在培养的非洲爪蟾细胞中,突变的Kif3a表达封闭了从ER到Golgi(高尔基体)的囊泡运输,阻止色素扩散,并且驱动蛋白2中的 Kap3的缺失与细胞外围N-钙黏蛋白转运的减少密切相关[39]。有趣的是,同型二聚体驱动蛋白2的亚基KIF17,与KIF3A在线虫感觉神经细胞的纤毛转运中联合作用。影响微管的聚合和稳定,以及上皮细胞形态的形成[40]。这些研究增加了KIF3A和异三聚体驱动蛋白2在纤毛外微管运输和微管动力学发挥作用的可能性。 Christopher等[36]观察表明,KIF3A对微管的调节行为具有意想不到的功能,对迁徙、侧膜形成以及管腔形成十分重要。微管的这两个功能很明显是由KIF3A控制的:微管动力学和微管取向。发现在KIF3A缺乏细胞中微管的极性紊乱,这与最近的一项研究报道一致,在果蝇神经元树突中驱动蛋白2是微管的取向所必须的[41]。驱动蛋白2与EB-1和抗原提呈细胞组成一复合物,引导微管向细胞体树突样生长,在轴突损伤后逆转树突的微管极性,因此,体内的KIF3A是微管极化所必须的。Christopher等[36]的发现和这些证据都一致认为驱动蛋白2普遍在微管取向中发挥作用。KIF3A功能不限于迁移。KIF3A和微管的动态性似乎在高阶极性如在胶原基质球和肾小管的生成具有重要作用。 KIF3A的缺失导致纤毛的缺失,并且KIF3A是研究纤毛功能的一个重要工具。普遍认为,敲除KIF3A后的表型变化被解释为纤毛丢失的后果[37]。基本纤毛基因的结构性缺失导致了中期胚胎致死和严重紊乱的中胚层发育[42]。 2.2.4 KIF3A在精子形成过程中的功能 男性的生殖能力依赖于正常精子的生成。精子形成是一个复杂的分化过程,其特征是单倍体细胞的减数分裂和奇妙的形态变化。精子的形成涉及微管网络活性的变化,微管网络维持着减数分裂,细胞分化,核的重塑,和鞭毛的形成。 Mari等[43]的研究表明,基于微管的顺行运输马达蛋白Kif3A是精子形成过程中精子尾部成型以及核成型所需的。KIF3A与KIF1结合蛋白(KBP)在小鼠睾丸中相互作用。 KIF3A是异三聚体驱动蛋白Ⅱ蛋白复合物中的一个马达蛋白,是正常精子形成和雄性生育力所需要的[44]。由于精子颈带组织和精子尾部形成的缺陷问题,KIF3A缺乏会导致头部形状的畸形。在纤毛形成和作用的过程中,体细胞内的KIF3A 在鞭毛内运输中起着重要的作用[45]。KIF3A在精子尾部成分MNS1的运输中起到一定作用[44]。 在两栖爬行动物东方蝾螈的精子发生过程中,N-末端驱动蛋白KIF3A参与精子鞭毛的形成[46]。在中华绒螯蟹和罗氏沼虾等甲壳类动物的精子形成过程中发现,Kifc1类似基因参与精核形变和顶体发生[47]。在嘉庚鞘精子发生过程中发现,KIF3A和KIF3B以二聚体的形式通过鞭毛内运输和微管套内运输来参与精细胞核形变及尾部的形成.总之,驱动蛋白在动物精子发生过程中发挥了不可替代的作用[8]。 2.2.5 KIF3A与某些肿瘤的关系 异常激活的Wnt/β-catenin信号通路是晚期前列腺癌的一个关键的因素,但是相比前列腺癌,许多其他癌症中激活Wnt信号通路后的遗传学改变是很少观察到的。前列腺癌调节Wnt信号通路的其他的分子机制仍有待明确。这表明,KIF3A马达驱动蛋白Ⅱ的亚基,在前列腺癌中起到激活Wnt信号通路的功能,见图1。KIF3A在大多数人类前列腺癌细胞株和原发肿瘤活检中是正向调节的。KIF3A表达水平与较高的格里森评分、TNM分期、前列腺癌的转移情况密切相关。此外,在良性的前列腺细胞中,KIF3A的外源性表达促进细胞生长,反之,癌细胞中KIF3A的沉默会减少细胞增殖、停止细胞生长以及细胞迁移或者入侵。在前列腺癌细胞中,KIF3A增加CK1依赖的DVL2磷酸化和β- catenin的激活,导致Wnt信号靶基因的转活,如cyclin D1、HEF1和基质金属蛋白酶9。这些研究结果支持了这一观点,在晚期前列腺癌中,正向调节的KIF3A通过kif3a-dvl2-β-catenin轴发挥作用,是Wnt信号通路异常活化原因之一[48]。"
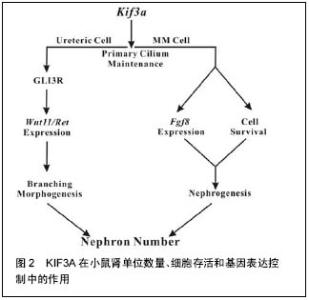
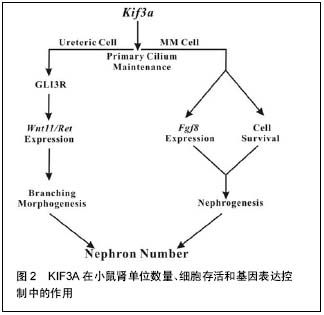
原生纤毛已发现在人类多形性胶质母细胞瘤肿瘤活检中被发现,并且衍生出细胞链。Lan等[49]用慢病毒载体创建3种患者来源的多形性胶质母细胞瘤非显性表达的细胞株,KIF3A被动形式(dnkif3a)。在所有未更改的细胞株中,大多数多形性胶质母细胞瘤细胞能够产生纤毛的后代,细胞中dnkif3a表达会溶解纤毛形成。小鼠异种移植中,未更改的和dnkif3a表达的细胞株显示出敏感度的差异,Shh信号通路的激活和肿瘤相关的生存变化。在一个细胞株中,通过dnkif3a的表达或使用环杷明抑制SMO的信号,Shh诱导的细胞增殖被阻止在体外。植入dnkif3a表达细胞的小鼠存活时间增多。在第2个细胞株中,dnkif3a的表达促进了细胞基线的增殖,同时令人惊讶的是,可在SHH诱导的细胞死亡中发现它。植入这些dnkif3a表达细胞的小鼠存活时间减少。最后,在第3个细胞株中,dnkif3a的表达对细胞的增殖、SHH的敏感度、小鼠的生存时间无影响。这些结果表明,KIF3A是GBM细胞纤毛形成中必不可少的,但其对胶质瘤细胞行为的作用是高度可变的[49]。 髓母细胞瘤细胞来源于失去了生长控制的颗粒神经元前体(GNPs)。在正常的发育过程中,GNPs对Sonic hedgehog(Shh)的响应是与修补(PTCH)受体结合的配体。如果PTCH基因的一个拷贝丢失,髓母细胞瘤可能形成,如人类格林综合征和PTCH+/-的小鼠。SHH信号适当的转导取决于原生纤毛。原生纤毛的缺失导致信号接收错误,以及无法正确激活SHH靶基因。KIF3A是马达驱动蛋白的组成部分,是原生纤毛的形成所必需的。这里使用他莫昔芬诱导消融新生的PTCH+/-小鼠小脑中GNPs的KIF3A,来表明KIF3A是髓母细胞瘤形成所必需的。为了探讨原生纤毛对肿瘤生成的重要性,分别敲除培养的细胞和肿瘤移植细胞的KIF3A。肿瘤模型的KIF3A损失导致其生长停滞和衰退。髓母细胞瘤的表现就好像它们依赖于原生纤毛的存在。研究结果强调了潜在的效用:破坏纤毛来治疗Hh通路相关的髓母细胞瘤[50]。 2.2.6 KIF3A在小鼠肾单位数量、细胞存活和基因表达控制中的作用 Li等[51]在小鼠的繁殖中使用Cre介导的重组产生KIF3A缺乏,主要是针对胚胎肾的输尿管和/或肾间质细胞。上述组织中的原生纤毛逐渐减少导致肾单位数量明显减少。值得注意的是,除了囊肿形成,输尿管上皮细胞的原生纤毛减少导致Wnt11和RET的表达下降,和输尿管分支减少。GLI3抑制剂的合成型表达(gli3d699/+)在纠正着这些异常。在胚胎后肾间充质细胞中,KIF3A不足限制了肾源性祖细胞的存活以及肾单位形成所必需的基因表达。同时,Li等[51]的数据表明,KIF3A通过显著的细胞谱系特异性机制控制肾单位数量。 小鼠肾脏KIF3A缺乏致肾单位形成减少,KIF3A细胞特异性的方式来控制肾单位数目,KIF3A通过 GLI3R依赖性的方式控制分支形态发生,KIF3A控制肾间充质细胞的存活,KIF3A的缺乏导致肾间质中成纤维细胞生长因子8的缺乏,KIF3A控制肾间充质细胞成纤维细胞生长因子8表达。 发现多囊肾疾病的蛋白突变定位于原生纤毛,确定原生纤毛为肾小管上皮细胞分化的关键[52]。直接支持纤毛依赖性功能,在输尿管上皮细胞谱系中,KIF3A肾脏特异性的灭活,抑制了纤毛形成,并诱导上皮囊肿[53]。肾单位形成可能因为NPHP2缺乏、纤毛集中蛋白和多囊肾病基因等而受损[54],原生纤毛可能在肾发育阶段发挥作用,控制肾单位形成以及在此之前的上皮细胞分化。 纤毛蛋白KIF3A、IFT88以及IFT20,均参与了鞭毛内运输[55],是肾纤毛形成所必须的;目前已知上述中任一个的失活均会导致囊性肾脏病[56]。Li等[51]的研究数据表明,在小鼠肾脏发育发病过程中,KIF3A的表达与原生纤毛均在输尿管细胞和肾间充质细胞中被发现。使用kif3aloxp等位基因,Cre介导的重组致使Cre重组酶表达的细胞中KIF3A总体的缺失。原生纤毛的缺失致使动力学减慢。然而,KIF3A的缺乏和原生纤毛数量的减少导致肾前体结构形成数量和最终成熟的肾单位减少。这种显性机制的实验研究支持KIF3A在小鼠肾发育过程中功能的模型。模型研究结果表明,KIF3A控制肾单位形成的功能具体在输尿管和肾间充质细胞[51]。在泌尿系中,KIF3A以gli3r依赖性反应控制着输尿管分支的形成。分枝数的控制是肾单位生成数量的一个关键因素。在肾间充质细胞中,KIF3A发挥两大显著的效果,见图2。首先,KIF3A控制细胞的存活,例如,KIF3A缺陷小鼠中肾胚芽发育成的肾单位更少,幸存的KIF3A缺陷肾间充质干细胞与野生型相比,很少能够参加肾源性结构形成。第二,在肾间充质干细胞中,KIF3A控制着关键基因的表达。"
| [1] Vale RD. The molecular motor toolbox for intracellar transport. Cell. 2003;112: 467-470. [2] Zhang HW,Wen ST, Zhang H , et al. A feedback- controlled Brownian ratchet operated by a temperature switch. Chin Phys B. 2012;21(7):078701. [3] Hirokawa N. Organelle transport along microtubules- the role of KIFs. Trends Cell Biol. 1996;6(4):135-141. [4] Hirokawa N, Noda Y, Okoda Y. Kinesin and dynein superfaimly proteins in organelle transport and cell division. Curr Opin Cell Biol. 1998;10(1):60-73. [5] Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6: 201-214. [6] Sharp DJ, Rogers GC, Seholey JM. Micotubule motors in mitosis. Nature.2000;407:41-47. [7] Miki H, Okada Y, Hirokawa N. Analysis of kinesin superfaimly: insights into structure and function. Trends Cell Biol. 2005;15(9):467-476. [8] Liu M, Xu N, Ruan S, et al. Resrarch Progress of Kinesin and Its Function. J Hangzhou Normal Univ. 2013;(1):40-44. [9] Hirokawa N, Takemura R. Kinesin superfaimly proteins and their various functions and dynamics. Exp Cell Res. 2004;301, 50-59. [10] Shi SH, Cheng T, Jan LY, et al. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004; 14: 2025-2032. [11] Yamazaki H, Nakata T, Okada Y, et al. Cloning and characterization of KAP3: a novel kinesin superfamily- associated protein of KIF3A/3B. Proc Natl Acad Sci USA. 1996;93: 8443-8448. [12] Whitehead JL, Wang SY, Bost-Usinger L, et al. Photoreceptor Localization of the KIF3A and KIF3B Subunits of the Heterotrimeric Microtubule Motor Kinesin II in Vertebrate Retina. Exp Eye Res. 1999;69: 491-503. [13] Aizawa H, Sekine Y, Takemura R, et al. Kinesin family in murine central nervous system. Cell Biol. 1992;119: 1287-1296. [14] Frazer KA, Ueda Y, Zhu Y, et al. Computational and biological analysis of 680 kb of DNA sequence from the human 5q31 cytokine gene cluster region. Gen Res. 1997;7:495-512. [15] Makalowski W, Zhang J, Boguski MS. Comparative analysis of 1196 orthologous mouse and human full-length mRNA and protein sequences. Gen Res. 1996;(9):846-857. [16] Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 2010;11: 331-344. [17] Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125: 439-442. [18] Marszalek JR, Ruiz-Lozano P, Roberts E et al. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA. 1999;96: 5043-5048. [19] Berbari NF, O’Connor AK, Haycraft CJ, et al. The primary cilium as a complex signaling center. Curr Biol. 2009; (19):526-535. [20] Davis, EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: new functional roles for an ancient organelle. Dev Cell. 2006;(11): 9-19. [21] Xiao Z, Zhang S, Mahlios J, et al. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. Biol Chem. 2006;(281): 30884-30895. [22] Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;(137):32-45. [23] Ni Q, Zhou SX, Li C, et al. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J Cell Sci. 2012;(125):1945-1957. [24] Christensen ST, Pedersen LB, Schneider L, et al. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;(8): 97-109. [25] Han YG, Spassky N, Romaguera-Ros M, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;(11):277-284. [26] Huang FD, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;(102): 11325-11330. [27] Kovacs JJ, Whalen EJ, Liu R, et al. Beta-arrestin- mediated localization of smoothened to the primary cilium.Science. 2008;( 320): 1777-1781. [28] Serra R. Role of intraflagellar transport and primary cilia in skelet al development. Anat Rec (Hoboken). 2008;(291): 1049-1061. [29] Veland IR, Awan A, Pedersen LB, et al. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009;(111): 39-53. [30] Wong SY, Seol AD, So PL, et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;(15): 1055-1061. [31] Li Q, Montalbetti N, Wu Y, et al. Polycystin-2 cation channel function is under the control of microtubular structures in primary cilia of renal epithelial cells. Biol Chem. 2006;281:37566-37575. [32] Xiao Z, Dallas M, Qiu N, et al. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011;25: 2418-2432. [33] Xiao Z, Zhang S, Cao L, et al. Conditional disruption of Pkd1 in osteoblasts results in osteopenia due to direct impairment of bone formation. Biol. Chem. 2010;285: 1177-1187. [34] Haycraft CJ, Zhang Q, Song B, et al. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307-316. [35] Kolpakova HE, Jinnin M, Hou B, et al. Kinesin-2 controls development and patterning of the vertebrate skeleton by Hedgehog- and Gli3-dependent mechanisms. Dev Biol. 2007;309: 273-284. [36] Christopher B, Fruzsina K, Bjoern B, et al. Kif3a Guides Microtubular Dynamics, Migration and Lumen Formation of MDCK Cells. PLoS ONE. 2013;8(5): e62165. [37] Huangfu D, Liu A, Rakeman AS, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426: 83-87. [38] Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11: 331-344. [39] Teng J, Rai T, Tanaka Y, et al. The KIF3 motor transports N-cadherin and organizes the developing neuroepithelium. Nat Cell Biol. 2005;7: 474-482. [40] Jaulin F, Kreitzer G. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol. 2010;190: 443-460. [41] Song B, Haycraft CJ, Seo HS, et al. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev Biol. 2007;305:202-216. [42] Murcia NS, Richards WG, Yoder BK, et al. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127: 2347-2355. [43] Mari SL, Noora K, Anu S. KIF1-binding protein interacts with KIF3A in haploid male germ cells. Reproduction. 2015;(150): 209-216. [44] Lehti MS, Kotaja N, Sironen A, et al. KIF3A is essential for sperm tail formation and manchette function. Mol Cell Endocrinol. 2013; 377:44-55. [45] Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180 23-29. [46] Hu J, Xu N, Tan FQ, et al. Molecular characterization of a Kif3A-like kineain gene in the testis of the Chinese fire-bellied newt Cynops orientalis. Mol Biol Rep. 2012; 39(4):4207-4214. [47] Wang YT, Mao H, Hou CC, et al. Characterization and expression pattern of Kifc1-like kinesin gene in the testis of the Macrobrachium nipponense with discussion of its relationship with atructure lamellar complex(LCx) and acroframosome(AFS). Mol Biol Rep. 2012;9(7):7591-7598. [48] Zun L, Ryan ER, Zemin W. KIF3a Promotes Proliferation and Invasion via Wnt Signaling in Advanced Prostate Cancer. Mol Cancer Res. 2014;12(4); 491-503. [49] Lan B, Hoang M, Loic PD, et al. Disruption of KIF3A in patient-derived glioblastoma cells: effects on ciliogenesis, hedgehog sensitivity, and tumorigenesis. Oncotarget. 2016;(7):7029-7043. [50] Monique TB, Eric WH, Matthew PS. Kif3a is Necessary for Initiation and Maintenance of Medulloblastoma. Carcinogenesis. 2013;34(6):1382-1392. [51] Li JC, Alevtina G, Lin C,et al. Kif3a Controls Murine Nephron Number Via GLI3 Repressor, Cell Survival, and Gene Expression in a Lineage-Specific Manner. PLOS ONE. 2013; 8: e65448. [52] Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. Am Soc Nephrol. 2007;18: 1381-1388. [53] Lin F, Hiesberger T, Cordes K, et al. Kidneyspecific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003; 100: 5286-5291. [54] Simons M, Gloy J, Ganner A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005; 37: 537-543. [55] Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in evelopment, homeostasis, and disease. Cell. 2009;137: 32-45. [56] Jonassen JA, San Agustin J, Follit JA, et al. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol. 2008;183: 377-384. [57] Ille F, Sommer L. Wnt signaling: multiple functions in neur development. Cell Mol Life Sci. 2005;62(10): 1100-1108. [58] Corbit KC, Shyer AE, Dowdle WE, et al. Kif3a constrains β-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10(1):70-76. [59] Val RD, Milligan RA. The way things move: looking under the hood of molecular motor protein.. Science. 2002; 288(5463):88-95. [60] Schliwa M, Woehlke G. Molecular motors. Nature. 2003;422(6933):759-765. [61] Ocbina PJ, Aanderson KV. Intraflagellar transport cilia and mammalian Hedgehog signalling : analysis in mouse embryonic fibroblasts. Dev Dyn. 2008;37(8):2030-2038. [62] Liu Z, Rebowe RE, Wang Z, et al. KIF3a promotes proliferation and invasion via Wnt signaling in advanced prostate cancer. Mol Cancer Res. 2014; 12(4):491-503. |
| [1] | Chen Huijing, Chen Yun, Deng Yuer, Gan Yanling, Zhan Wengang, Tan Qijia, Xie Caijun, Li Cong, Zhang Zhiqiang. Short- and long-term effects of olfactory ensheathing cells in the treatment of chronic spinal cord injury: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(9): 1468-1476. |
| [2] | Wei Weibing1, Zhou Xiangxing1, Zhou Binbin2, Li Yijun1, Zou Zhilong1, Yang Hui1 . Evaluation of lower limb function in rat models of spinal cord injury at different segments [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(7): 1073-1077. |
| [3] | Wei Weibing, Zou Zhilong, Zhou Binbin, Li Bolin, Qin Jingyu, Feng Zhenfen, Li Jiannan, Ye Liangying,Wu Rongmi. Selection of injury segments in a rat model of spinal cord injury: network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(4): 650-656. |
| [4] | Deng Guiying1, 2, Zeng Gaofeng3, Cen Zhongxi1, Gao Yunbing1, Cao Baichuan1, Huang Jianhua1, Zong Shaohui1 . Effect of miRNA-136-5p on inflammatory factors in rat models of acute spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(15): 2397-2402. |
| [5] | Huang Jianhua1, Zeng Gaofeng2, Cen Zhongxi1, Deng Guiying1, Gao Yunbing1, Zong Shaohui1 . Changes of cytokines in peripheral blood within 48 hours after acute spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(15): 2409-2414. |
| [6] | He Zhijiang, Zhu Lei, Cheng Shixiang, Huang Kui, Chen Cao, Sun Minglin. Neurotrophin-3 modified hyaluronan-methylcellulose hydrogel promotes neurological function in rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(14): 2202-2207. |
| [7] | Cheng Jianping, Li Hua, Li Xiongjie. Bone marrow mesenchymal stem cell transplantation combined with Lindera glauca leaf extract attenutes inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(13): 1975-1981. |
| [8] | Wei Weibing, Zhou Binbin, Li Bolin, Qin Jingyu, Feng Zhenfen, Li Jiannan. Methods for establishing rat models of spinal cord injury: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(12): 1949-1954. |
| [9] | Li Ren-bin, Yu Guang-shu, Lin Yan-bin, Zhou Jia-feng, Huang Yi-qi, Zheng Wei. Edaravone effects on the expression levels of collagen type I and IV after spinal cord injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(8): 1235-1240. |
| [10] | Wang Ji-chao, Zhu Jun-peng, Wu Xue-ping. Physical activities promote recovery from spinal cord injury in patients [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(8): 1287-1293. |
| [11] | Zhang Jian-jun, Wang Dong, Shi Huan-chang, Yang Wei-shan, Wang Lin. Mild hypothermia with subarachnoid transplantation of neural stem cells to repair spinal cord injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(5): 686-691. |
| [12] | Xiao Xue-fei1, 3, Li Juan-juan2, Huang Hui1, Zhang Xiang1, Huang Yao1, Li Jing-hui1, Yu Hua-lin1. Gastrodin effects on the neural functional recovery and expression of glial fibrillary acidic protein in a rat model of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(4): 558-563. |
| [13] | Sun Nian-yi1, 2, Xiong Xing-juan1, He Yu3, Wang Wen-chun2, Zhang An-ren2. Every-other-day fasting improves motor functional recovery of rats with clip-compression injury of the spinal cord [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(4): 564-569. |
| [14] | Wang Wen-zhao, Su Yan-lin, Li Hong-fei, Shen Lin, Chen Jia-nan, Pan Xin-da, Jia Tang-hong. miR-21a-5p knockdown promotes the locomotor function recovery of mouse models of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(32): 5180-5185. |
| [15] | Lu Yu-bao, Yang Yang, Wang Yun-chang, Chen Guo-hu, Yuan Lin-hui, Li Chun-yan, Guan Xin, Ma Zhan-jun. Bone marrow mesenchymal stem cells in the treatment of spinal cord injury: research hotspots and directions [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(29): 4736-4742. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||