Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (16): 4088-4104.doi: 10.12307/2026.706
Previous Articles Next Articles
Treatment of acute myeloid leukemia with corynoline: network pharmacology analysis of potential mechanisms and experimental validation
Zhou Wu1, 2, Zhang Jingxin2, Liu Yuancheng2, Hu Chenglong2, Wang Siqi2, Xu Jianxia2, Huang Hai1, 2, Wei Sixi1, 2
- 1Clinical Laboratory Center, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2School of Medical Laboratory Science, Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Received:2025-06-27Accepted:2025-09-08Online:2026-06-08Published:2025-11-26 -
Contact:Wei Sixi, PhD., Chief physician, Doctoral supervisor, Clinical Laboratory Center, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; School of Medical Laboratory Science, Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Zhou Wu, MS candidate, Clinical Laboratory Center, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; School of Medical Laboratory Science, Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:Key Laboratory Project for Chronic Disease Biomarkers Research at Guizhou Medical University, No. [2024] fy004 (to WSX)
CLC Number:
Cite this article
Zhou Wu, Zhang Jingxin, Liu Yuancheng, Hu Chenglong, Wang Siqi, Xu Jianxia, Huang Hai, Wei Sixi. Treatment of acute myeloid leukemia with corynoline: network pharmacology analysis of potential mechanisms and experimental validation[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4088-4104.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
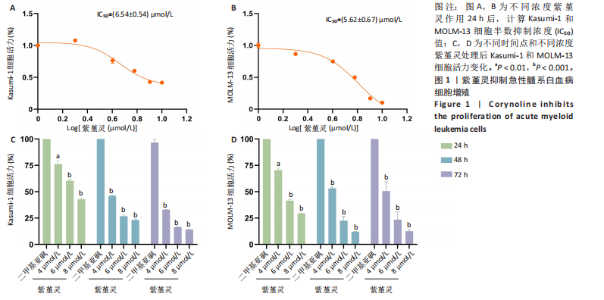
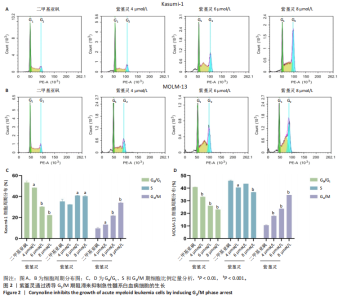
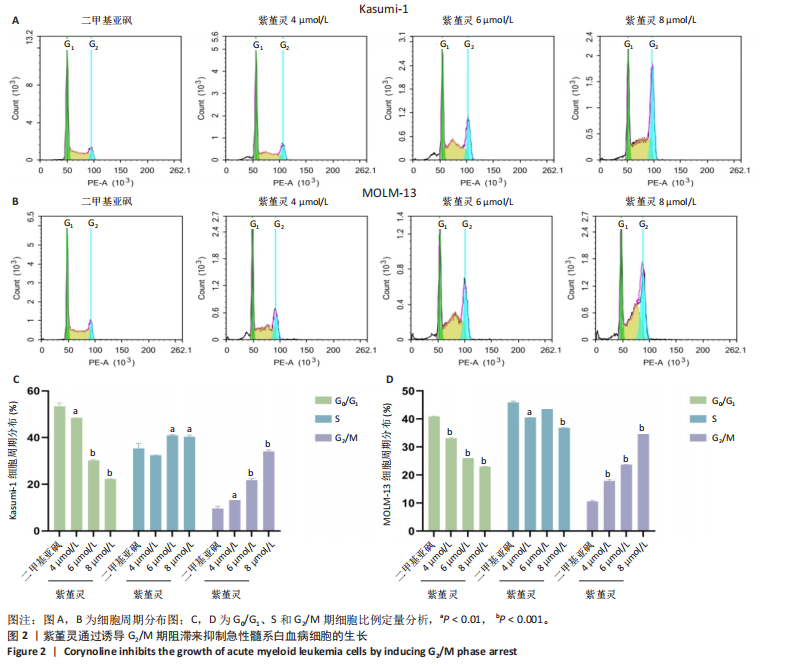
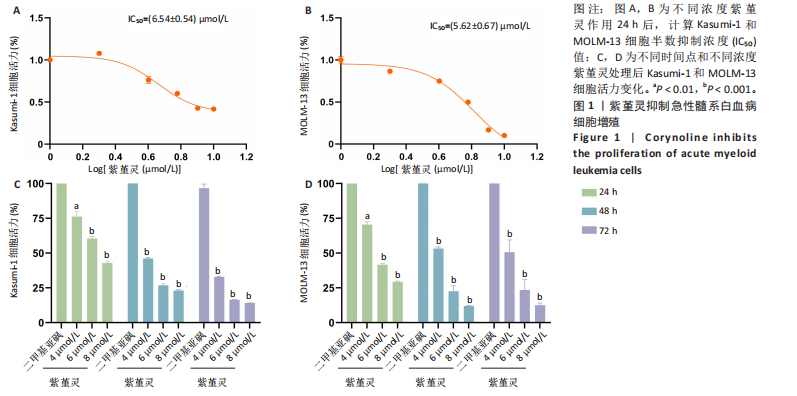
2.1 紫堇灵抑制Kasumi-1与MOLM-13细胞增殖 为评估紫堇灵对急性髓系白血病细胞增殖的抑制作用,采用CCK-8法检测Kasumi-1和MOLM-13细胞在不同处理条件下的细胞活力。结果显示,紫堇灵处理24 h后,Kasumi-1与MOLM-13细胞的IC??分别为(6.54± 0.54) μmol/L和(5.62±0.67) μmol/L(图1A,B)。在此基础上,进一步采用低(4 μmol/L)、中(6 μmol/L)和高(8 μmol/L)浓度的紫堇灵处理细胞,分别于24,48 和72 h时点检测细胞活力。结果表明,随着紫堇灵浓度的升高,细胞活力在各时间点均呈现明显下降趋势(图1C,D)。以上结果提示,紫堇灵可在一定时间范围内以浓度依赖方式抑制Kasumi-1与MOLM-13细胞的增殖。 2.2 紫堇灵通过诱导G2/M期阻滞来抑制Kasumi-1与MOLM-13细胞生长 为进一步探讨紫堇灵抑制细胞增殖的机制,采用流式细胞术分析其对细胞周期分布的影响,见图2。结果显示,紫堇灵处理24 h后,Kasumi-1细胞G2/M期的细胞比例在4,6,8 μmol/L浓度下分别为(13.34±0.25)%,(21.78±0.64)%,(34.20±0.72)%,均高于对照组(9.67±0.91)%(图 2A,C)。在MOLM-13细胞中,G2/M期的细胞比例分别为(17.88±0.72)%,(23.69±0.28)%,(34.73±0.78)%,亦明显高于对照组(10.56±0.32)%(图 2B,D)。上述结果提示,紫堇灵通过诱导G2/M期阻滞从而抑制白血病细胞的生长。 2.3 紫堇灵诱导Kasumi-1与MOLM-13细胞凋亡 采用Annexin V-APC/7-AAD染色联合流式细胞术检测紫堇灵对细胞凋亡的影响,见图3。结果显示,在"
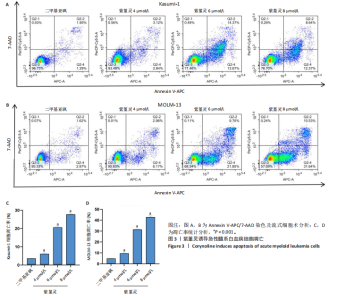
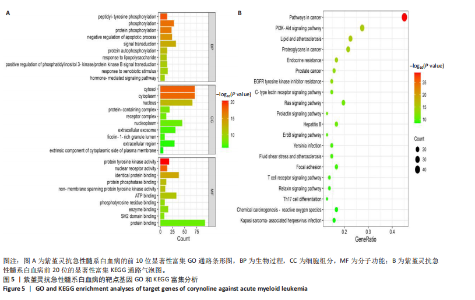
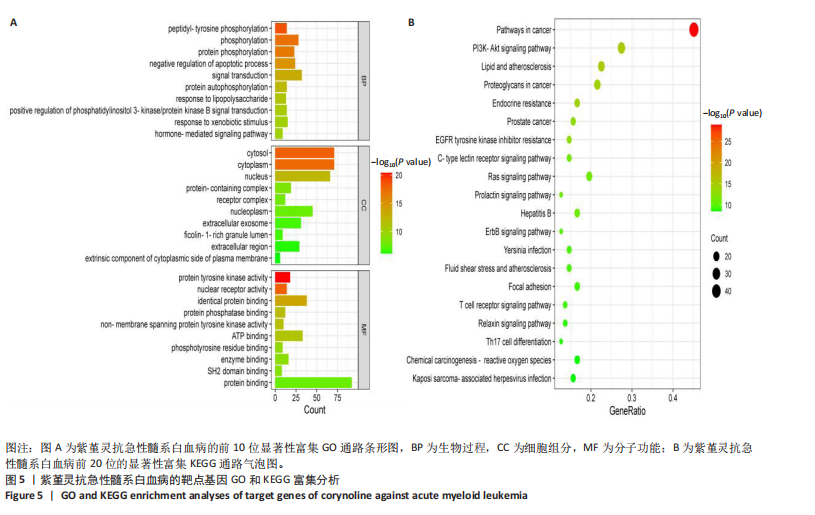
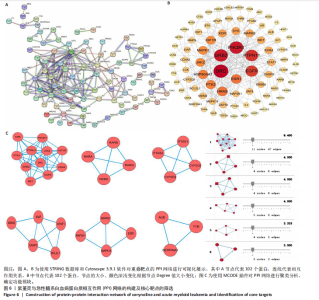
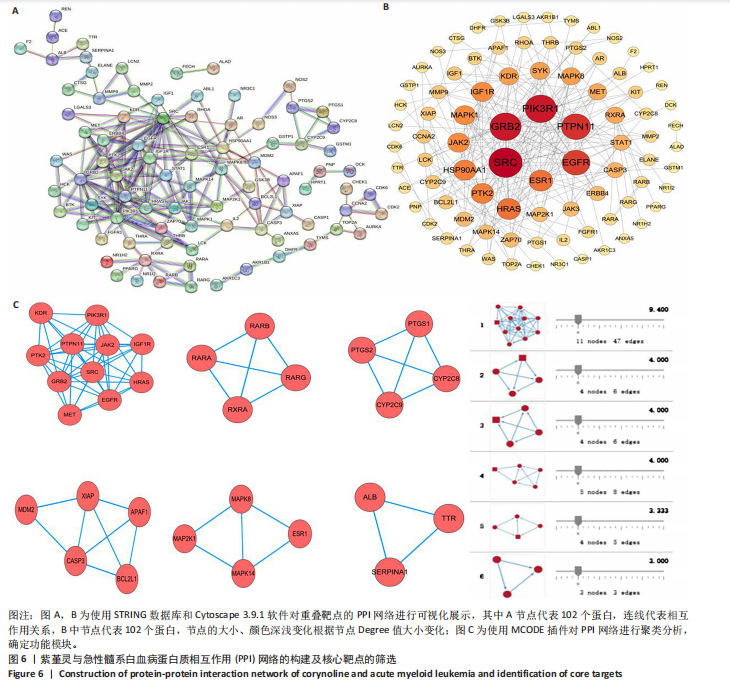
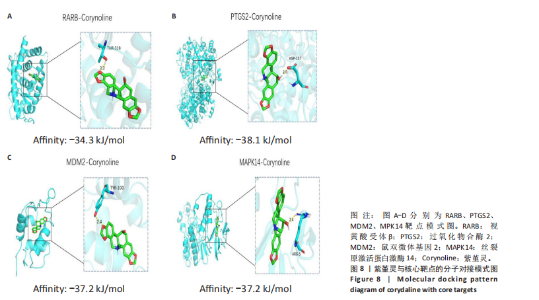
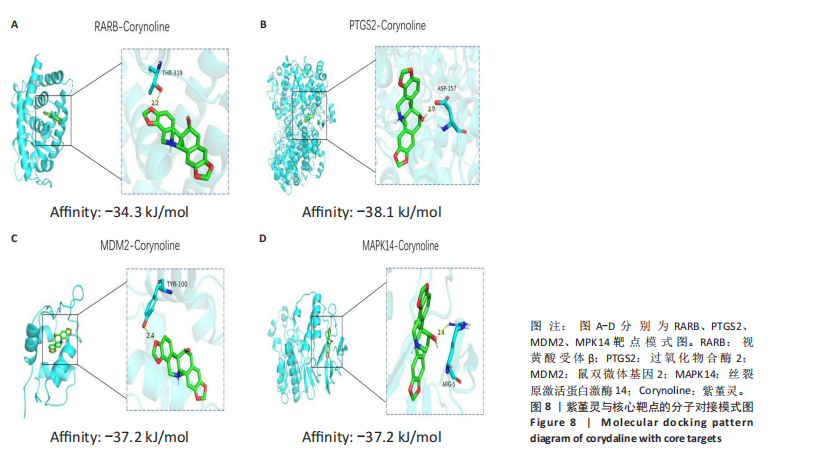
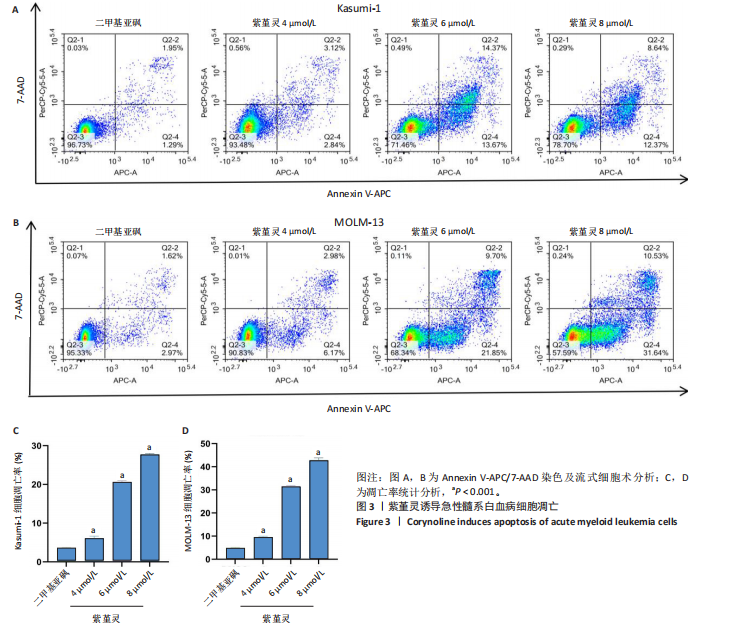
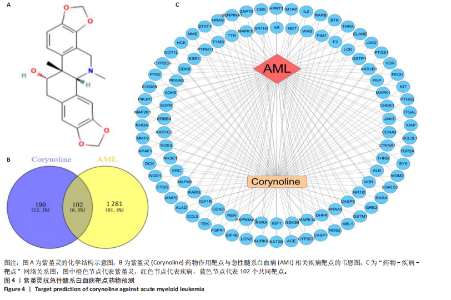
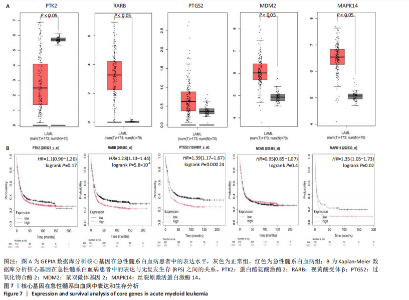
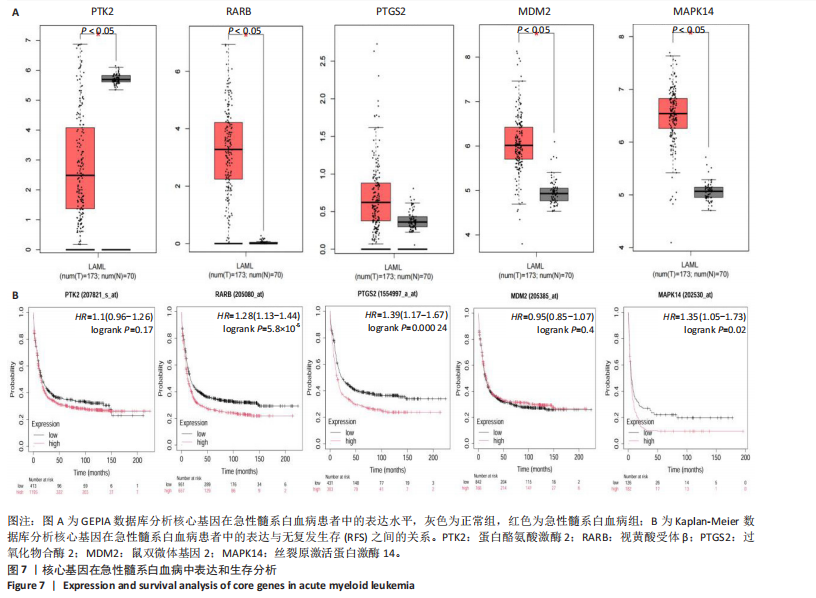
Kasumi-1细胞中,4,6,8 μmol/L紫堇灵处理24 h后,凋亡率分别为(5.93±0.71)%,(20.62±0.33)%,(27.75±0.24)%,明显高于对照组(3.47±0.21)%(图 3A,C);MOLM-13细胞的凋亡率分别为(9.55±0.37)%,(31.00±0.27)%,(42.88±0.86)%,也显著高于对照组(4.85±0.25)%(图 3B,D)。结果表明,紫堇灵可浓度依赖性诱导急性髓系白血病细胞凋亡,且MOLM-13对紫堇灵更敏感,因此被选为后续机制研究的模型细胞。 2.4 紫堇灵抗急性髓系白血病的药物靶点预测 基于紫堇灵抗急性髓系白血病的药理作用,研究通过网络药理学方法进一步探讨其可能作用机制。通过PubChem数据库获取紫堇灵的化学结构式(图4A),并利用PharmMapper、SwissTargetPrediction及TCMSP等数据库对其潜在药物靶点进行预测,共预测出292个靶点。同时,整合GeneCards、DrugBank和PharmGKB数据库检索预测出1 383个与急性髓系白血病相关的疾病基因。采用韦恩图分析方法对两者交集筛选,获得102个共同作用靶点(图4B)。为进一步揭示紫堇灵与急性髓系白血病之间的作用机制,使用Cytoscape 3.9.1 软件构建“药物-疾病-靶点”相互作用网络(图4C)。 2.5 GO和KEGG富集分析 为明确紫堇灵调控急性髓系白血病的生物学过程及可能通路,将上述102个交集靶点导入DAVID数据库进行GO和KEGG富集分析。GO分析共识别出463条生物过程(BP)、54条细胞组分(CC)及116条分子功能(MF)富集项,选取P值前10的GO术语绘制条形图展示(图5A)。结果显示,生物过程(BP)主要富集于蛋白磷酸化、细胞凋亡的负调控及磷脂酰肌醇3-激酶-蛋白激酶B信号通路等过程;细胞组分(CC)主要分布于细胞质、细胞核及蛋白质复合体;分子功能(MF)涉及蛋白酪氨酸激酶活性、核受体活性及蛋白结合等功能。KEGG富集结果显示共有144条信号通路显著富集,筛选前20位通路绘制气泡图(图5B)。其中磷脂酰肌醇3-激酶-蛋白激酶B信号通路、癌症相关蛋白聚糖、脂质代谢与动脉粥样硬化等为主要通路。 2.6 蛋白质相互作用网络的构建及核心靶点的筛选 利用STRING数据库,基于102个交叉靶点构建了蛋白质相互作用网络,并通过Cytoscape 3.9.1 软件进行可视化展示(图 6A,B)。该网络由86个节点和215条边组成。使用MCODE插件进行聚类分析,确定了6个紧密连接的子网络:聚类1(11个节点,47条边)、聚类2(4个节点,6条边)、聚类3(4个节点,6条边)、聚类4(5个节点,8条边)、聚类5(4个节点,5条边)以及聚类6(3个节点,3条边)。基于节点度(Degree)、介数中心性等拓扑参数,确定了5个核心靶点基因:蛋白酪氨酸激酶2(Protein tyrosine kinase 2,PTK2)、视黄酸受体β、过氧化物合酶2、鼠双微体基因2和丝裂原激活蛋白激酶14(图6C),这些基因在网络中处于关键调控位置,可能参与紫堇灵抗急性髓系白血病的主要生物过程。 2.7 核心基因在急性髓系白血病中表达和生存分析 为探究核心靶点的临床意义,利用GEPIA2数据库分析蛋白酪氨酸激酶2、视黄酸受体β、过氧化物合酶2、鼠双微体基因2和丝裂原激活蛋白激酶14的表达水平进行分析。结果显示,与正常对照组相比,急性髓系白血病样本中视黄酸受体β、鼠双微体基因2和丝裂原激活蛋白激酶14的表达显著上调(图7A),提示这些基因可能作为紫堇灵治疗急性髓系白血病的潜在靶点。通过Kaplan-Meier Plotter数据库进行的生存分析表明,视黄酸受体β、过氧化物合酶2和丝裂原激活蛋白激酶14的高表达水平与急性髓系白血病患者较差的总体生存率显著相关(图7B)。这些发现表明,紫堇灵可能通过调控疾病进展中的关键基因发挥其抗急性髓系白血病作用,从而突显这些分子作为治疗干预靶点的重要价值。综合研究结果提示,视黄酸受体β、过氧化物合酶2、鼠双微体基因2和丝裂原激活蛋白激酶14可能是介导紫堇灵抗急性髓系白血病活性的关键分子靶标。 2.8 分子对接分析 为了验证网络药理学预测的潜在治疗效果,采用分子对接的方法来评估小分子药物与核心靶点的结合亲和力。结果显示对视黄酸受体β、过氧化物合酶2、鼠双微体基因2和丝裂原激活蛋白激酶14靶点与紫堇灵相应的结合能分别为-34.3,-38.1,-37.2和-37.2 kJ/mol,见图8。结合能< -20.9 kJ/mol表明结合活性良好,结合能< -29.3 kJ/mol表明结合活性强[35]。紫堇灵与视黄酸受体β、过氧化物合酶2、鼠双微体基因2和丝裂原激活蛋白激酶14蛋白结合能均< -29.3 kJ/mol,表明结合活性强。分子对接结果提示视黄酸受体β、过氧化物合酶2、鼠双微体基因2和丝裂原激活蛋白激酶14蛋白与紫堇灵相互作用是发挥的治疗急性髓系白血病的潜在作用机制。 "
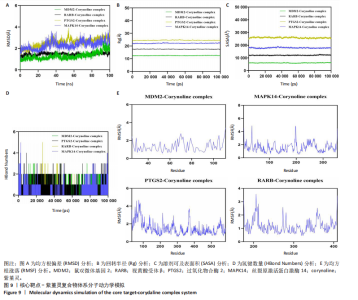
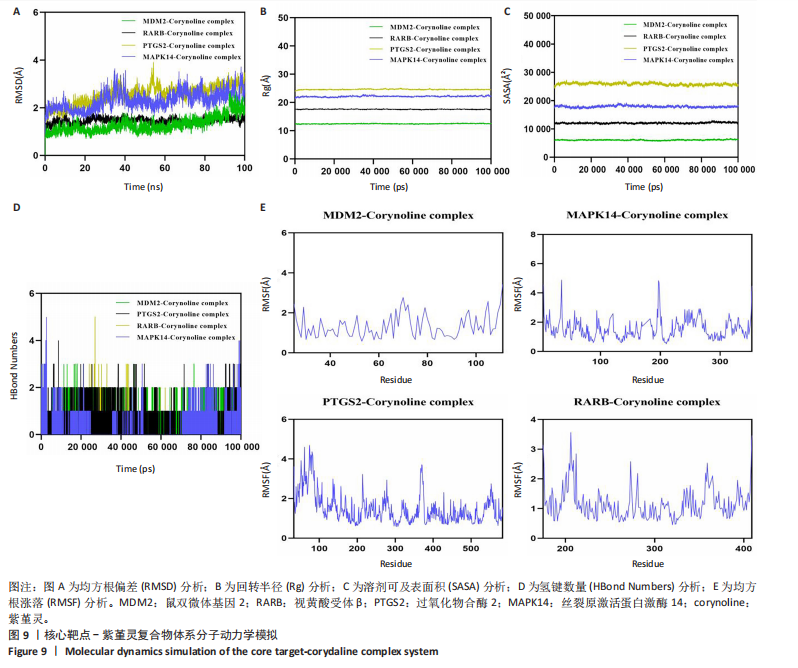
2.9 分子动力学模拟 为评估紫堇灵与靶蛋白复合物的稳定性,研究进行了100 ns的分子动力学模拟。均方根偏差(root mean square deviation,RMSD)轨迹分析表明,鼠双微体基因2-紫堇灵和视黄酸受体β-紫堇灵复合物在大约10 ns后达到稳定状态,平均均方根偏差值分别为-1.6 ?和-1.5 ?。相比之下,过氧化物合酶2-紫堇灵和丝裂原激活蛋白激酶14-紫堇灵复合物在约98 ns后达到稳定状态,其均方根偏差值分别为3.1 ?和2.7 ?(图9A),显示出良好的整体稳定性。为进一步表征复合物的构象动态变化,研究开展了回转半径(Rg)和溶剂可及表面积(SASA)分析(图9B,C)。结果表明,在模拟过程中,所有复合物未发生显著的结构收缩或扩张,提示其整体构象稳定。氢键分析显示,各复合物在大部分模拟时间内均维持约2个氢键(图9D),表明分子间相互作用稳定。此外,均方根涨落(root mean square fluctuation,RMSF)分析显示,大多数残基的均方根涨落值低于3 ?(图9E),提示复合物具有较低的构象柔性和较强的结构稳定性。综合上述分子动力学模拟结果,紫堇灵与鼠双微体基因2、视黄酸受体β、过氧化物合酶2和丝裂原激活蛋白激酶14靶蛋白形成的复合物均表现出良好的稳定性,进一步从分子层面支持了其潜在的抗急性髓系白血病活性。 2.10 紫堇灵通过调控鼠双微体基因2-p53轴及抑制磷脂酰肌醇3-激酶/蛋白激酶B通路发挥抗急性髓系白血病作用 为探讨紫堇灵的分子机制,首先通"

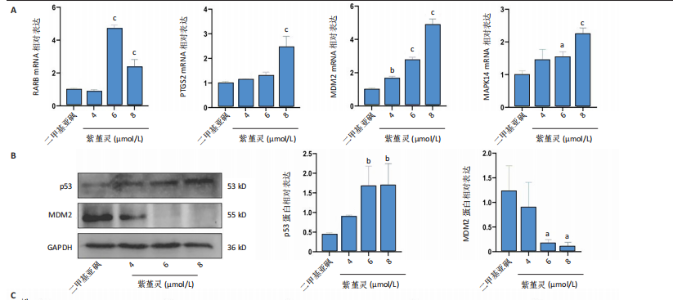
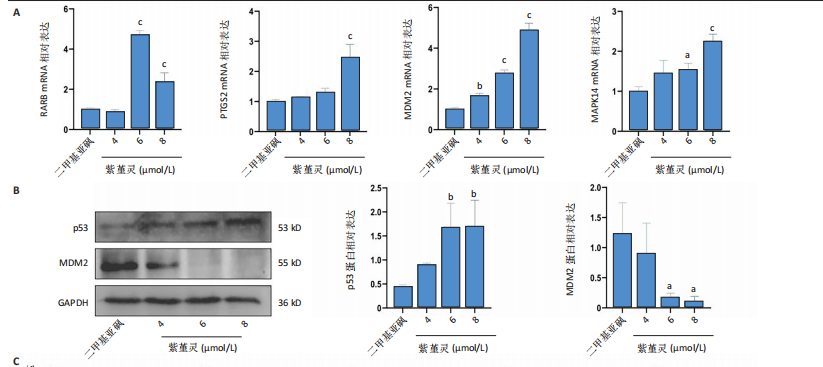
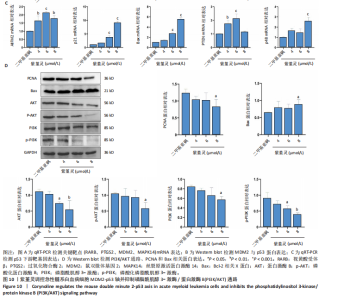
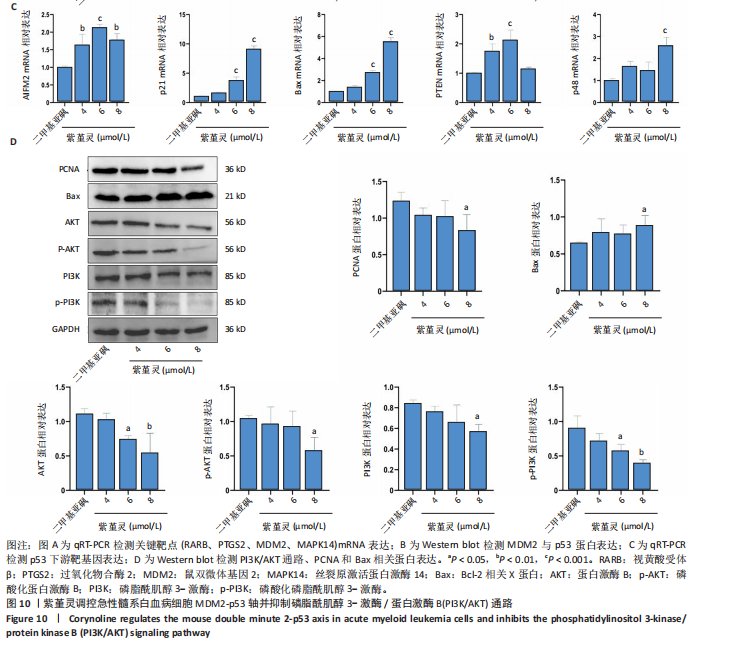
过 qRT-PCR 检测其处理 MOLM-13细胞24 h后核心靶点表达情况。结果显示,视黄酸受体β、过氧化物合酶2、鼠双微体基因2与丝裂原激活蛋白激酶14 mRNA水平均显著上调,其中鼠双微体基因2 表达呈现浓度依赖性上调趋势(图10A)。然而,Western blot 检测显示随着药物浓度升高,鼠双微体基因2蛋白水平反而呈下降趋势,同时p53蛋白表达上调(图10B),提示紫堇灵可能通过促进鼠双微体基因2降解而间接稳定并激活p53。鼠双微体基因2作为p53的主要负调控因子,可通过促进其泛素化及后续的蛋白酶体降解发挥作用[36];p53作为一个转录因子,p53的再激活可通过调控下游基因的转录参与细胞DNA 修复、周期调节、凋亡和衰老等生物学过程[37]。进一步分析p53下游靶基因表达,qRT-PCR 结果显示 AIFM2、p21、Bax、p48等基因均被上调,其中p21和Bax呈显著浓度依赖性上调(图10C),提示紫堇灵可通过激活 p53/p21/Bax 信号轴诱导细胞凋亡与周期阻滞。 结合此前 KEGG 富集分析,提示紫堇灵可能调控磷脂酰肌醇3-激酶/蛋白激酶B通路。Western blot结果进一步证实其可浓度依赖性抑制磷脂酰肌醇3-激酶、蛋白激酶B及其磷酸化形式(磷酸化磷脂酰"
| [1] BHANSALI RS, PRATZ KW, LAI C. Recent advances in targeted therapies in acute myeloid leukemia. J Hematol Oncol. 2023;16(1):29. [2] DÖHNER K, THIEDE C, JAHN N, et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood. 2020;135(5):371-380. [3] ZHANG N, WU J, WANG Q, et al. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023;13(1):82. [4] LIU H. Emerging agents and regimens for AML. J Hematol Oncol. 2021;14(1):49. [5] KOGAN SC. Curing APL: differentiation or destruction?. Cancer Cell. 2009;15(1):7-8. [6] LUO H, VONG CT, CHEN H, et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med. 2019; 14:48. [7] ZHAO W, ZHENG X D, TANG P Y, et al. Advances of antitumor drug discovery in traditional Chinese medicine and natural active products by using multi-active components combination. Med Res Rev. 2023; 43(5):1778-1808. [8] NAEEM A, HU P, YANG M, et al. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules. 2022;27(23):8367. [9] YANG C, ZHANG C, WANG Z, et al. Corynoline Isolated from Corydalis bungeana Turcz. Exhibits Anti-Inflammatory Effects via Modulation of Nfr2 and MAPKs. Molecules. 2016;21(8):975. [10] KIM DK. Inhibitory effect of corynoline isolated from the aerial parts of Corydalis incisa on the acetylcholinesterase. Arch Pharm Res. 2002; 25(6):817-819. [11] FANG ZZ, ZHANG YY, GE GB, et al. Identification of cytochrome P450 (CYP) isoforms involved in the metabolism of corynoline, and assessment of its herb-drug interactions. Phytother Res. 2011;25(2): 256-263. [12] LIU B, SU K, WANG J, et al. Corynoline Exhibits Anti-inflammatory Effects in Lipopolysaccharide (LPS)-Stimulated Human Umbilical Vein Endothelial Cells through Activating Nrf2. Inflammation. 2018; 41(5):1640-1647. [13] SHI Y, YUAN Q, CHEN Y, et al. Corynoline inhibits esophageal squamous cell carcinoma growth via targeting Pim-3. Phytomedicine. 2024;123:155235. [14] LI SL, KONG XY, FANG Y. [(+)-corynoline Regulates the Proliferation,Stemness and Apoptosis of Triple Negative Breast Cancer Cells]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2022;44(2):244-252. [15] YI C, LI X, CHEN S, et al. Natural product corynoline suppresses melanoma cell growth through inducing oxidative stress. Phytother Res. 2020;34(10):2766-2777. [16] LI S, ZHANG B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2): 110-120. [17] LI X, LIU Z, LIAO J, et al. Network pharmacology approaches for research of Traditional Chinese Medicines. Chin J Nat Med. 2023;21(5):323-332. [18] FANG T, LIU L, LIU W. Exploring the mechanism of fraxetin against acute myeloid leukemia through cell experiments and network pharmacology.BMC Complement Med Ther. 2024;24(1):226. [19] JIAO Y, SHI C, SUN Y. Unraveling the Role of Scutellaria baicalensis for the Treatment of Breast Cancer Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. Int J Mol Sci. 2023;24(4):3594. [20] KIM S, CHEN J, CHENG T, et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49(D1): D1388-D1395. [21] WANG X, SHEN Y, WANG S, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45(W1):W356-W360. [22] DAINA A, MICHIELIN O, ZOETE V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357-W364. [23] RU J, LI P, WANG JA, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [24] UniProt: the Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025;53(D1):D609-D617. [25] HAMOSH A, SCOTT AF, AMBERGER JS, et al. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33(Database issue): D514-D517. [26] KNOX C, WILSON M, KLINGER CM, et al. DrugBank 6.0: the DrugBank Knowledgebase for 2024. Nucleic Acids Res. 2024;52(D1): D1265-D1275. [27] SAFRAN M, CHALIFA-CASPI V, SHMUELI O, et al. Human Gene-Centric Databases at the Weizmann Institute of Science: GeneCards, UDB, CroW 21 and HORDE. Nucleic Acids Res. 2003;31(1):142-146. [28] SHERMAN BT, HAO M, QIU J, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022;50(W1):W216-W221. [29] TANG Z, KANG B, LI C, et al. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556-W560. [30] GYŐRFFY B.Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation (Camb). 2024;5(3):100625. [31] MORRIS GM, HUEY R, LINDSTROM W, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility.J Comput Chem. 2009;30(16):2785-2791. [32] BERMAN H M, WESTBROOK J, FENG Z, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28(1):235-242. [33] JO S, KIM T, IYER VG, et al. CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem. 2008;29(11):1859-1865. [34] MARK P, NILSSON L. Structure and dynamics of liquid water with different long-range interaction truncation and temperature control methods in molecular dynamics simulations.J Comput Chem. 2002; 23(13):1211-1219. [35] JIA G, JIANG X, LI Z, et al. Decoding the Mechanism of Shen Qi Sha Bai Decoction in Treating Acute Myeloid Leukemia Based on Network Pharmacology and Molecular Docking. Front Cell Dev Biol. 2021:9:796757. [36] SHANGARY S, WANG S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14(17):5318-5324. [37] MARVALIM C, DATTA A, LEE SC. Role of p53 in breast cancer progression: An insight into p53 targeted therapy. Theranostics. 2023;13(4):1421-1442. [38] CASSIER P A, CASTETS M, BELHABRI A, et al. Targeting apoptosis in acute myeloid leukaemia. Br J Cancer. 2017;117(8):1089-1098. [39] YANG X, WANG J. Precision therapy for acute myeloid leukemia. J Hematol Oncol. 2018;11(1):3. [40] BULLINGER L, DöHNER K, DöHNER H. Genomics of Acute Myeloid Leukemia Diagnosis and Pathways. J Clin Oncol. 2017;35(9):934-946. [41] GRIMWADE D, IVEY A, HUNTLY BJ. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance.Blood. 2016;127(1):29-41. [42] GAO X, ZUO X, MIN T, et al. Traditional Chinese medicine for acute myelocytic leukemia therapy: exploiting epigenetic targets. Front Pharmacol. 2024;15:1388903. [43] MILLER WH JR, SCHIPPER HM, LEE JS, et al. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62(14):3893-3903. [44] SHEN ZX, SHI ZZ, FANG J, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2004;101(15):5328-5335. [45] ESTEY E, GARCIA-MANERO G, FERRAJOLI A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107(9):3469-3473. [46] LALLEMAND-BREITENBACH V, DE THÉ H. Retinoic acid plus arsenic trioxide, the ultimate panacea for acute promyelocytic leukemia?. Blood. 2013;122(12):2008-2010. [47] BURNETT AK, RUSSELL NH, HILLS RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295-1305. [48] HUANG Z, YANG Y, FAN X, et al. Network pharmacology-based investigation and experimental validation of the mechanism of scutellarin in the treatment of acute myeloid leukemia. Front Pharmacol. 2022;13:952677. [49] WANG X, WANG Y, CHEN J, et al. On the mechanism of wogonin against acute monocytic leukemia using network pharmacology and experimental validation. Sci Rep. 2024;14(1):10114. [50] ZHAO L, ZHANG H, LI N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. [51] JIANG M, LI J, WU J, et al. Case report: A rare case of TBL1XR1-RARB positive acute promyelocytic leukemia in child and review of the literature.Front Oncol. 2022;12:1028089. [52] FANG D D, TANG Q, KONG Y, et al. MDM2 inhibitor APG-115 exerts potent antitumor activity and synergizes with standard-of-care agents in preclinical acute myeloid leukemia models. Cell Death Discov. 2021;7(1):90. [53] KONOPLEVA M Y, RöLLIG C, CAVENAGH J, et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022;6(14):4147-4156. [54] MATOU-NASRI S, NAJDI M, ALSAUD NA, et al. Blockade of p38 MAPK overcomes AML stem cell line KG1a resistance to 5-Fluorouridine and the impact on miRNA profiling. PLoS One. 2022;17(5):e0267855. [55] YUAN TL, CANTLEY LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497-510. [56] BERTACCHINI J, HEIDARI N, MEDIANI L, et al. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci. 2015;72(12): 2337-2347. [57] LI J, QU P, ZHOU XZ, et al. Pimozide inhibits the growth of breast cancer cells by alleviating the Warburg effect through the P53 signaling pathway. Biomed Pharmacother. 2022;150:113063. [58] HE J, ZHU G, GAO L, et al. Fra-1 is upregulated in gastric cancer tissues and affects the PI3K/Akt and p53 signaling pathway in gastric cancer.Int J Oncol. 2015;47(5):1725-1734. [59] JAMAL SME, ALAMODI A, WAHL RU, et al. Melanoma stem cell maintenance and chemo-resistance are mediated by CD133 signal to PI3K-dependent pathways. Oncogene. 2020;39(32):5468-5478. [60] YAO Y, ZHANG Q, LI Z, et al. MDM2: current research status and prospects of tumor treatment. Cancer Cell Int. 2024;24(1):170. [61] BRUMMER T, ZEISER R. The role of the MDM2/p53 axis in antitumor immune responses. Blood. 2024;143(26):2701-2709. [62] KOJIMA K, KONOPLEVA M, SAMUDIO I J, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood.2005;106(9):3150-3159. [63] SHANGARY S, WANG S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223-241. [64] HU J, CAO J, TOPATANA W, et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J Hematol Oncol. 2021; 14(1):157. [65] LIU F, LI X, YAN H, et al. Downregulation of CPT2 promotes proliferation and inhibits apoptosis through p53 pathway in colorectal cancer.Cell Signal. 2022;92:110267. [66] FREEDMAN DA, WU L, LEVINE AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999;55(1):96-107. |
| [1] | Zhou Sirui, Xu Yukun, Zhao Kewei. Ideas and methods of anti-melanogenesis of Angelica dahurica extracellular vesicles [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1747-1754. |
| [2] | Liu Anting, Lu Jiangtao, Zhang Wenjie, He Ling, Tang Zongsheng, Chen Xiaoling. Regulation of AMP-activated protein kinase by platelet lysate inhibits cadmium-induced neuronal apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1800-1807. |
| [3] | Yuan Xiaoshuang, Yang Xu, Yang Bo, Chen Xiaoxu, Tian Ting, Wang Feiqing, Li Yanju, Liu Yang, Yang Wenxiu. Effect of conditioned medium of diffuse large B-cell lymphoma cells on proliferation and apoptosis of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1632-1640. |
| [4] | He Jiale, Huang Xi, Dong Hongfei, Chen Lang, Zhong Fangyu, Li Xianhui. Acellular dermal matrix combined with adipose-derived stem cell exosomes promotes burn wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1699-1710. |
| [5] | Chen Yulin, He Yingying, Hu Kai, Chen Zhifan, Nie Sha Meng Yanhui, Li Runzhen, Zhang Xiaoduo , Li Yuxi, Tang Yaoping. Effect and mechanism of exosome-like vesicles derived from Trichosanthes kirilowii Maxim. in preventing and treating atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1768-1781. |
| [6] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [7] | Jia Jinwen, Airefate·Ainiwaer, Zhang Juan. Effects of EP300 on autophagy and apoptosis related to allergic rhinitis in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1439-1449. |
| [8] | Guo Ying, Tian Feng, Wang Chunfang. Potential drug targets for the treatment of rheumatoid arthritis: large sample analysis from European databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1549-1557. |
| [9] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [10] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Advance in the mechanisms underlying miRNAs in steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1207-1214. |
| [11] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| [12] | Bao Zhuoma, Hou Ziming, Jiang Lu, Li Weiyi, Zhang Zongxing, Liu Daozhong, Yuan Lin. Effect and mechanism by which Pterocarya hupehensis skan total flavonoids regulates the proliferation, migration and apoptosis of fibroblast-like synoviocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 816-823. |
| [13] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [14] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Mechanism by which vascular endothelial growth factor A targets regulation of angiogenesis in the treatment of steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 671-679. |
| [15] | Chen Ling, Mao Qiuhua, Xu Pu, Zhang Wenbo. Effect of water-soluble matrix of nano-pearl powder on proliferation, migration and apoptosis of mouse fibroblasts#br# [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 338-344. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||