Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (7): 1818-1827.doi: 10.12307/2026.065
Previous Articles Next Articles
Development and application of human amniotic membrane in tissue engineering
Zhu Jing¹, Zhai Xiguo¹, Wu Qizhen², Wang Yupei³, Lyu Ling⁴, Hou Qinzheng¹
- 1College of Life Sciences, Northwest Normal University, Lanzhou 730070, Gansu Province, China; 2Perinatal Medicine Center, Gansu Provincial Maternity and Child-care Hospital/Gansu Provincial Central Hospital, Lanzhou 730050, Gansu Province, China; 3Medical Genetics Center, Gansu Provincial Maternity and Child-care Hospital/Gansu Provincial Central Hospital, Lanzhou 730050, Gansu Province, China; 4LDR Unit, International Medical Department, Gansu Provincial Maternity and Child-care Hospital/Gansu Provincial Central Hospital, Lanzhou 730050, Gansu Province, China
-
Received:2024-12-30Revised:2025-05-16Accepted:2025-06-20Online:2026-03-08Published:2025-08-20 -
Contact:Hou Qinzheng, Professor, Doctoral supervisor, College of Life Sciences, Northwest Normal University, Lanzhou 730070, Gansu Province, China Lyu Ling, MS, Chief physician, LDR Unit, International Medical Department, Gansu Provincial Maternity and Child-care Hospital/Gansu Provincial Central Hospital, Lanzhou 730050, Gansu Province, China -
About author:Zhu Jing, Master candidate, College of Life Sciences, Northwest Normal University, Lanzhou 730070, Gansu Province, China -
Supported by:Gansu Provincial Science and Technology Program, Nos. 22YF7WA092 (to WQZ), 23YFFA0045 (to WYP), 25YFFA057 (to WYP); Gansu Provincial Health Industry Scientific Research Program, No. GSWSKY2021-021 (to WYP); Lanzhou Science and Technology Plan Project, No. 2023-NQ-199 (to WYP)
CLC Number:
Cite this article
Zhu Jing, Zhai Xiguo, Wu Qizhen, Wang Yupei, Lyu Ling, Hou Qinzheng. Development and application of human amniotic membrane in tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1818-1827.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
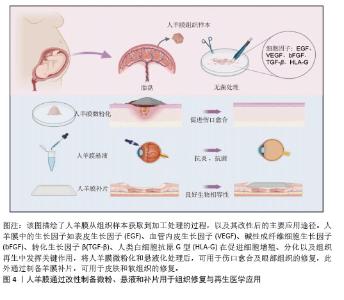
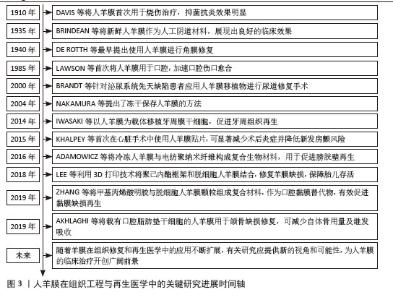
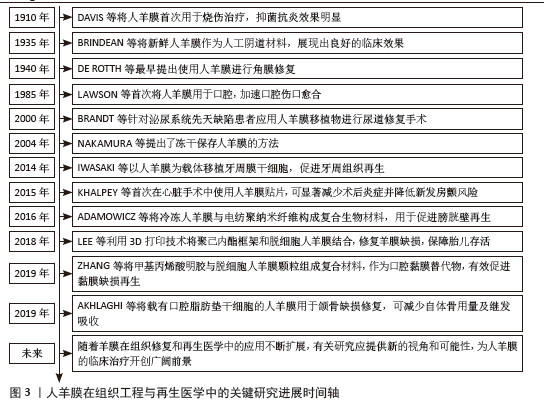
2.2 人羊膜的组织结构 人羊膜来自胎盘,内层包裹羊水和胎儿,在整个妊娠期为胎儿提供支持和保护。人羊膜厚度为20-500 μm[9],由上皮层、基底膜和无血管间充质组织组成,各层结构独特、功能互补。上皮层位于最外层与羊水相邻,由均一的立方上皮细胞构成,含有少量胞浆内细胞器,产生的细胞因子可促进组织修复、上皮化和细胞增殖,在组织损伤后可参与修复过程,通过抑制炎症反应实现人羊膜的低免疫原性[10-11]。基底膜位于上皮层下方,是连接上皮层和基质的关键结构,含蛋白聚糖、层粘连蛋白、纤维连接蛋白以及Ⅰ、Ⅲ、Ⅳ、Ⅴ和Ⅶ型胶原,增强人羊膜的结构稳定性,提高抗拉能力[12]。无血管间充质组织含有蛋白聚糖和胶原纤维网络,将人羊膜与绒毛膜分隔开,提供弹性和缓冲性能,网状胶原纤维和弹性纤维形成松散且具有弹性的网状层,使人羊膜在获得外力时能够弹性伸展而不易破裂[13-14]。 2.3 人羊膜的生物学功能 随着研究的深入和临床需求的增长,人羊膜用于眼表组织的治疗已经扩展到角膜溃疡、结膜重建、干眼症、翼状胬肉切除术后的修复等多个方面[15],在心胸外科领域用于修复组织缺损,如心包和胸膜等[16],在妇科领域用于治疗先天性阴道缺失或阴道切除后的重建等[17-18]。人羊膜通过分泌碱性成纤维细胞生长因子促进细胞增殖、维持干细胞特性;同时释放出的血管内皮生长因子、表皮生长因子和脑源性神经营养因子可促进血管生成并增加血管内皮细胞的通透性;此外,人羊膜中的白细胞介素8、干扰素γ、单核细胞趋化蛋白1参与免疫细胞的募集和炎症调控,增强组织修复过程中的免疫协调作用,为组织再生提供了免疫支持[19],这些因子通过调控细胞行为、促进血管生成和调节炎症微环境等途径,促进组织的修复和再生,进一步凸显了人羊膜在再生医学中的重要价值。 2.4 新鲜人羊膜制备与储存技术 通过剖宫产手术从健康孕妇的胎盘中采集人羊膜,采集前需对母体进行检测,排除人类免疫缺陷性病毒(HIV)、乙肝病毒(HBV)、丙肝病毒(HCV)、巨细胞病毒、疱疹病毒、弓形体、梅毒及其他传染病的存在,采集过程严格规范,并且所有的采集操作是在获得孕妇知情同意的前提下进行。对于人羊膜的临床应用,若将未经任何处理的人羊膜直接用于临床治疗会存在较高的交叉感染风险。在常规操作流程中,取出人羊膜后,需用无菌生理盐水或磷酸盐缓冲液进行反复冲洗,直至表面不再有明显血迹,随后将清洗干净的人羊膜保存在无菌4 ℃环境,确保人羊膜组织的活性和结构在短期内得到维持[20]。对人羊膜进行适当的处理如冷冻保存技术(即冻存人羊膜)或脱细胞处理工艺(去除细胞成分,仅保留细胞外基质结构)可有效延长使用期限并减少免疫反应发生[21-22]。 目前最常见的储存方法是将新鲜人羊膜放于基础培养基(DMEM)与甘油1∶1形成的混合液中于-80 ℃保存,可防止人羊膜组织冷冻损伤,能更好地保存基底膜成分,显微观察发现冷冻保存后的人羊膜上皮细胞层无明显丢失,上皮组织仍然保持单层柱状/立方状,进一步探究发现在保存1周、3个月和9个月后,表皮生长因子、转化生长因子β和碱性成纤维生长因子水平均无显著下降,Ⅳ型胶原蛋白和层粘连蛋白水平在低温处理后略有降低[23]。将超低温冷冻处理后的人羊膜用于创面修复,在术后1个月可完全吸收[24]。快速冷冻法能够有效保留人羊膜中的生物活性成分,但冻存后的人羊膜需要在低温条件下保存,限制了其储存和运输。与此不同,脱水法使人羊膜水分流失的同时加入组织溶解保护剂,防止细胞过多损伤,维持人羊膜的主要形态学特征,显微观察发现单层上皮细胞和基底膜变化最小,基质细胞有显著减少,其他细胞没有明显丢失,蛋白质损失较少,脱水处理后的人羊膜可在室温保存,机械强度更高,更容易储存和运输[25]。基于传统保存方法,2023年OGLIARI等[26]将新鲜人羊膜无菌处理后,上皮层朝下放在无菌纱布或凡士林纱布这类支撑材料上,将人羊膜固定在聚四氟乙烯管中,在高效的气相氮气罐中保存,使用时在室温下去除冷冻保护剂进行解冻。与直接放在低温保护剂中不同的是,此方法增加了吸收层与无菌纱布层,以确保人羊膜每一层都与低温保护剂溶液有接触,这种方法储存的人羊膜在解冻后细胞活力高于其他研究,更好地保留了活细胞的细胞活力、结构完整性,同时与伤口愈合相关的蛋白分泌也没有受到冻存和解冻的影响。 2.5 人羊膜的改性技术及其应用 为了提升人羊膜在组织修复、临床治疗、药物递送等领域中的适应性和应用效果,可通过物理(如冻干、辐射处理)和化学(如交联、表面修饰等)等方法对人羊膜进行加工,改变其表面性质,提高其力学强度、溶解性以及生物可用性,进而提高人羊膜中有效成分的利用率,达到优化治疗的效果,见图4。 将人羊膜液化后形成人羊膜悬液,在这一过程中保留了人羊膜自身具有的优势,在眼科修复中可降低角膜损伤引发的炎症反应,促进角膜愈合并减少角膜细胞凋亡;人羊膜凝胶凭借高黏附性和高水合作用,可有效缓冲外界刺激、减少因摩擦或者干燥导致的细胞损伤,在皮肤损伤修复中表现出优异的治疗效果;人羊膜微粉因其较小的颗粒直径,具有更高的渗透能力,可以深入抵达受损组织区域,持续地为损伤部位释放生长因子和促进修复的分子,在创伤修复和免疫调节中发挥了积极作用;人羊膜补片呈现为固态形式,有效改善人羊膜力学强度较低的缺陷,为组织修复提供物理支持,适用于器官再生领域以及需要长期支撑的创伤修复。 "
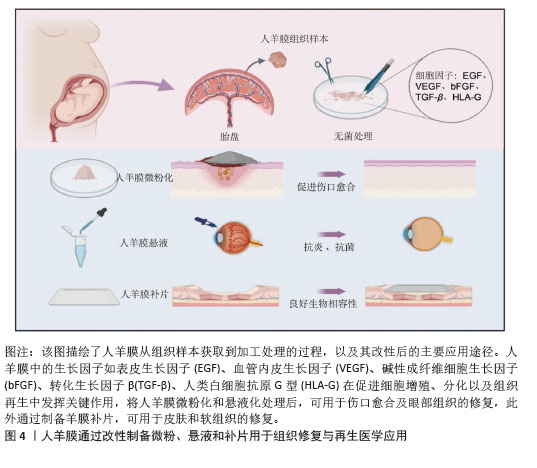
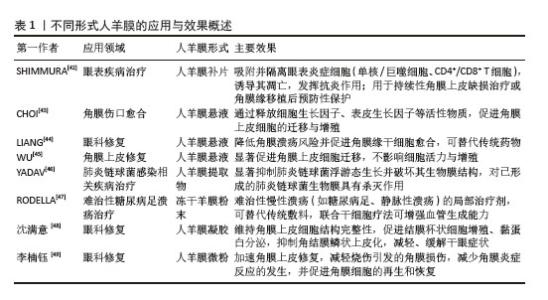
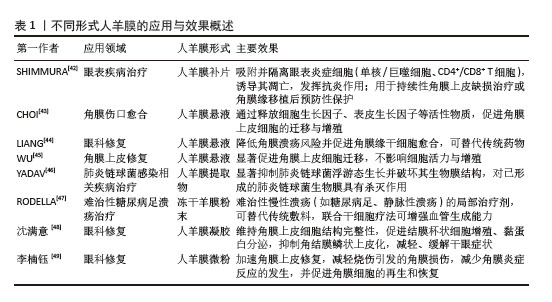
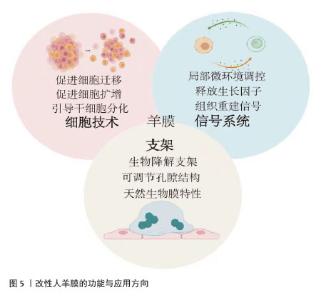
2.5.1 微粉化人羊膜在组织修复中的应用 相较于新鲜人羊膜,MAMEDE等[27]发现微粉化处理促进了人羊膜中有效因子的释放,并未破坏人羊膜中的有效成分,通过减轻炎症和伤口感染来促进伤口愈合。人羊膜微粉化是一种将人羊膜处理成微小颗粒的方法,通常将新鲜人羊膜无菌处理后,-80 ℃冷冻保存24 h,随后将培养皿置于预冷的玻璃冻干器中进行48 h的冻干处理,冻干完成后,在室温下将人羊膜研磨成粉末。微粉化后的人羊膜更易于黏附在伤口表面,可防止浆液性分泌物积聚,并作为屏障防止细菌渗透,有效减少伤口部位的细菌负荷和感染风险[28-29],ELISA检测显示微粉化处理后人羊膜中的纤维连接蛋白、层粘连蛋白、趋化因子及蛋白酶等并未被破坏[30]。 目前人羊膜微粉主要应用于骨关节炎治疗、慢性伤口愈合、骨修复和再生医学领域[31],临床试验结果表明,人羊膜粉末有助于减少炎症反应,刺激代谢过程,促进组织愈合。使用微粉化人羊膜复合物修复烧伤创面,可促进新生血管再生,加速创面愈合并减轻疼痛;人羊膜微粉化应用于组织工程,可作为生物活性支架促进细胞增殖,改善间充质干细胞体外扩增效率低、移植后存活率低的问题[32],微粉化颗粒直径大小对组织愈合、药物释放和细胞反应有显著影响,微粉直径小易被细胞吞噬,促进巨噬细胞活化,加速炎症清除和组织修复,微粉直径大更适合作为细胞支架,有利于细胞黏附、增殖和分化。但不同大小的人羊膜微粉颗粒降解速率不同,微粉直径过大可能降解缓慢,影响药效释放;过小则可能快速降解,无法长时间提供治疗效果[33]。 2.5.2 人羊膜悬液在组织修复中的应用 在眼科疾病的治疗中,使用抗生素是必要的治疗方法,但抗生素会引起上皮细胞毒性,导致再上皮化的快速发生,不利于眼部伤口恢复。2005年BONCI等[34]发现人羊膜悬浮液可减缓眼部炎症,滴入后上皮细胞完整性和透明度显著提高。2010年SHEHA等[35]将人羊膜提取物制成滴眼液用于辅助治疗急性眼部化学烧伤,减少因烧伤诱导的炎症递质的释放,降低由烧伤导致的角膜溃疡和穿孔的风险,2018年BARADARAN-RAFII等[36]将人羊膜提取物滴眼液作为一种眼表疾病治疗药物,替代传统的辅助药物促进上皮细胞愈合。人羊膜提取物滴眼液可防止角膜瘢痕形成、角膜融化,为眼表疾病治疗提供一种新的辅助治疗思路[37]。 人羊膜悬液中发现的生长因子可以成功穿透角膜,对活化的淋巴细胞有免疫抑制作用。人羊膜悬液已经用于治疗干眼症、化学烧伤、延迟上皮化、角膜缘干细胞功能缺陷、瘢痕性眼表疾病、大疱性角膜病变和角膜溃疡,成为治疗轻度和中度急性化学烧伤的有效辅助治疗方法。但到目前为止,没有研究对人羊膜提取眼药水的术后长期影响进行评估,制造工艺仍未标准化,生产人羊膜提取眼药水的最佳方法还有待研究。 2.5.3 人羊膜补片在组织修复中的应用 合成网片如四氟乙烯等在体内支持正常组织的穿透与融合,存在降解慢、生物毒性等缺点;生物网片由猪、牛和人的胶原组织制成,裂开率较低,近年来被用于修复结肠、直肠等,但二者都存在抗张能力较低、价格高、植入易发生感染、出血等问题[38]。近年来,人羊膜作为嵌体移植物覆盖在合成网片侵蚀部分,展现出了良好的治疗优势,用于修复复杂阴道、盆底肌、小肠黏膜、软组织缺损等[39]。人羊膜通常经表面活性剂、高渗盐水、脂肪酶和核酸酶处理,保留细胞外基质后用于替代传统补片。在组织修复早期阶段,人羊膜补片在贴附部位有抗黏附作用,可帮助形成紧密附着的屏障结构,防止网片和缝合线粘连,表现出良好的生物相容性和快速的集成性,DI LORETO等[40]用干燥的人羊膜作为抗黏附层,可减少肠梗阻、肠瘘的发生。与普通的丝纤维素膜相比,人羊膜补片会释放更多的肝细胞生长因子,抑制转化生长因子β1的表达,促进血管内皮生长因子诱导血管生成,减少植入后的炎症反应[41]。但原始人羊膜补片存在力学性能差、容易破裂或移位等问题,通常需要和其他材料相结合提供长期支撑,且植入后由于伤口类型不同可能存在降解过快或过慢等问题,影响其支架作用和药物释放。 改性后的人羊膜展现出更多的优势,在各类临床应用场景中得到了广泛的关注,见表1。"
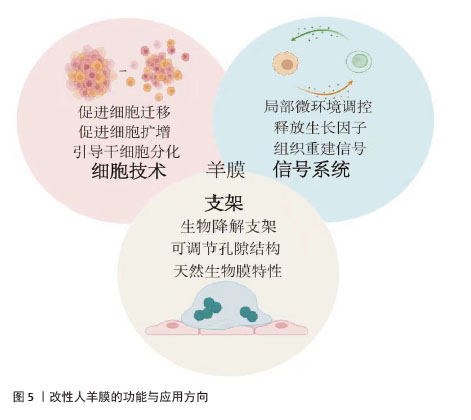
2.6 人羊膜衍生组织工程学材料的加工和性能 对于无法恢复功能的损伤,可以选择自体、同种异体或异种移植材料替代受损组织[50],替代材料应具有生物相容性、可生物降解性、适当的孔隙率和机械强度,支持植入细胞的生长、增殖和吸收。合成支架满足特定的化学、生物、物理和力学特性,为细胞提供生长支架并调节细胞活性,不干扰免疫表型或分化能力,为细胞生长提供所需的微环境[51]。优化生物材料性能对于提高支架治疗效果至关重要。人羊膜衍生的组织工程学材料具有三大功能:一是通过细胞技术促进细胞的迁移、增殖和分化,促进组织修复;二是通过信号系统调节局部微环境并释放生长因子,促进细胞增殖和组织重建;三是作为生物降解支架,提供可调节的结构和天然生物特性,支持细胞生长并推动组织修复与再生。这些功能使人羊膜在再生医学中展现出广泛应用潜力,见图5。"
| [1] DAVIS JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307-398. [2] NIKNEJAD H, PEIROVI H, JORJANI M, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88-99. [3] MOHAMMADI AA, SEYED JAFARI SM, KIASAT M, et al. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns. 2013; 39(2):349-353. [4] AZUARA-BLANCO A, PILLAI CT, DUA HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83(4):399-402. [5] CHEN HJ, PIRES RT, TSENG SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84(8):826-833. [6] GÜNDÜZ K, UÇAKHAN OO, KANPOLAT A, et al. Nonpreserved human amniotic membrane transplantation for conjunctival reconstruction after excision of extensive ocular surface neoplasia. Eye (Lond). 2006; 20(3):351-357. [7] GHOLIPOURMALEKABADI M, SEIFALIAN AM, URBANSKA AM, et al. 3D Protein-Based Bilayer Artificial Skin for the Guided Scarless Healing of Third-Degree Burn Wounds in Vivo. Biomacromolecules. 2018;19(7): 2409-2422. [8] TRITZ J, RAHOUADJ R, DE ISLA N, et al. Designing a three-dimensional alginate hydrogel by spraying method for cartilage tissue engineering. Soft Matter. 2010;6(20): 5165-5174. [9] TSENG SC, ESPANA EM, KAWAKITA T, et al. How does amniotic membrane work? Ocul Surf. 2004;2(3):177-187. [10] LEAL-MARIN S, KERN T, HOFMANN N, et al. Human Amniotic Membrane: A review on tissue engineering, application, and storage. J Biomed Mater Res B Appl Biomater. 2021; 109(8):1198-1215. [11] LITWINIUK M, GRZELA T. Amniotic membrane: new concepts for an old dressing. Wound Repair Regen. 2014;22(4): 451-456. [12] ARRIZABALAGA JH, NOLLERT MU. Human Amniotic Membrane: A Versatile Scaffold for Tissue Engineering. ACS Biomater Sci Eng. 2018;4(7):2226-2236. [13] HU Z, LUO Y, NI R, et al. Biological importance of human amniotic membrane in tissue engineering and regenerative medicine. Mater Today Bio. 2023;22: 100790. [14] MUNOZ-TORRES JR, MARTÍNEZ-GONZÁLEZ SB, LOZANO-LUJÁN AD, et al. Biological properties and surgical applications of the human amniotic membrane. Front Bioeng Biotechnol. 2023;10:1067480. [15] MELLER D, PAUKLIN M, THOMASEN H, et al. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011; 108(14):243-248. [16] ZMIJEWSKI M, PIETRASZEK A. The application of deep-frozen and radiation-sterilized human amnion as a biological dressing to prevent prolonged air leakage in thoracic surgery. Ann Transplant. 2005; 10(3):17-20. [17] NISOLLE M, DONNEZ J. Vaginoplasty using amniotic membranes in cases of vaginal agenesis or after vaginectomy. J Gynecol Surg. 1992;8(1):25-30. [18] ROSHANRAVAN R, GHAHRAMANI L, HOSSEINZADEH M, et al. A new method to repair recto-vaginal fistula: Use of human amniotic membrane in an animal model. Adv Biomed Res. 2014;3:114. [19] GHOLIPOURMALEKABADI M, FARHADIHOSSEINABADI B, FARAJI M, et al. How preparation and preservation procedures affect the properties of amniotic membrane? How safe are the procedures? Burns. 2020;46(6): 1254-1271. [20] DRAGÚŇOVÁ J, KABÁT P, CUCOROVÁ V, et al. Deep frozen amniotic membrane used as a scaffold and/or carrier for different cell types. Cell Tissue Bank. 2019;20(1):35-48. [21] TODA A, OKABE M, YOSHIDA T, et al. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105(3): 215-228. [22] WAGNER M, WALTER P, SALLA S, et al. Cryopreservation of amniotic membrane with and without glycerol additive. Graefes Arch Clin Exp Ophthalmol. 2018; 256(6):1117-1126. [23] LAMON M, BERTOLIN M, TROJAN D, et al. Cryopreservation of human amniotic membrane for ocular surface reconstruction: a comparison between protocols. Cell Tissue Bank. 2022;23(4): 851-861. [24] JOHN S, KESTING MR, PAULITSCHKE P, et al. Development of a tissue-engineered skin substitute on a base of human amniotic membrane. J Tissue Eng. 2019;10: 2041731418825378. [25] FENELON M, MAUREL DB, SIADOUS R, et al. Comparison of the impact of preservation methods on amniotic membrane properties for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2019;104: 109903. [26] OGLIARI KS, GRUDZINSKI PB, DA SILVA CG, et al. A novel method to pack cryopreserved amniotic membrane for wound dressing-the pathway through validation of a new biological product. Biomed Mater. 2023;18(4):045004. [27] MAMEDE AC, CARVALHO MJ, ABRANTES AM, et al. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2): 447-458. [28] MAJIDNIA E, AHMADIAN M, SALEHI H, et al. Development of an electrospun poly(ε-caprolactone)/collagen-based human amniotic membrane powder scaffold for culturing retinal pigment epithelial cells. Sci Rep. 2022;12(1):6469. [29] JAHANAFROOZ Z, BAKHSHANDEH B, BEHNAM ABDOLLAHI S, et al. Human amniotic membrane as a multifunctional biomaterial: recent advances and applications. J Biomater Appl. 2023;37(8): 1341-1354. [30] LEI J, PRIDDY LB, LIM JJ, et al. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv Wound Care (New Rochelle). 2017;6(2):43-53. [31] RIBOH JC, SALTZMAN BM, YANKE AB, et al. Human Amniotic Membrane-Derived Products in Sports Medicine: Basic Science, Early Results, and Potential Clinical Applications. Am J Sports Med. 2016;44(9): 2425-2434. [32] ZHOU Z, XUN J, WU C, et al. Acceleration of burn wound healing by micronized amniotic membrane seeded with umbilical cord-derived mesenchymal stem cells. Mater Today Bio. 2023;20:100686. [33] REECE DS, BURNSED OA, PARCHINSKI K, et al. Reduced Size Profile of Amniotic Membrane Particles Decreases Osteoarthritis Therapeutic Efficacy. Tissue Eng Part A. 2020;26(1-2):28-37. [34] BONCI P, BONCI P, LIA A. Suspension made with amniotic membrane: clinical trial. Eur J Ophthalmol. 2005;15(4):441-445. [35] SHEHA H, LIANG L, HASHEM H. Amniotic membrane extract for acute ocular chemical burns. Tech Ophthalmol.2010;8(4):146-150. [36] BARADARAN-RAFII A, ASL NS, EBRAHIMI M, et al. The role of amniotic membrane extract eye drop (AMEED) in in vivo cultivation of limbal stem cells. Ocul Surf. 2018;16(1):146-153. [37] SABATER-CRUZ N, FIGUERAS-ROCA M, FERRÁN-FUERTES M, et al. Amniotic membrane extract eye drops for ocular surface diseases: use and clinical outcome in real-world practice. Int Ophthalmol. 2021;41(9):2973-2979. [38] LIU H, ZHOU Z, LIN H, et al. Synthetic Nanofiber-Reinforced Amniotic Membrane via Interfacial Bonding. ACS Appl Mater Interfaces. 2018;10(17):14559-14569. [39] LAU HH, JOU QB, HUANG WC, et al. Amniotic Membrane Graft in the Management of Complex Vaginal Mesh Erosion. J Clin Med. 2020;9(2):356. [40] DI LORETO FP, MANGIONE A, PALMISANO E, et al. Dried human amniotic membrane as an antiadherent layer for intraperitoneal placing of polypropylene mesh in rats. Surg Endosc. 2013;27(4):1435-1440. [41] LIU Z, ZHU X, ZHU T, et al. Evaluation of a Biocomposite Mesh Modified with Decellularized Human Amniotic Membrane for Intraperitoneal Onlay Mesh Repair. ACS Omega. 2020;5(7):3550-3562. [42] SHIMMURA S, SHIMAZAKI J, OHASHI Y, et al. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20(4):408-413. [43] CHOI JA, JIN HJ, JUNG S, et al. Effects of amniotic membrane suspension in human corneal wound healing in vitro. Mol Vis. 2009;15:2230-2238. [44] LIANG L, LI W, LING S, et al. Amniotic membrane extraction solution for ocular chemical burns. Clin Exp Ophthalmol. 2009;37(9):855-863. [45] WU MF, STACHON T, LANGENBUCHER A, et al. Effect of Amniotic Membrane Suspension (AMS) and Amniotic Membrane Homogenate (AMH) on Human Corneal Epithelial Cell Viability, Migration and Proliferation In Vitro. Curr Eye Res. 2017; 42(3):351-357. [46] YADAV MK, GO YY, KIM SH, et al. Antimicrobial and Antibiofilm Effects of Human Amniotic/Chorionic Membrane Extract on Streptococcus pneumoniae. Front Microbiol. 2017;8:1948. [47] RODELLA A, POZZOBON M, RIGON M, et al. Topical application of lyophilized and powdered human amniotic membrane promotes diabetic ulcer healing. Wound Medicine. 2019;27(1):100171. [48] 沈满意,马晓萍.羊膜凝胶对大鼠干眼的治疗作用及其作用机制[J].复旦学报(医学版),2019,46(2):217-225. [49] 李楠钰,张文佳,胡竹林.羊膜在眼表疾病治疗的研究进展[J]. 国际眼科纵览, 2019,43(2):94-99. [50] DADKHAH TEHRANI F, FIROUZEH A, SHABANI I, et al. A Review on Modifications of Amniotic Membrane for Biomedical Applications. Front Bioeng Biotechnol. 2021;8:606982. [51] XUE W, DU J, LI Q, et al. Preparation, Properties, and Application of Graphene-Based Materials in Tissue Engineering Scaffolds. Tissue Eng Part B Rev. 2022; 28(5):1121-1136. [52] ZHANG Y, ZHANG C, LI Y, et al. Evolution of biomimetic ECM scaffolds from decellularized tissue matrix for tissue engineering: A comprehensive review. Int J Biol Macromol. 2023;246:125672. [53] MAO Y, HOFFMAN T, SINGH-VARMA A, et al. Antimicrobial Peptides Secreted From Human Cryopreserved Viable Amniotic Membrane Contribute to its Antibacterial Activity. Sci Rep. 2017; 7(1):13722. [54] CASTELLANOS G, BERNABÉ-GARCÍA Á, MORALEDA JM, et al. Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta. 2017;59: 146-153. [55] RYZHUK V, ZENG XX, WANG X, et al. Human amnion extracellular matrix derived bioactive hydrogel for cell delivery and tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;85:191-202. [56] MURPHY SV, SKARDAL A, SONG L, et al. Solubilized Amnion Membrane Hyaluronic Acid Hydrogel Accelerates Full-Thickness Wound Healing. Stem Cells Transl Med. 2017;6(11):2020-2032. [57] KAFILI G, TAMJID E, NIKNEJAD H, et al. Development of bioinspired nanocomposite bioinks based on decellularized amniotic membrane and hydroxyethyl cellulose for skin tissue engineering. Cellulose. 2024; 31(5):2989-3013. [58] SHI P, GAO M, SHEN Q, et al. Biocompatible surgical meshes based on decellularized human amniotic membrane. Mater Sci Eng C Mater Biol Appl. 2015;54:112-119. [59] AMENSAG S, GOLDBERG L, O’MALLEY KA, et al. Pilot assessment of a human extracellular matrix-based vascular graft in a rabbit model. J Vasc Surg. 2017;65(3): 839-847.e1. [60] HORTENSIUS RA, EBENS JH, HARLEY BA. Immunomodulatory effects of amniotic membrane matrix incorporated into collagen scaffolds. J Biomed Mater Res A. 2016;104(6):1332-1342. [61] ADAMOWICZ J, POKRYWCZYŃSKA M, TWORKIEWICZ J, et al. New Amniotic Membrane Based Biocomposite for Future Application in Reconstructive Urology. PLoS One. 2016;11(1):e0146012. [62] ESMAEILI Z, NOKHBEDEHGHAN Z, ALIZADEH S, et al. Biomimetic amniotic/silicone-based bilayer membrane for corneal tissue engineering. Materials Design. 2024;237:112614. [63] NOGAMI M, KIMURA T, SEKI S, et al. A Human Amnion-Derived Extracellular Matrix-Coated Cell-Free Scaffold for Cartilage Repair: In Vitro and In Vivo Studies. Tissue Eng Part A. 2016;22(7-8): 680-688. [64] HOSSEINI SA, ASHOURI S, KERMANI F, et al. Bioactive glasses-reinforced human amniotic membrane for tissue engineering applications. Materials Letters. 2024;358:135786. [65] KUO CK, LI WJ, MAUCK RL, et al. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18(1):64-73. [66] ILANCHERAN S, MOODLEY Y, MANUELPILLAI U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. 2009;30(1):2-10. [67] KOIZUMI N, INATOMI T, SUZUKI T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108(9):1569-1574. [68] NASEROLESLAMI M, ABOUTALEB N, MOKHTARI B. Amniotic membrane mesenchymal stem cells labeled by iron oxide nanoparticles exert cardioprotective effects against isoproterenol (ISO)-induced myocardial damage by targeting inflammatory MAPK/NF-κB pathway. Drug Deliv Transl Res. 2021;11(1):242-254. [69] NASEROLESLAMI M, ABOUTALEB N. Human amniotic membrane mesenchymal stem cells exert cardioprotective effects against isoproterenol (ISO)-induced myocardial injury through suppression of inflammation and modulation of inflammatory MAPK/NF-κB pathway. Cell Tissue Bank. 2022;23(1): 67-77. [70] KIM HG, CHOI OH. Neovascularization in a mouse model via stem cells derived from human fetal amniotic membranes. Heart Vessels. 2011;26(2):196-205. [71] HUANG J, ZHANG W, YU J, et al. Human amniotic mesenchymal stem cells combined with PPCNg facilitate injured endometrial regeneration. Stem Cell Res Ther. 2022; 13(1):17. [72] LIU H, JIANG C, LA B, et al. Human amnion-derived mesenchymal stem cells improved the reproductive function of age-related diminished ovarian reserve in mice through Ampk/FoxO3a signaling pathway. Stem Cell Res Ther. 2021;12(1):317. |
| [1] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [2] | Zhou Hongli, Wang Xiaolong, Guo Rui, Yao Xuanxuan, Guo Ru, Zhou Xiongtao, He Xiangyi. Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1962-1970. |
| [3] | Tan Jing, Li Li, Wang Liangliang, Qin Xiangyu. Bionic functional coating improves the integration of titanium implants and skin tissue interface [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2014-2022. |
| [4] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [5] | Liu Yang, Liu Donghui , Xu Lei, Zhan Xu, Sun Haobo, Kang Kai. Role and trend of stimuli-responsive injectable hydrogels in precise myocardial infarction therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2072-2080. |
| [6] | Lai Yu, Chen Yueping, Zhang Xiaoyun. Research hotspots and frontier trends of bioactive materials in treating bone infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2132-2144. |
| [7] | Jin Dongsheng, Zhao Zhanghong, Zhu Ziyin, Zhang Sen, Sun Zuyan, Deng Jiang. Effects of icariin-loaded microsphere-three-dimensional scaffold on osteogenic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1658-1668. |
| [8] | Ding Yifan, Yin Wenjie, Zhang Li, Yuan Shuya, Sun Guoju, Zhang Naili, Zhao Dongmei, Ma Lina. Repair of segmental bone defect of rabbit radius by decalcified bone matrix loaded with adipose-derived stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1679-1686. |
| [9] | Liu Xingyu, Li Lijie. Secretome of stem cells from human exfoliated deciduous teeth: a new hotspot in tissue engineering and stem cell therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1858-1868. |
| [10] | Hu Jing, Zhu Ling, Xie Juan, Kong Deying, Liu Doudou. Autophagy regulates early embryonic development in mice via affecting H3K4me3 modification [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1147-1155. |
| [11] | Liu Xinyue, Li Chunnian, Li Yizhuo, Xu Shifang. Regeneration and repair of oral alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1247-1259. |
| [12] | Wang Mingqi, Feng Shiya, Han Yinhe, Yu Pengxin, Guo Lina, Jia Zixuan, Wang Xiuli. Construction and evaluation of a neuralized intestinal mucosal tissue engineering model in vitro [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 892-900. |
| [13] | Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan. Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 936-948. |
| [14] | Yang Hu, Zheng Yu, Jia Chengming, Wang Tong, Zhang Guangfei, Ji Yaoyao. Immune microenvironment regulates bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 701-710. |
| [15] | Bai Xiangyu, Huo Feng, Hao Yan, Wang Zecheng, Guo Xiaoyu. Platelet-derived growth factor BB-loaded chitosan/reduced graphene oxide scaffold for repairing alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 329-337. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||