Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (37): 5602-5608.doi: 10.3969/j.issn.2095-4344.2016.37.019
Previous Articles Next Articles
Roles of skeletal muscle growth factors, myosin, and collagen in the repair of injured skeletal muscle
Feng Jian
- Department of Sports, Chang'an University, Xi’an 710064, Shaanxi Province, China
-
Received:2016-06-23Online:2016-09-09Published:2016-09-09 -
About author:Feng Jian, Master, Lecturer, Department of Sports, Chang'an University, Xi’an 710064, Shaanxi Province, China
CLC Number:
Cite this article
Feng Jian. Roles of skeletal muscle growth factors, myosin, and collagen in the repair of injured skeletal muscle[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(37): 5602-5608.
share this article
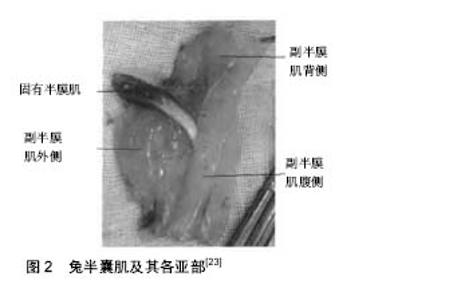
2.1 影响骨骼肌损伤后修复质量的生长因子 2.1.1 胰岛素样生长因子 骨骼肌损伤后再生能力有限,自然愈合质量不可靠,易发生再次损伤,而依靠成肌细胞增殖获得的修复常常是不完全的,严重影响了肌肉运动功能。近年来研究发现胰岛素样生长因子与骨骼肌的损伤修复有密切联系。在胰岛素样生长因子的作用下,成肌细胞加速增殖和分化,促进骨骼肌再生修复,同时增强再生肌肉的收缩力。胰岛素样生长因子因其化学结构与胰岛素相似而得名,它是一类既具有促细胞分化和增殖活性又具有胰岛素样作用的多肽。胰岛素样生长因子由具有特定功能的配体胰岛素样生长因子Ⅰ和胰岛素样生长因子Ⅱ、受体和结合蛋白组成,在个体生长和细胞分化方面有着重要的作用,在正常生理活动中也有极为重要的意义。体内外研究表明,它们调控着细胞的生长和增殖。胰岛素样生长因子是一种强力的促细胞分裂素,其作用决定于胰岛素样生长因子与其受体的相互作用。现已发现,许多疾病如心血管疾病、代谢综合征、骨质疏松等与胰岛素样生长因子系统密切相关[15-16]。研究和利用这一因子,解决与之密切相关的多种疾病,将为临床医学提供新的思路。许多组织在不同阶段都有胰岛素样生长因子的表达。胰岛素样生长因子不仅是内分泌因子,还能在组织局部起作用,使许多组织的功能进一步分化。近期研究表明,运动对骨骼肌组织内胰岛素样生长因子的分泌有调节作用,从而引起骨骼肌形态及代谢方面的运动性变化适应[15]。 胰岛素样生长因子是与组织形态维持以及预防细胞死亡的重要因素,也是在局部肌肉修复过程中激活肌肉卫星细胞并且调节蛋白合成。胰岛素样生长因子Ⅰ和Ⅱ通过两种主要的细胞表面受体Ⅰ型和Ⅱ型和许多结合蛋白发挥生物学作用。骨骼肌损伤后再生过程中,胰岛素样生长因子Ⅰ或胰岛素样生长因子Ⅱ的表达水平升高[15]。在成肌细胞整个增生过程中,胰岛素样生长因子Ⅱ一直呈低水平表达,在分化阶段升高到巅峰状态。如果外源给予胰岛素样生长因子Ⅰ,可能可以延缓肌萎缩进展,延缓衰老。但局部直接注射胰岛素样生长因子效应往往短暂,效果并不理想。 胰岛素样生长因子I是生长激素发挥促生长作用的重要调节因子。其与胰岛素结构相似并具有细胞分化和增殖功能的多肽,其生物学活性受胰岛素样生长因子I受体及胰岛素结合蛋白的调节。胰岛素样生长因子I具有调控细胞凋亡、影响蛋白质的合成、调节骨骼肌生长和修复、保护神经系统等生物学功能。生长激素具有促进体内蛋白质沉积、骨骼生长等功能。虽然许多组织都能表达胰岛素样生长因子Ⅰ,但它们各自的功能不同。骨骼肌组织表达的胰岛素样生长因子Ⅰ对骨骼肌损伤后的再生、修复非常重要,也是通过适当干预保持肌肉质量的重要调节因素。骨量的获得或丢失受遗传和环境因素调节,胰岛素样生长因子Ⅰ在这两个过程中起着关键性的作用。在过去的10余年中,关于胰岛素样生长因子I在细胞生长及其生命过程中的作用的研究已取得了巨大的进步,研究者们目前已确定组织特异性胰岛素样生长因子调节系统的结构和特性。骨骼肌能够根据环境刺激的变化改变其质量,可以通过细胞融合或提高蛋白质水平来增加它的大小。在骨骼肌中,力刺激诱导胰岛素样生长因子Ⅰ发生选择性剪接,产生能够激活卫星细胞而使细胞增殖的生长因子[16]。 2.1.2 成纤维细胞生长因子 刘建华等[17]利用大鼠坐骨神经钳夹损伤模型,观察碱性成纤维细胞生长因子对大鼠坐骨神经损伤后腓肠肌功能恢复的作用。结果显示治疗组大鼠受损神经所支配之骨骼肌诱发电位及骨骼肌收缩力的恢复率较对照组明显加快,表明碱性成纤维细胞生长因子能促进大鼠坐骨神经损伤后骨骼肌功能恢复。同样柏志全等[18]观察碱性成纤维细胞生长因子对大鼠坐骨神经损伤后腓肠肌功能恢复的作用,研究人员以钳夹损伤左侧坐骨神经大鼠为模型,治疗组肌肉注射碱性成纤维细胞生长因子,对照组注射等渗盐水,结果与对照组相比,治疗组腓肠肌诱发电位幅值恢复率明显加快;腓肠肌单收缩力和强直收缩力的恢复率也显著加强。说明碱性成纤维细胞生长因子能促进大鼠坐骨神经损伤后骨骼肌运动功能的恢复。 成纤维细胞生长因子2是具有成肌细胞增殖的特性的多效细胞因子。L8-成肌细胞遗传修饰藻酸盐包封的成纤维细胞生长因子2修复骨骼肌损伤后细胞增殖增加,细胞凋亡减少。脂联素表达增加, p75(NTR) 表达也有变化,成肌球细胞计数增加,成纤维细胞生长因子2的过度表达成肌细胞不能显着地改善肌肉力量,但能够调节受伤肌肉组织的细胞增殖以及凋亡,调节脂肪生成[19]。骨骼肌损伤骨骼肌变性包括早微血管改变和炎症细胞浸润,而且受多种生长因子的控制。碱性成纤维细胞生长因子,胰岛素样生长因子1和转化生长因子β1在骨骼肌损伤血管发生和炎症反应中起不同的作用。在损伤端注入特异性抗生长因子的中和抗体,碱性成纤维细胞生长因子的免疫中和减少毛细管、巨噬细胞和肥大细胞的数量,和延迟坏死的肌纤维细胞吞噬;胰岛素样生长因子1和转化生长因子β1免疫中和促进肌肉重建,巨噬细胞浸润和坏死的肌纤维吞噬。胰岛素样生长因子1中和减少肥大细胞的数量,并没有修改T细胞或嗜中性粒细胞,转化生长因子β1的中和增加所有这些细胞的数量[16]。 2.1.3 肝细胞生长因子 肝细胞生长因子存在于骨骼肌,通过激活静止卫星细胞刺激卫星细胞增殖并促进骨骼肌再生。骨骼肌损伤1 h后可以检测到肝细胞生长因子的表达,同时激活肝细胞生长因子蛋白酶,骨骼肌损伤后肝和脾中肝细胞生长因子mRNA有表达,脾脏肝细胞生长因子蛋白的水平增加,说明内分泌肝细胞生长因子诱导因子的骨骼肌再生中起重要作用,非肌肉器官衍生的肝细胞生长因子在卫星细胞活化/增殖等骨骼肌再生过程中起重要作用[20]。 肝素结合生长因子在肌管形成阶段起重要作用,肝细胞生长因子是由相对分子质量35 000和70 000的2条多肽链组成的蛋白因子,存在于肝脏与肝外多种组织和细胞,能刺激肝细胞的DNA合成,它还参与多种细胞的增殖、迁移,对各类肿瘤的侵袭转移有着重要的诱导作用。肝细胞生长因子不同于再生肝和胚胎肝细胞胞棱中的肝刺激物质,具有刺激肝细胞分裂、促进细胞运动、启动肝再生等多种生理作用,是一个具有强烈丝裂原活性以及细胞运动与形态调节的多效性细胞因子。目前的研究表明,能够特异性促进肝细胞生长的因子分为两种血源性肝细胞生长因子和肝源性肝细胞刺激因子[21]。 2.2 肌球蛋白在骨骼肌中的表达与作用 肌球蛋白是一种多功能的蛋白是一个六聚体的生物大分子,由2条相对分子质量为19 400-21 200的重链亚基和2对相对分子质量20 000左右的的轻链亚基构成。肌球蛋白轻链是肌球蛋白的组成成分之一,作为结构和调节蛋白在肌纤维的发生分化、肌肉运动和代谢等过程中发挥重要生理功能。而肌球蛋白重链具有肌球蛋白分子运动马达和细丝形成的作用,可以分为2个功能性结构域即氨基端具有马达功能的头部球状区域和羧基端具有丝状形成能力的一个具有α双螺旋长杆状结构域。而ATPase酶解和肌动蛋白结合位点都位于重链头部区域Subfragment-1部位的2个表面环附近。肌球蛋白重链是横纹肌收缩蛋白的重要组成成分。已发现肌球蛋白重链有多种亚型,在不同的机械负荷下可相互转化,呈现出对外界机械信号的高度适应性[22]。机械生长因子可能在机械负荷和肌球蛋白重链亚型转变间发挥化学桥梁的功能。骨骼肌的收缩特性部分是由肌球蛋白重链亚型决定的。 骨骼肌肌纤维类型的构成比较复杂,呈单一性分布的较少。有研究在通过肌球蛋白重链的亚型的分类方法检测家兔半膜肌各亚部的肌纤维型的构成情况[23],结果显示家兔半膜肌4个亚部中,固有半膜肌只含有Ⅰ型肌纤维,表达肌球蛋白重链Ⅰ型和少量肌球蛋白重链Ⅱa型基因。副半膜肌腹侧含有少量Ⅰ型肌纤维,表达少量肌球蛋白重链Ⅰ型基因,其余主要含有Ⅱ型肌纤维,表达肌球蛋白重链Ⅱa、肌球蛋白重链Ⅱb和肌球蛋白重链Ⅱx型基因;而其背侧和外侧只含有快肌,仅表达肌球蛋白重链Ⅱa、肌球蛋白重链Ⅱb和肌球蛋白重链Ⅱx型基因(图2)。同样蔡永清等[24]家兔的半腱肌由近侧亚部和远侧亚部连续排列组成,各有一条肌神经的第一级分支支配。家兔的半膜肌则由固有半膜肌和副半膜肌两部分组成,其中副半膜肌又分为背侧,腹侧和外侧3个平行排列的亚部。"
| [1] Lu H, Huang D, Ransohoff RM,et al. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair.FASEB J. 2011;25(10):3344-3355. [2] Chien SH, Chen SK, Lin SY,et al. Repair method and healing of skeletal muscle injury. Gaoxiong Yi Xue Ke Xue Za Zhi.1991;7(9):481-488. [3] Mu X, Peng H, Pan H, et al. Study of muscle cell dedifferentiation after skeletal muscle injury of mice with a Cre-Lox system.PLoS One.2011;6(2):e16699. [4] Castiglioni A,Corna G,Rigamonti E,et al.Rovere- Querini P.FOXP3+ T Cells Recruited to Sites of Sterile Skeletal Muscle Injury Regulate the Fate of Satellite Cells and Guide Effective Tissue Regeneration.PLoS One.2015;10(6):e0128094. [5] 王光.细胞生长因子对失神经骨骼肌萎缩的作用机制分析[J].医学综述,2010,16(18):2754-2757. [6] Nicholas J, Voss JG, Tsuji J,et al.Time course of chemokine expression and leukocyte infiltration after acute skeletal muscle injury in mice.Innate Immun. 2015 Apr;21(3):266-274. [7] Radi ZA, Koza-Taylor PH, Bell RR,et al. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury.Am J Pathol. 2011;179(1):240-247 [8] de Almeida P, Tomazoni SS, Frigo L,et al.What is the best treatment to decrease pro-inflammatory cytokine release in acute skeletal muscle injury induced by trauma in rats: low-level laser therapy, diclofenac, or cryotherapy? Lasers Med Sci.2014;29(2):653-658. [9] Wirsdörfer F, Bangen JM, Pastille E,et al. Breaking the co-operation between bystander T-cells and natural killer cells prevents the development of immunosuppression after traumatic skeletal muscle injury in mice.Clin Sci (Lond). 2015;128(11):825-838. [10] Gumucio JP, Flood MD, Phan AC,et al.Targeted inhibition of TGF-β results in an initial improvement but long-term deficit in force production after contraction-induced skeletal muscle injury. J Appl Physiol (1985). 2013 Aug 15;115(4):539-45. [11] Park JK, Ki MR, Lee EM,et al. Losartan improves adipose tissue-derived stem cell niche by inhibiting transforming growth factor-β and fibrosis in skeletal muscle injury. Cell Transplant.2012;21(11):2407-2424. [12] 黄人健,彭宝珍,周国瑛,等.人心肌肌球蛋白轻链1与重链和肌动蛋白的结合[J].生物化学与生物物理学报,2001, (1):41-45. [13] Fuchs B, Zumstein M, Regenfelder F,et al. Upregulation of alpha-skeletal muscle actin and myosin heavy polypeptide gene products in degenerating rotator cuff muscles.J Orthop Res.2008; 26(7):1007-11. [14] Onuoha GN, Alpar EK, Laprade M,et al. Effects of bone fracture and surgery on plasma myosin heavy chain fragments of skeletal muscle. Clin Invest Med. 1999;22(5):180-184. [15] Lu H, Huang D, Saederup N,et al. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury.FASEB J. 2011; 25(1):358-369. [16] Lefaucheur JP, Gjata B, Lafont H,et al.Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-beta 1.J Neuroimmunol. 1996 Oct;70(1):37-44. [17] 刘建华,柏志全,黎昭洪,等.bFGF对大鼠坐骨神经损伤后骨骼肌功能恢复的促进作用[J].广州医药,1999,30(3): 15-17. [18] 柏志全,刘建华,王子栋,等.bFGF对大鼠坐骨神经损伤后腓肠肌功能恢复的影响[J].暨南大学学报(自然科学与医学版),1999(4):1-3. [19] Stratos I, Madry H, Rotter R,et al. Fibroblast growth factor-2-overexpressing myoblasts encapsulated in alginate spheres increase proliferation, reduce apoptosis, induce adipogenesis, and enhance regeneration following skeletal muscle injury in rats. Tissue Eng Part A. 2011;17(21-22):2867-2877. [20] Suzuki S, Yamanouchi K, Soeta C,et al.Skeletal muscle injury induces hepatocyte growth factor expression in spleen. Biochem Biophys Res Commun. 2002;292(3):709-714. [21] Ikutomo M, Sakakima H, Matsuda F, et al. Midkine- deficient mice delayed degeneration and regeneration after skeletal muscle injury.Acta Histochem. 2014; 116(2):319-326. [22] Jerkovic R, Argentini C, Serrano-Sanchez A,et al. Early myosin switching induced by nerve activity in regenerating slow skeletal muscle.Cell Struct Funct. 1997;22(1):147-153. [23] 陈渊,钟天来,李寿田,等.家兔半膜肌各亚部肌球蛋白重链表达差异的实验研究[J].遵义医学院学报,2011,(5): 448-452. [24] 蔡永清,李名扬,靳仕信.家兔半腱肌和半膜肌的亚部化及肌纤维型构成的研究[J].大连医科大学学报,1988, 10(2): 45-49. [25] Andrew C,FRY Michael H,FERKIN Brian K,等.雌、雄草原田鼠外周骨骼肌肌球蛋白重链的表达[J].动物学报, 2008, (1):104-110. [26] Smith HK, Plyley MJ, Rodgers CD,et al.Expression of developmental myosin and morphological characteristics in adult rat skeletal muscle following exercise-induced injury.Eur J Appl Physiol Occup Physiol.1999;80(2):84-91. [27] Zhang J,Dhoot GK.Localized and limited changes in the expression of myosin heavy chains in injured skeletal muscle fibers being repaired. Muscle Nerve. 1998;21(4):469-81. [28] Koh IH, Kang HJ, Jeon SW,et al. Passive skeletal muscle excursion after tendon rupture correlates with increased collagen content in muscle.Yonsei Med J. 2014;55(5):1395-1399. [29] Yun YR, Lee S, Jeon E,et al. Fibroblast growth factor 2-functionalized collagen matrices for skeletal muscle tissue engineering.Biotechnol Lett. 2012;34(4): 771-778. [30] Baptista J, Martins MD, Pavesi VC,et al. Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed Laser Surg. 2011;29(1):11-17. [31] Koskinen SO, Wang W, Ahtikoski AM,et al. Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content.Am J Physiol Regul Integr Comp Physiol. Am J Physiol Regul Integr Comp Physiol. 2001;280(5): R1292-300. [32] Chen YH, Peng YL, Wang Y,et al.TGF-β1-induced synthesis of collagen fibers in skeletal muscle-derived stem cells.J Huazhong Univ Sci Technolog Med Sci. 2013;33(2):238-243. [33] de Souza TO, Mesquita DA, Ferrari RA, et al. Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Lasers Med Sci.2011;26(6):803-814. [34] 石艺平.人源Ⅲ型胶原蛋白在毕赤酵母中表达及性质研究[D].福建:福建师范大学, 2014:1-82. |
| [1] | Shen Jinbo, Zhang Lin. Micro-injury of the Achilles tendon caused by acute exhaustive exercise in rats: ultrastructural changes and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1190-1195. |
| [2] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [3] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [4] | Liu Liu, Zhou Qingzhu, Gong Zhuo, Liu Boyan, Yang Bin, Zhao Xian. Characteristics and manufacturing techniques of collagen/inorganic materials for constructing tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 607-613. |
| [5] | Xu Xiaoming, Chen Yan, Song Qian, Yuan Lu, Gu Jiaming, Zhang Lijuan, Geng Jie, Dong Jian. Human placenta derived mesenchymal stem cell gel promotes the healing of radiation skin damage in SD rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3976-3980. |
| [6] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [7] | Chen Lei, Zheng Rui, Jie Yongsheng, Qi Hui, Sun Lei, Shu Xiong. In vitro evaluation of adipose-derived stromal vascular fraction combined with osteochondral integrated scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3487-3492. |
| [8] | Yang Li, Li Xueli, Song Jinghui, Yu Huiqian, Wang Weixia. Effect of cryptotanshinone on hypertrophic scar of rabbit ear and its related mechanism [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(20): 3150-3155. |
| [9] | Liu Zhendong, Wang Rui, Li Xiaolei, Wang Jingcheng. Review of interferon alpha-2b inhibiting scar formation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 317-321. |
| [10] | Chen Siyu, Li Yannan, Xie Liying, Liu Siqi, Fan Yurong, Fang Changxing, Zhang Xin, Quan Jiayu, Zuo Lin. Thermosensitive chitosan-collagen composite hydrogel loaded with basic fibroblast growth factor retards ventricular remodeling after myocardial infarction in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2472-2478. |
| [11] | Chen Liang, Meng Shu, Cheng Guoping, Ding Yi . Effects of fish scale collagen membrane on adhesion, proliferation and osteogenic differentiation of rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2494-2499. |
| [12] | Zhang Hui, Wang Shaohua, Wang Qian, Wang Hui, Gan Hongquan, Cui Yishuang, Li Qijia, Wang Zhiqiang. Porous tantalum coated with RGD polypeptide can activate the integrin/focal adhesion kinase signaling pathway of MG63 cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2535-2540. |
| [13] | Li Shao, Liang Yongkang, Gao Yi, Peng Qing. Establishment and functional in vitro characteristics of three-dimensional collagen HepaRG microsphere [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2541-2547. |
| [14] | Liu Yunyi, Wang Bo, Wang Lin. Effects of post-exercise gastrocnemius needling on Achilles tendon degeneration in obese rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(14): 2211-2218. |
| [15] | Liu Zhigang, Guo Qinggong, Chen Jingtao. Effect of Capparis spinosa total alkaloid on proliferation and apoptosis of nucleus pulposus cells in an intervertebral disc degeneration rat model [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(11): 1699-1704. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||