Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (18): 4557-4567.doi: 10.12307/2026.678
Matrix metalloproteinase 9 mediates mitophagy to regulate osteogenesis and myogenesis
Wang Siwei1, 2, Yao Xiaosheng1, 2, Qi Xiaonan2, Wang Yu2, Cui Haijian2, Zhao Jiaxuan2
- 1Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China; 2Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China
-
Received:2025-06-19Accepted:2025-09-02Online:2026-06-28Published:2025-12-01 -
Contact:Yao Xiaosheng, PhD, Chief physician, Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China; Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China -
About author:Wang Siwei, PhD candidate, Associate chief technologist, Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China; Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China -
Supported by:National Natural Science Foundation of China (Youth Program), No. 82305275 (to QXN); Application Basic Research Project of the Department of Science and Technology of Liaoning Province, No. 2023JH2/101700225 (to WSW)
CLC Number:
Cite this article
Wang Siwei, Yao Xiaosheng, Qi Xiaonan, Wang Yu, Cui Haijian, Zhao Jiaxuan. Matrix metalloproteinase 9 mediates mitophagy to regulate osteogenesis and myogenesis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4557-4567.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
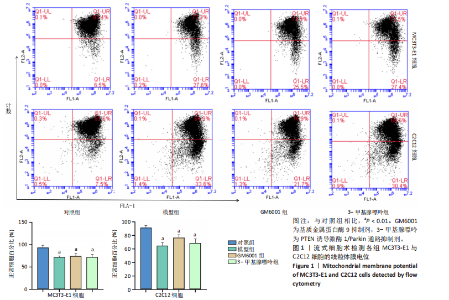
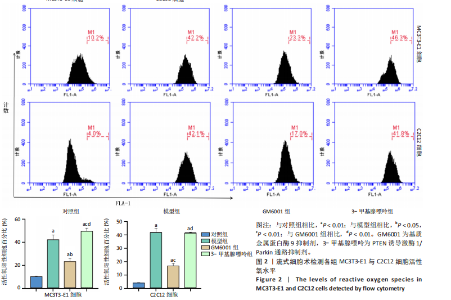
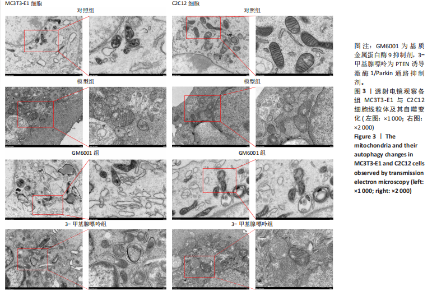
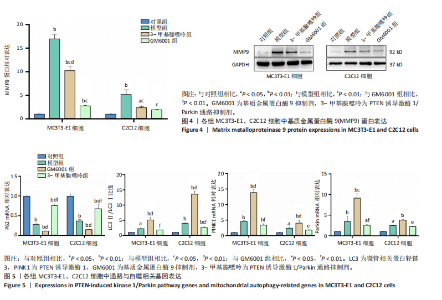
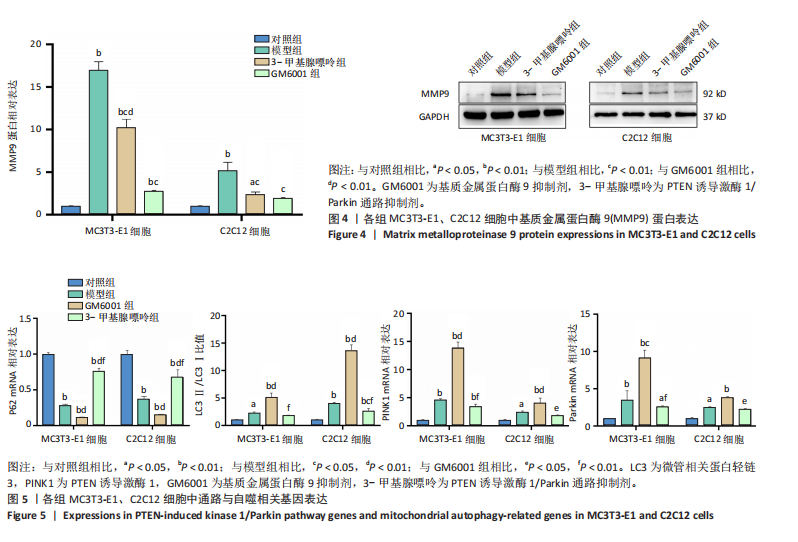
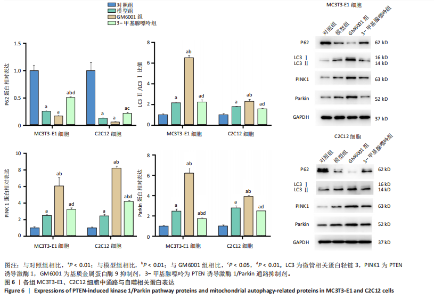
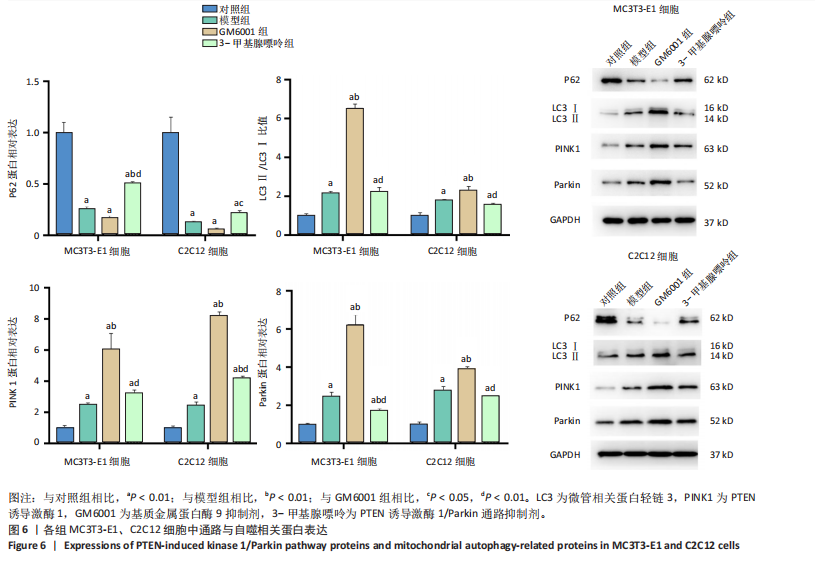
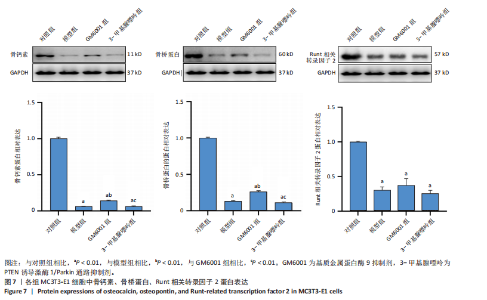
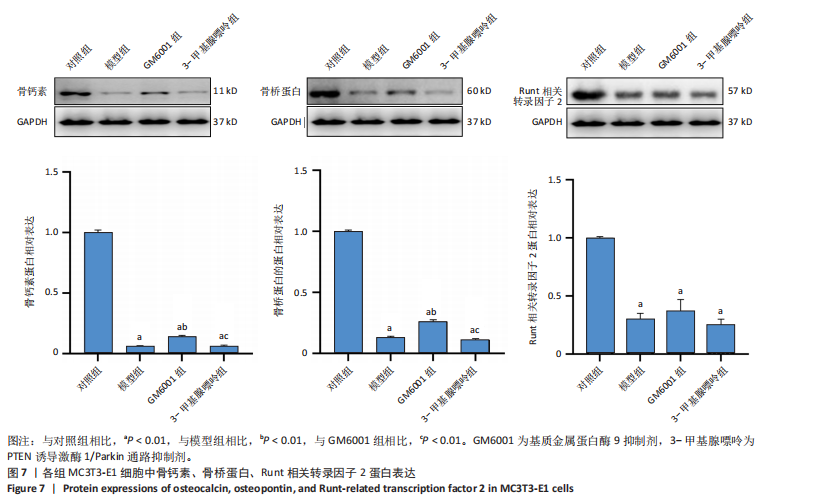
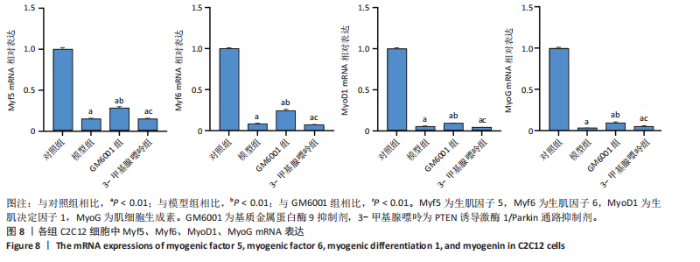
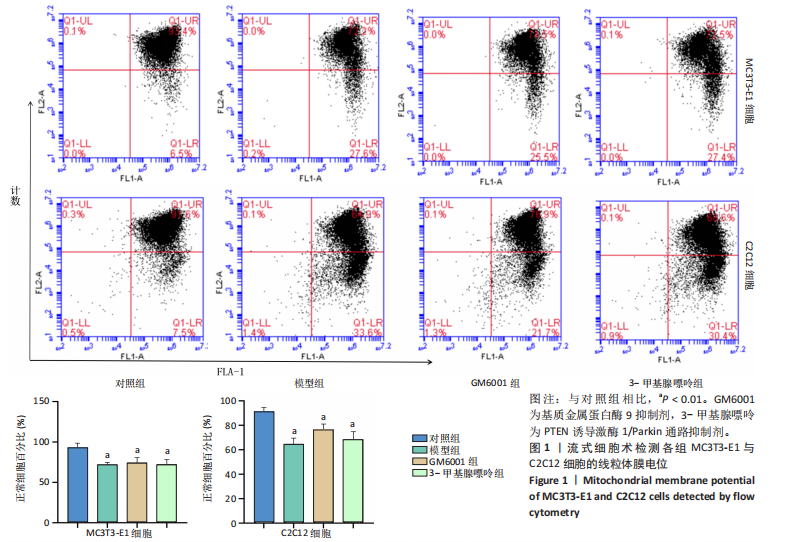
2.1 流式细胞术检测线粒体膜电位 在MC3T3-E1与C2C12细胞系中,模型组、3-甲基腺嘌呤组、GM6001组线粒体膜电位较对照组显著降低(P < 0.01),各干预组间无显著性差异,见图1。 2.2 流式细胞术检测活性氧水平 在MC3T3-E1与C2C12细胞系中,与对照组相比,模型组、3-甲基腺嘌呤组、GM6001组活性氧阳性细胞百分比显著升高(P < 0.01);与模型组相比,GM6001组活性氧阳性细胞百分比降低(P < 0.05,P < 0.01);与GM6001组相比,3-甲基腺嘌呤组活性氧阳性细胞百分比显著升高(P < 0.01),见图2。 2.3 透射电镜观察细胞中的线粒体损伤及自噬情况 对照组线粒体呈椭圆形或棒状,形态较为规则,膜呈典型的双层膜结构,嵴呈现板层状形态排列,没有或有较少的空泡结构。模型组线粒体表现出典型的损伤特征,线粒体体积显著增大、肿胀,嵴的数量减少,排列紊乱,部分嵴出现断裂甚至完全消失,线粒体内出现大小不等的空泡,部分受损线粒体被自噬体包裹。与模型组相比,GM6001组与3-甲基腺嘌呤组部分线粒体体积表现增大,形态不规则程度较低,肿胀现象不甚明显,内膜结构重塑,嵴的数量增加,断裂或消失的嵴得到修复,线粒体内的空泡数量减少,空泡体积减小,GM6001组上述现象更为明显。GM6001组自噬体显著聚集,3-甲基腺嘌呤组自噬体聚集减弱,见图3。 2.4 Western blot检测基质金属蛋白酶9蛋白表达 在MC3T3-E1与C2C12细胞系中,模型组基质金属蛋白酶9蛋白表达较对照组升高(P < 0.01),应用基质金属蛋白酶9抑制剂GM6001后基质金属蛋白酶9蛋白表达降低(P < 0.01)。在MC3T3-E1细胞系中,应用通路抑制剂3-甲基腺嘌呤后基质金属蛋白酶9蛋白表达较GM6001组升高(P < 0.01),差异有显著性意义(P < 0.05),见图4。 2.5 qRT-PCR及Western blot检测通路及自噬相关基因的mRNA及蛋白表达 在MC3T3-E1与C2C12细胞系中,模型组P62 mRNA表达较对照组降低,微管相关蛋白轻链3Ⅱ/微管相关蛋白轻链3Ⅰ比值较对照组升高,通路相关基因PTEN诱导激酶1、Parkin mRNA表达较对照组升高;应用基质金属蛋白酶9抑制剂GM6001后,P62 mRNA表达较模型组降低,微管相关蛋白轻链3Ⅱ/微管相关蛋白轻链3Ⅰ比值较模型组升高,通路相关基因PTEN诱导激酶1、Parkin mRNA表达较模型组升高;应用通路抑制剂3-甲基腺嘌呤后,P62 mRNA表达较GM6001组升高,微管相关蛋白轻链3Ⅱ/微管相关蛋白轻链3Ⅰ较GM6001组降低,PTEN诱导激酶1、Parkin mRNA表达较GM6001组降低,差异有显著性意义(P < 0.05,P < 0.01),见图5。 在MC3T3-E1与C2C12细胞系中,模型组自噬相关蛋白P62表达较对照组降低,微管相关蛋白轻链3Ⅱ/微管相关蛋白轻链3Ⅰ蛋白比值较对照组升高,通路蛋白PTEN诱导激酶1、Parkin表达较对照组升高;应用基质金属蛋白酶9抑制剂GM6001后,自噬相关蛋白P62表达较模型组降低、微管相关蛋白轻链3Ⅱ/微管相关蛋白轻链3Ⅰ蛋白比值较模型组升高,通路蛋白PTEN诱导激酶1、Parkin表达较模型组升高;应用线粒体自噬抑制剂3-甲基腺嘌呤后,自噬相关蛋白P62表达较GM6001组升高,微管相关蛋白轻链3Ⅱ/微管相关蛋白轻链3Ⅰ蛋白比值较GM6001组降低,通路蛋白PTEN诱导激酶1、Parkin表达较GM6001组降低,差异有显著性意义(P < 0.05,P < 0.01),见图6。 2.6 基质金属蛋白酶9抑制剂对细胞成骨和成肌的影响 在MC3T3-E1细胞系中,模型组、GM6001组、3-甲基腺嘌呤组骨钙素、骨桥蛋白、Runt相关转录因子2蛋白表达均较对照组显著降低,成骨分化受抑制;GM6001组骨钙素、骨桥蛋白的蛋白表达较模型组升高,3-甲基腺嘌呤组骨钙素、骨桥蛋白的蛋白表达较GM6001组降低,差异有显著性意义(P < 0.01),见图7。在C2C12细胞系中,模型组、GM6001组、3-甲基腺嘌呤组生肌因子5、生肌因子6、生肌决定因子1、肌细胞生成素mRNA表达较对照组降低,GM6001组上述成肌基因表达较模型组升高;3-甲基腺嘌呤组上述成肌基因表达较GM6001组降低,差异有显著性意义(P < 0.01),见图8。"
| [1] LIJIE G, YUEYUE Z, NAN Z, et al. Mitsugumin 53 promotes mitochondrial autophagy through regulating Ambra1 expression in C2C12 myoblast cells. Cell Biol Int. 2019;43(3):290-298. [2] LU Y, LI Z, ZHANG S, et al. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics. 2023;13(2):736-766. [3] DONG Y, ZHANG X. Targeting cellular mitophagy as a strategy for human cancers. Front Cell Dev Biol. 2024;12:1431968. [4] ZHENG F, ZHONG J, CHEN K, et al. PINK1-PTEN axis promotes metastasis and chemoresistance in ovarian cancer via non-canonical pathway. J Exp Clin Cancer Res. 2023;42(1):295. [5] HAN R, LIU Y, LI S, et al. PINK1-PRKN mediated mitophagy: differences between in vitro and in vivo models. Autophagy. 2023;19(5):1396-1405. [6] XIE W, CHEN M, LOOR JJ, et al. AMPK-endoplasmic reticulum stress axis contributes to lipopolysaccharide-caused mitochondrial dysfunction by regulating mitochondria-associated membrane function in bovine hepatocytes. J Dairy Sci. 2023;106(7):5146-5164. [7] KAPOOR C, VAIDYA S, WADHWAN V, et al. Seesaw of matrix metalloproteinases (MMPs). J Cancer Res Ther. 2016;12(1):28-35. [8] BHARATHA M, NANDANA MB, PRAVEEN R, et al. Unconjugated bilirubin and its derivative ameliorate IMQ-induced psoriasis-like skin inflammation in mice by inhibiting MMP9 and MAPK pathway. Int Immunopharmacol. 2024;130:111679. [9] XIA Q, DU Z, CHEN M, et al. A protein complex of LCN2, LOXL2 and MMP9 facilitates tumour metastasis in oesophageal cancer. Mol Oncol. 2023;17(11):2451-2471. [10] GAAFAR NAG, ASLANI M, AGHAZADEH Z, et al. The Oral Administration Effect of Drug Mannuronic Acid (M2000) on Gene Expression of Matrix and Tissue Inhibitor of Metalloproteinases in Rheumatoid Arthritis Patients. Curr Drug Discov Technol. 2020;17(5):704-710. [11] RASHID ZA, BARDAWEEL SK. Novel Matrix Metalloproteinase-9 (MMP-9) Inhibitors in Cancer Treatment. Int J Mol Sci. 2023;24(15):12133. [12] ZHU G, CHEN W, TANG CY, et al. Knockout and Double Knockout of Cathepsin K and Mmp9 reveals a novel function of Cathepsin K as a regulator of osteoclast gene expression and bone homeostasis. Int J Biol Sci. 2022;18(14):5522-5538. [13] COFFEY EC, ASTUMIAN M, ALROWAISHED SS, et al. Lysosomal Function Impacts the Skeletal Muscle Extracellular Matrix. J Dev Biol. 2021;9(4):52. [14] YAN C, SHI Y, YUAN L, et al. Mitochondrial quality control and its role in osteoporosis. Front Endocrinol (Lausanne). 2023;14:1077058. [15] LEE SY, AN HJ, KIM JM, et al. PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis. Stem Cell Res Ther. 2021;12(1):589. [16] LEDUC-GAUDET JP, REYNAUD O, HUSSAIN SN, et al. Parkin overexpression protects from ageing-related loss of muscle mass and strength. J Physiol. 2019;597(7):1975-1991. [17] SU X, YANG D, HU Y, et al. Berberine suppressed sarcopenia insulin resistance through SIRT1-mediated mitophagy. Open Life Sci. 2023; 18(1):20220648. [18] BADRELDIN AA, BAGHERI L, ZHANG B, et al. Relative mRNA and protein stability of epigenetic regulators in musculoskeletal cell culture models. Gene. 2021;766:145032. [19] WALKER CL, POMATTO LCD, TRIPATHI DN, et al. Redox Regulation of Homeostasis and Proteostasis in Peroxisomes. Physiol Rev. 2018; 98(1):89-115. [20] DONG Y, HOU Q, LEI J, et al. Quercetin Alleviates Intestinal Oxidative Damage Induced by H2O2 via Modulation of GSH: In Vitro Screening and In Vivo Evaluation in a Colitis Model of Mice. ACS Omega. 2020; 5(14):8334-8346. [21] 陶乐维,韩煦,陈清光,等.基于FOXO1的抗氧化作用探讨六味地黄方对去卵巢大鼠及H2O2诱导的氧化损伤MC3T3-E1细胞的干预作用[J].上海中医药杂志,2023,57(7):13-20. [22] CHEN T, WANG H, JIANG C, et al. PKD1 alleviates oxidative stress-inhibited osteogenesis of rat bone marrow-derived mesenchymal stem cells through TAZ activation. J Cell Biochem. 2021;122(11):1715-1725. [23] DENG W, DING Z, WANG Y, et al. Dendrobine attenuates osteoclast differentiation through modulating ROS/NFATc1/ MMP9 pathway and prevents inflammatory bone destruction. Phytomedicine. 2022; 96:153838. [24] ZHU L, TANG Y, LI XY, et al. Proteolytic regulation of a galectin-3/Lrp1 axis controls osteoclast-mediated bone resorption. J Cell Biol. 2023;222(4):e202206121. [25] ZHANG H, LIU L, JIANG C, et al. MMP9 protects against LPS-induced inflammation in osteoblasts. Innate Immun. 2020;26(4):259-269. [26] HU C, JI F, LV R, et al. Putrescine promotes MMP9-induced angiogenesis in skeletal muscle through hydrogen peroxide/METTL3 pathway. Free Radic Biol Med. 2024;212:433-447. [27] PHAN TTT, LIN YC, CHOU YT, et al. Tumor suppressor p53 restrains cancer cell dissemination by modulating mitochondrial dynamics. Oncogenesis. 2022;11(1):26. [28] LIU C, LIU R, CAO Z, et al. Identification of MMP9 as a Novel Biomarker to Mitochondrial Metabolism Disorder and Oxidative Stress in Calcific Aortic Valve Stenosis. Oxid Med Cell Longev. 2022;2022:3858871. [29] CAO L, LIU J, ZHANG L, et al. Curcumin inhibits H2O2-induced invasion and migration of human pancreatic cancer via suppression of the ERK/NF-κB pathway. Oncol Rep. 2016;36(4):2245-2251. [30] 王思微,姚啸生,戚晓楠,等.线粒体相关蛋白IGF-1、MMP9、PKM的在骨质疏松症患者中临床意义及诊断价值[J/OL].重庆医科大学学报,1-6[2025-05-29].https://doi.org/10.13406/j.cnki.cyxb.003764. [31] ASHRAFI G, SCHWARZ TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1): 31-42. [32] ZHOU TY, MA RX, LI J, et al. Review of PINK1-Parkin-mediated mitochondrial autophagy in Alzheimer’s disease. Eur J Pharmacol. 2023;959:176057. [33] NARENDRA DP, YOULE RJ. The role of PINK1-Parkin in mitochondrial quality control. Nat Cell Biol. 2024;26(10):1639-1651. [34] BARODIA SK, CREED RB, GOLDBERG MS. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res Bull. 2017;133:51-59. [35] KANE LA, LAZAROU M, FOGEL AI, et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205(2): 143-153. [36] YI J, WANG HL, LU G, et al. Spautin-1 promotes PINK1-PRKN-dependent mitophagy and improves associative learning capability in an alzheimer disease animal model. Autophagy. 2024;20(12):2655-2676. [37] HUANG T, ZUO L, WALCZYŃSKA KS, et al. Essential roles of matrix metalloproteinases in axolotl digit regeneration [published correction appears in Cell Tissue Res. 2021;386(1):207. [38] 朱汉民,王松,肖文琳,等.线粒体自噬调控骨代谢[J].中国组织工程研究,2025,29(8):1676-1683. [39] LI Y, ZHANG H, YU C, et al. New Insights into Mitochondria in Health and Diseases. Int J Mol Sci. 2024;25(18):9975. |
| [1] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [2] | Li Zhenyu, Zhang Siming, Bai Jiaxiang, Zhu Chen. Osthole improves osteogenic differentiation function of bone marrow mesenchymal stem cells under high-glucose conditions [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1641-1648. |
| [3] | Jin Dongsheng, Zhao Zhanghong, Zhu Ziyin, Zhang Sen, Sun Zuyan, Deng Jiang. Effects of icariin-loaded microsphere-three-dimensional scaffold on osteogenic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1658-1668. |
| [4] | Zou Yulian, Chen Chaopei, Huang Haixia, Lan Yuyan, Liu Min, Huang Ting. Resveratrol promotes osteogenic differentiation of bone marrow mesenchymal stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1669-1678. |
| [5] | Xia Linfeng, Wang Lu, Long Qianfa, Tang Rongwu, Luo Haodong, Tang Yi, Zhong Jun, Liu Yang. Human umbilical cord mesenchymal stem cell-derived exosomes alleviate blood-brain barrier damage in mice with septic encephalopathy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1711-1719. |
| [6] | Zhang Haiwen, Zhang Xian, Xu Taichuan, Li Chao. Bibliometric and visual analysis of the research status and trends of senescence in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1580-1591. |
| [7] | Zhou Jian, Zhang Tao, Zhou Weili, Zhao Xingcheng, Wang Jun, Shen Jie, Qian Li, Lu Ming. Effects of resistance training on quadriceps mass and knee joint function in patients with osteoporosis and sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1081-1088. |
| [8] | Sun Jiahe, Shi Jipeng, Zhu Tianrui, Quan Helong, Xu Hongqi. Effect of exercise intervention in elderly individuals with sarcopenia and its comorbidities: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 997-1007. |
| [9] | He Qiwang, , , Chen Bo, Liang Fuchao, Kang Zewei, Zhou Yuan, Ji Anxu, Tang Xialin, . Relationship between Alzheimer’s disease and sarcopenia and body mass index: analysis of GWAS datasets for European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1036-1046. |
| [10] | Li Guangzheng, Li Wei, Zhang Bochun, Ding Haoqin, Zhou Zhongqi, Li Gang, Liang Xuezhen. A prediction model for sarcopenia in postmenopausal women: information analysis based on the China Health and Retirement Longitudinal Study database [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 849-857. |
| [11] | Zhou Zixiang, Zhao Baoxiang. Research progress in the relationship between nontraumatic necrosis of the femoral head and lipid metabolism and its treatment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 680-690. |
| [12] | Sun Danhe, Guo Xiaoling, Zhao Lingzhou. Construction and osteogenic activity of titanium dioxide nanotube and polydopamine composite coating on titanium implants [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5167-5177. |
| [13] | Yu Le, Nan Songhua, Shi Zijian, He Qiqi, Li Zhenjia, Cui Yinglin. Mechanisms underlying mitophagy, ferroptosis, cuproptosis, and disulfidptosis in Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4446-4456. |
| [14] | Fu Jingyue, Zhou Qinfeng, Li Muzhe, Ma Yong, Pan Yalan, Sun Jie, Huang Xiangyang, Guo Yang. Preparation and evaluation of an animal model of osteoporosis and osteoarthritis comorbidity in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4299-4308. |
| [15] | Wu Jiazhou, Qian Tao, Liu Zexian, Wu Yanbin, He Ying, Li Yazhou, Peng Jiang. Three-dimensional culture of stromal vascular fraction self-assembles into complex vascularized osteogenic organoids [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2681-2690. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||