Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (8): 1929-1939.doi: 10.12307/2026.017
Previous Articles Next Articles
Metal organic framework/carboxymethyl chitosan-oxidized sodium alginate/platelet-rich plasma hydrogel promotes healing of diabetic infected wounds
Liu Hongjie1, Mu Qiuju2, Shen Yuxue1, Liang Fei1, Zhu Lili1, 2
- 1Clinical Laboratory Science Teaching and Research Department, School of Clinical Laboratory Science, Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Department of Blood Transfusion, Affiliated Hospital of Guizhou Medical University, Guiyang 550000, Guizhou Province, China
-
Received:2024-11-13Accepted:2025-01-06Online:2026-03-18Published:2025-07-15 -
Contact:Zhu Lili, Senior technologist, Clinical Laboratory Science Teaching and Research Department, School of Clinical Laboratory Science, Guizhou Medical University, Guiyang 550004, Guizhou Province, China; Department of Blood Transfusion, Affiliated Hospital of Guizhou Medical University, Guiyang 550000, Guizhou Province, China -
About author:Liu Hongjie, Master candidate, Junior technologist, Clinical Laboratory Science Teaching and Research Department, School of Clinical Laboratory Science, Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:Guizhou Provincial Science and Technology Plan Project, No. ZK[2024]184 (to ZLL)
CLC Number:
Cite this article
Liu Hongjie, Mu Qiuju, Shen Yuxue, Liang Fei, Zhu Lili. Metal organic framework/carboxymethyl chitosan-oxidized sodium alginate/platelet-rich plasma hydrogel promotes healing of diabetic infected wounds[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1929-1939.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
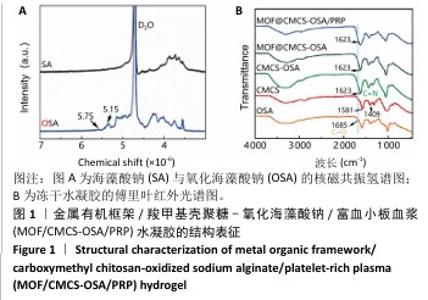
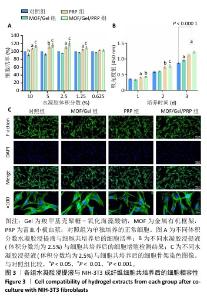
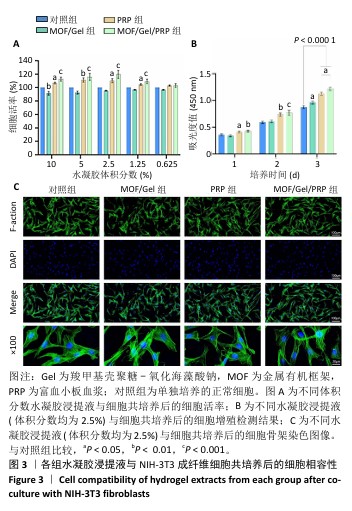
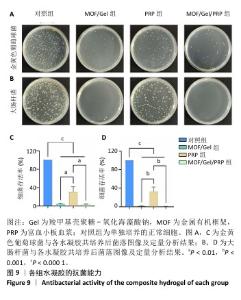
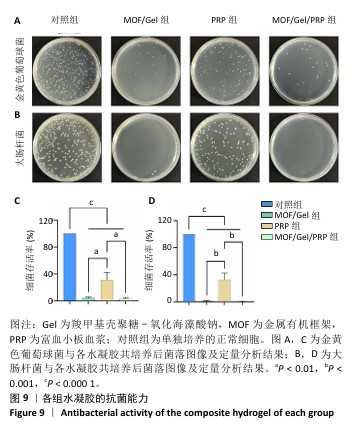
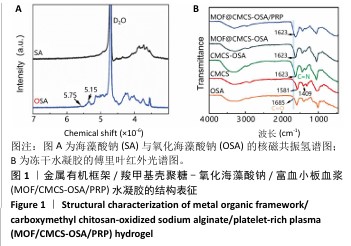
2.1 水凝胶结构及形貌表征 采用核磁共振氢谱观察氧化海藻酸钠的氧化过程(图1A),在5.15×10-6和5.75×10-6之间观察到羟基和醛基形成的半缩醛峰,表明海藻酸钠的α-羟基被成功氧化成二醛。傅里叶变换红外光谱分析以检查水凝胶形成(图1B),发现氧化海藻酸钠和羧甲基壳聚糖混合后它们特征性的峰值消失,在1 623 cm-1处出现了一个新的亚胺结构峰值,证实了席夫碱反应的发生;在氧化海藻酸钠、金属有机框架材料与富血小板血浆的凝胶反应中,于1 623 cm-1处也能够观察到席夫碱键的形成,从而证实了几种物质的成功交联。扫描电镜下可见羧甲基壳聚糖-氧化海藻酸钠、金属有机框架/羧甲基壳聚糖-氧化海藻酸钠和金属有机框架/羧甲基壳聚糖-氧化海藻酸钠/富血小板血浆水凝胶都具有相互交联且均匀的多孔结构,富血小板血浆的引入进一步提高了水凝胶的交联密度,使水凝胶内部结构更加紧密(图2)。"
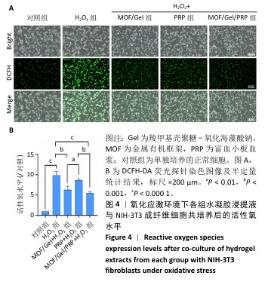
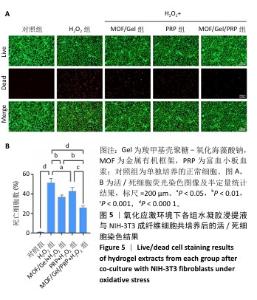
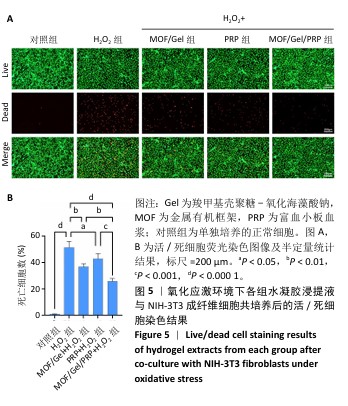
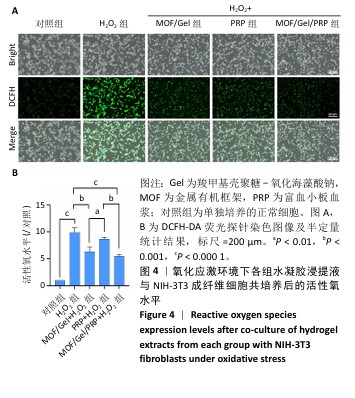
2.3 水凝胶的活性氧清除能力 DCFH-DA荧光探针检测结果显示,暴露于H2O2后,NIH-3T3成纤维细胞中活性氧水平增加(P < 0.000 1),达到对照组的9.9倍;与H2O2组相比,MOF/Gel+H2O2组和MOF/Gel/PRP+H2O2组细胞内活性氧水平显著下降(P < 0.001,P < 0.000 1),分别下降了1.5倍和1.8倍;与富血小板血浆+H2O2组相比,MOF/Gel+H2O2组和MOF/Gel/PRP+H2O2组细胞内活性氧水平下降(P < 0.01,P < 0.001),见图4,提示MOF/Gel水凝胶与MOF/Gel/PRP水凝胶具有强大的抗氧化能力。"
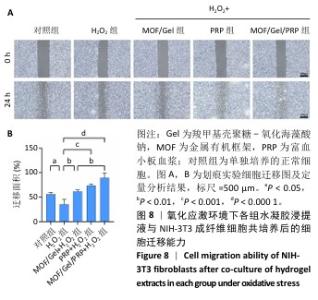
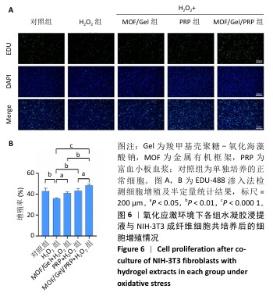
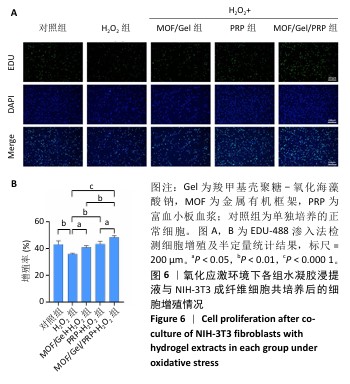
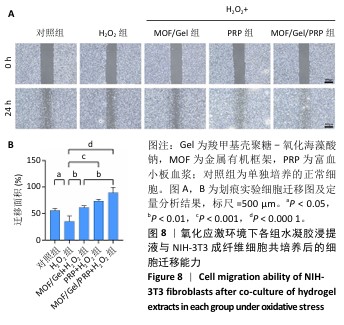
2.7 复合水凝胶对氧化应激条件下细胞迁移的影响 细胞迁移在组织修复和再生过程中发挥着关键作用,此次研究通过细胞划痕实验探究不同处理下NIH-3T3成纤维细胞的迁移能力。H2O2处理显著抑制了细胞迁移,仅有30.01%的细胞迁移面积,而对照组细胞迁移面积为55.89%;与H2O2组相比,MOF/Gel+H2O2组、富血小板血浆+H2O2组细胞迁移能力有所增强,细胞迁移面积分别达到了61.21%,73.66%,而MOF/Gel/PRP+H2O2组细胞迁移面积最高,达到了89.53%,表明MOF/Gel水凝胶与富血小板血浆具有协同作用,显著刺激了细胞在氧化应激状态下的细胞迁移能力,见图8。以上结果强调了MOF/Gel水凝胶协同富血小板血浆作为有效治疗剂的潜力,有助于提升细胞迁移能力,克服氧化应激对创面愈合的负面影响。"
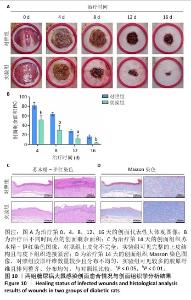
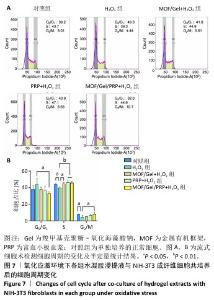
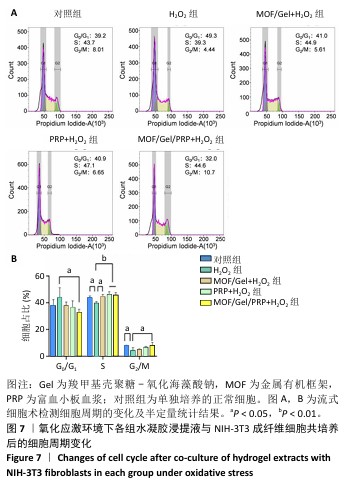
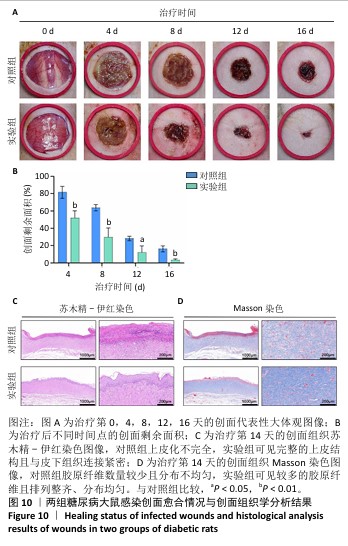
2.9 水凝胶对糖尿病大鼠感染创面的修复作用 两组大鼠治疗不同时间的创面大体观,见图10A,治疗第4天,对照组创面存在出明显的细菌感染,实验组未观察到细菌生物膜的形成。对照组创面愈合速率较慢,治疗第16天仍有16%的创面剩余面积,实验组治疗第16天的创面剩余面积仅为3.4%,实验组治疗第4,8,12,16天的创面剩余面积均小于对照组(P < 0.05或P < 0.01),见图10B。治疗第14天,苏木精-伊红染色结果显示,对照组上皮化不完全,实验组可见完整的上皮结构且与皮下组织连接紧密,见图10C;Masson染色结果显示,对照组胶原纤维数量较少且分布不均匀,实验组可见较多的胶原纤维且排列整齐、分布均匀,见图10D。这些结果提示MOF/Gel/PRP水凝胶展现出优异的促创面愈合效果。"
| [1] XIONG Y, CHU X, YU T, et al. Reactive Oxygen Species-Scavenging Nanosystems in the Treatment of Diabetic Wounds. Adv Healthc Mater. 2023;12(25):2300779. [2] DUNNILL C, PATTON T, BRENNAN J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017; 14(1):89-96. [3] LIANG Y, HE J, GUO B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15(8):12687-12722. [4] SILVA JC, PITTA MG, PITTA IR, et al. New peroxisome proliferator-activated receptor agonist (GQ-11) improves wound healing in diabetic mice. Adv Wound Care. 2019;8(9):417-428. [5] LIU K, ZHANG H, WANG Z, et al. Carrier-Free, Injectable Hydrogel Formed by Self-Assembled Nanofibers of Antimicrobial and Anti-inflammatory Peptide for Wound Healing. ACS App Nano Mater. 2024;7(14):16585-16598. [6] CHEN J, LIU Y, CHENG G, et al. Tailored hydrogel delivering niobium carbide boosts ROS‐scavenging and Antimicrobial activities for diabetic wound healing. Small. 2022;18(27):2201300. [7] ZHENG SY, WAN XX, KAMBEY PA, et al. Therapeutic role of growth factors in treating diabetic wound. World J Diabetes. 2023;14(4):364. [8] XU D, WU L, YAO H, et al. Catalase‐like nanozymes: Classification, catalytic mechanisms, and their applications. Small. 2022;18(37):2203400. [9] WU A, LI M, CHEN Y, et al. Multienzyme Active Manganese Oxide Alleviates Acute Liver Injury by Mimicking Redox Regulatory System and Inhibiting Ferroptosis. Adv Healthc Mater. 2024;13(11):2302556. [10] HU S, WANG L, LI J, et al. Catechol-modified and MnO2-nanozyme-reinforced hydrogel with improved antioxidant and antibacterial capacity for periodontitis treatment. ACS Biomater Sci Eng. 2023;9(9):5332-5346. [11] SUN H, YU X, MA X, et al. MnOx-CeO2 catalyst derived from metal-organic frameworks for toluene oxidation. Catal Today. 2020;355:580-586. [12] PAN W, WANG W, WANG P, et al. Sustained delivery of extracellular vesicles using UiO-66-NH2 crosslinked hydrogel for accelerating chronic diabetic wound-healing. Mater Design. 2024;238:112688. [13] ZHU J, QIU W, YAO C, et al. Water-stable zirconium-based metal-organic frameworks armed polyvinyl alcohol nanofibrous membrane with enhanced antibacterial therapy for wound healing. J Colloid Interf Sci. 2021;603:243-251. [14] FAN YL, LIU HJ, WANG ZL, et al. A One-Nano MOF-Two-Functions Strategy Toward Self-healing, Anti-inflammatory, and Antibacterial Hydrogels for Infected Wound Repair. Chem Eng J. 2024;497:155037. [15] BI X, BAI Q, LIANG M, et al. Silver peroxide nanoparticles for combined antibacterial sonodynamic and photothermal therapy. Small. 2022;18(2): 2104160. [16] ZHANG Y, KANG J, CHEN X, et al. Ag nanocomposite hydrogels with immune and regenerative microenvironment regulation promote scarless healing of infected wounds. J Nanobiotechnol. 2023;21(1):435. [17] EVERTS P, ONISHI K, JAYARAM P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020; 21(20):7794. [18] FANG J, WANG X, JIANG W, et al. Platelet-rich plasma therapy in the treatment of diseases associated with orthopedic injuries. Tissue Eng Part B Rev. 2020;26(6):571-585. [19] CL K, JEYARAMAN M, JEYARAMAN N, et al. Antimicrobial Effects of Platelet-Rich Plasma and Platelet-Rich Fibrin: A Scoping Review. Cureus. 2023;15(12):e51360. [20] ZHANG Y, WANG ZL, DENG ZP, et al. An extracellular matrix-inspired self-healing composite hydrogel for enhanced platelet-rich plasma-mediated chronic diabetic wound treatment. Carbohyd Polym. 2023;315:120973. [21] SHANG S, ZHUANG K, CHEN J, et al. A bioactive composite hydrogel dressing that promotes healing of both acute and chronic diabetic skin wounds. Bioact Mater. 2024;34:298-310. [22] KŁOSIŃSKI KK, WACH RA, KRUCZKOWSKA W, et al. Carboxymethyl Chitosan Hydrogels for Effective Wound Healing—An Animal Study. J Funct Biomater. 2023;14(9):473. [23] JIANG Y, GUO S, JIAO J, et al. A biphasic hydrogel with self-healing properties and a continuous layer structure for potential application in osteochondral defect repair. Polymers (Basel). 2023;15(12):2744. [24] KIRK T, AHMED A, ROGNONI E. Fibroblast memory in development, homeostasis and disease. Cells. 2021;10(11):2840. [25] WOODLEY JP, LAMBERT DW, ASENCIO IO. Reduced Fibroblast Activation on Electrospun Polycaprolactone Scaffolds. Bioengineering (Basel). 2023; 10(3):348. [26] WOODLEY JP, LAMBERT DW, ASENCIO I O. Understanding fibroblast behavior in 3D biomaterials. Tissue Eng Part B Rev. 2022;28(3):569-578. [27] 梅和平,石孟琼,陈茂华,等.地连二心颗粒对H2O2 致小鼠成纤维细胞损伤的保护作用研究[J].中药药理与临床,2017,33(1):139-144. [28] WU M, TU J, HUANG J, et al. Exosomal IRF1-loaded rat adipose-derived stem cell sheet contributes to wound healing in the diabetic foot ulcers. Mol Med. 2023;29(1):60. [29] 胡晶晶 . 具有 ROS 清除作用的水凝胶包裹小檗碱 @ NMOF 促进糖尿病足创面愈合 [D]. 广州 : 南方医科大学 ,2022. [30] DENG L, DU C, SONG P, et al. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid Med Cell Longev. 2021;2021(1):8852759. [31] LONG M, ROJO DE LA VEGA M, WEN Q, et al. An essential role of NRF2 in diabetic wound healing. Diabetes. 2016;65(3):780-793. [32] YAN R, ZHANG X, XU W, et al. ROS-Induced Endothelial Dysfunction in the Pathogenesis of Atherosclerosis. Aging Dis. 2024;16(1):250-268. [33] WANG Y, XIE R, LI Q, et al. A self-adapting hydrogel based on chitosan/oxidized konjac glucomannan/AgNPs for repairing irregular wounds. Biomater Sci. 2020;8(7):1910-1922. [34] ZHENG Z, LI M, SHI P, et al. Polydopamine-modified collagen sponge scaffold as a novel dermal regeneration template with sustained release of platelet-rich plasma to accelerate skin repair: a one-step strategy. Bioact Mater. 2021;6(8):2613-2628. [35] ZHOU J, CHEN N, LIAO J, et al. Ag-activated metal-organic framework with peroxidase-like activity synergistic Ag+ release for safe bacterial eradication and wound healing. Nanomaterials (Basel). 2022;12(22):4058. [36] DASTNESHAN A, RAHIMINEZHAD S, MEZAJIN M N, et al. Cefazolin encapsulated UIO-66-NH2 nanoparticles enhance the antibacterial activity and biofilm inhibition against drug-resistant S. aureus: In vitro and in vivo studies. Chem Eng J. 2023;455:140544. [37] KESHK WA, ZAHRAN SM. Mechanistic role of cAMP and hepatocyte growth factor signaling in thioacetamide-induced nephrotoxicity: Unraveling the role of platelet rich plasma. Biomed Pharmacother. 2019;109:1078-1084. [38] RANIA N, ISLAM IH, ATEF M, et al. Effect of platelet rich plasma on an experimental rat model of adriamycin induced chronic kidney disease. Med J Cairo Univ. 2019;87:2207-2217. [39] BURANASIN P, MIZUTANI K, IWASAKI K, et al. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS One. 2018;13(8):e0201855. [40] ELMONGY NF, MEAWAD SB, ELSHORA SZ, et al. Platelet-rich plasma ameliorates neurotoxicity induced by silver nanoparticles in male rats via modulation of apoptosis, inflammation, and oxidative stress. J Biochem Mol Toxicl. 2023;37(9):e23420. [41] ZHENG WQ, ZHANG JH, LI ZH, et al. Mammalian mitochondrial translation infidelity leads to oxidative stress–induced cell cycle arrest and cardiomyopathy. Proc Natl Acad Sci U S A. 2023;120(37):e2309714120. [42] THANGAPAZHAM RL, DARLING TN, MEYERLE J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15(5):8407-8427. [43] YI W, BAO Q, XU D, et al. ETS1 Expression in Diabetic Foot Ulcers: Implications for Fibroblast Phenotype and Wound Healing Through the PP2A/YAP Pathway. J Inflamm Res. 2024;17:7373-7388. [44] DEVEREAUX J, NURGALI K, KIATOS D, et al. Effects of platelet-rich plasma and platelet-poor plasma on human dermal fibroblasts. Maturitas. 2018;117: 34-44. [45] WEI S, WANG Z, LIANG X, et al. A composite hydrogel with antibacterial and promoted cell proliferation dual properties for healing of infected wounds. AM J Transl Res. 2023;15(7):4467. [46] HUANG P, HE Y, HUANG C, et al. MOF@platelet-rich plasma antimicrobial GelMA dressing: structural characterization, bio-compatibility, and effect on wound healing efficacy. RSC Adv. 2024;14(41):30055-30069. |
| [1] | Liu Yang, Liu Donghui , Xu Lei, Zhan Xu, Sun Haobo, Kang Kai. Role and trend of stimuli-responsive injectable hydrogels in precise myocardial infarction therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2072-2080. |
| [2] | Guo Yuchao, Ni Qianwei, Yin Chen, Jigeer·Saiyilihan, Gao Zhan . Quaternized chitosan hemostatic materials: synthesis, mechanism, and application [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2091-2100. |
| [3] | Lai Yu, Chen Yueping, Zhang Xiaoyun. Research hotspots and frontier trends of bioactive materials in treating bone infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2132-2144. |
| [4] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [5] | Chen Ling, Mao Qiuhua, Xu Pu, Zhang Wenbo. Effect of water-soluble matrix of nano-pearl powder on proliferation, migration and apoptosis of mouse fibroblasts#br# [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 338-344. |
| [6] | Liu Xiaohong, Zhao Tian, Mu Yunping, Feng Wenjin, Lyu Cunsheng, Zhang Zhiyong, Zhao Zijian, Li Fanghong. Acellular dermal matrix hydrogel promotes skin wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 395-403. |
| [7] | Wang Zilin, Mu Qiuju, Liu Hongjie, Shen Yuxue, Zhu Lili. Protective effects of platelet-rich plasma hydrogel on oxidative damage in L929 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 771-779. |
| [8] | Zhao Hongxia, Sun Zhengwei, Han Yang, Wu Xuechao , Han Jing. Osteogenic properties of platelet-rich fibrin combined with gelatin methacryloyl hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 809-817. |
| [9] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [10] | Dong Meilin, Du Haiyu, Liu Yuan. Quercetin-loaded carboxymethyl chitosan hydrogel promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 692-699. |
| [11] |
Zhang Bo, Zhang Zhen, Jiang Dong.
Tannic acid modified interpenetrating network hydrogel promotes tissue remodeling of ruptured Achilles tendon after surgery#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 721-729.
|
| [12] | Li Yonghang, Li Wenming, Yan Caiping, Wang Xingkuan, Xiang Chao, Zhang Yuan, Jiang Ke, Chen Lu. Critical bone defect repaired with anti-fibrosis and “H”-type core-shell bionic scaffold [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3420-3431. |
| [13] | He Rui, Li Chongyi, Wang Ruiyao, Zeng Dan, Fan Daidi. Application of MXene-based hydrogels in wound repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3486-3493. |
| [14] | Liu Zhongyu, Li Wenya, Fan Yonghong, Lyu Shuang, Pei Juan, Chen Yaqin, Liu Beiyu, Sun Hongyu. Methacrylated dermal extracellular matrix hydrogel promotes repair of abdominal wall defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2074-2082. |
| [15] | Zhao Wenqi, Yu Haichi, Song Yiru, Yuan Tianyang, Liu Qinyi. Platelet-rich plasma and hydrogel for spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2189-2200. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||