Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (16): 4077-4087.doi: 10.12307/2026.679
Previous Articles Next Articles
Sonic hedgehog signaling pathway regulates the formation and septation of the outflow tract in the embryoic mouse heart
Yao Kaining, Yan Yunan, Zhou Yifan, Shi Liang, Cao Ximei, Zeeshan Rahim, Yang Yanping
- Department of Histology and Embryology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2025-04-27Accepted:2025-08-19Online:2026-06-08Published:2025-11-26 -
Contact:Yang Yanping, PhD, Professor, Department of Histology and Embryology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Yao Kaining, MS candidate, Department of Histology and Embryology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
Supported by:Applied Basic Research Program of Department of Science and Technology of Shanxi Province (General Programs), No. 202303021221135 (to YYP); Applied Basic Research Program of Department of Science and Technology of Shanxi Province (General Programs), No. 202303021211247 (to SL); Graduate Education Innovation Program of Shanxi Province, No. 2023JG085 (to SL); Shanxi Province Higher School Teaching Reform Innovation Program, No. J20230486 (to SL)
CLC Number:
Cite this article
Yao Kaining, Yan Yunan, Zhou Yifan, Shi Liang, Cao Ximei, Zeeshan Rahim, Yang Yanping. Sonic hedgehog signaling pathway regulates the formation and septation of the outflow tract in the embryoic mouse heart[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4077-4087.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
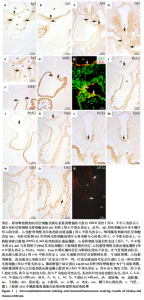
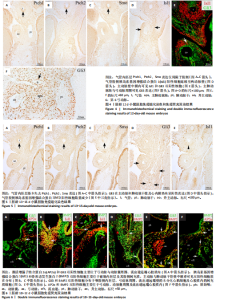
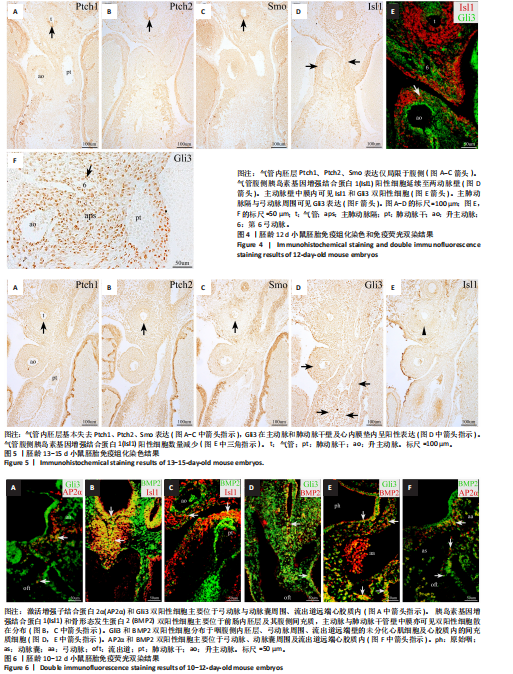
2.1 音猬因子信号通路组分与AP2α在第二生心区、心脏神经嵴及流出道的时空表达模式 胚龄10 d小鼠胚胎心管已成襻,可见流出道远端、近端分别与动脉囊、右心室相连,流出道壁由外向内依次为心肌膜、心胶质及内皮,见图1A。位于动脉囊背侧的原始咽横断面呈扁椭圆形,它腹侧的内胚层细胞为单层立方或柱状,呈较强Isl1阳性,内胚层腹侧可观察到少量间充质细胞散在分布,部分呈Isl1 阳性,此外,Isl1阳性细胞还分布于鳃弓核心间充质、心包腔背侧壁及相连的流出道远端,见图1A,B;向心管静脉端移行,可见咽尾端横断面呈矢状走行的椭圆形,并延续为尚未分隔的食管与气管,气管腹侧部内胚层细胞表达Isl1,相邻Isl1 阳性间充质细胞数量较咽头端增多并延续至心背系膜与心房壁,见图1C,D。心肌肌球蛋白重链在Isl1阳性的流出道远端壁、心房背侧壁呈弱阳性表达,见图1C与图1E-G,说明此期第二生心区祖细胞向心管动脉端与静脉端添加心肌细胞。免疫荧光双染可见前肠腹侧内胚层、心包腔背侧壁及流出道远端壁部分Isl1阳性细胞同时表达音猬因子,见图1H。Ptch1、Ptch 2、Smo并未在咽头端内胚层与咽间充质内表达,而是出现于气管腹侧内胚层、流出道心肌,见图1I-M,说明音猬因子受体蛋白与呼吸内胚层发生密切相关;下游信号Gli3在前肠内胚层及腹侧间充质、弓动脉周围、心包腔背侧壁、流出道与心房壁各层广泛表达,见图1N,O;在流出道近段可见部分内皮细胞与相邻的间充质细胞相连,正在进行上皮-间充质转化,参与流出道心内膜垫的形成,见图1O。AP2α用于标记正在迁移的心脏神经嵴细胞,此期AP2α阳性表达分布于弓动脉周围、动脉囊腹侧及与之相连的流出道远端心胶质内,见图1P,说明神经嵴与内皮来源细胞共同参与形成心内膜垫,与之相邻的心肌表达Ptch1、Ptch 2、Smo;而Gli3不仅见于第二生心区,也见于心脏神经嵴以及二者在流出道的衍生细胞。 与胚龄10 d小鼠胚胎相比,胚龄10.5 d小鼠胚胎动脉囊相邻的咽腹侧Isl1阳性间充质细胞明显可见,见图2A,B,咽腹侧壁正中内胚层突向腹侧形成“v”形呼吸内胚层头段,Ptch1、Ptch 2、Smo开始在呼吸内胚层头段呈阳性表达,三者在与流出道远端相连的心包腔背侧壁也呈阳性表达,流出道心内膜垫间充质细胞增多,见图2C-E;向心脏静脉端移行,可见正在与食管分隔的气管腹侧Isl1阳性细胞分布与胚龄10 d小鼠胚胎相似,见图2F。心包腔背侧壁与呼吸内胚层腹侧的第二生心区祖细胞继续分别向流出道远端、心房与静脉窦延续,见图2A,F。Gli3、AP2α表达与胚龄10 d小鼠胚胎相似,见图2G,H。 对于胚龄11-11.5 d小鼠胚胎,动脉囊突入心包腔形成心肌肌球蛋白重链阴性的流出道非心肌部,见图3A。呼吸内胚层向腹侧增长,除了继续表达Isl1、音猬因子外,Ptch1、Ptch2、Smo亦呈强阳性,其两侧Isl1阳性细胞急剧增多形成锥形区,并继续向流出道非心肌部延续,见图3B-F。说明音猬因子配体与受体高表达出现于呼吸内胚层形成过程中,而呼吸内胚层增长伴随相邻第二生心区细胞的增多。两条螺旋状走行的心内膜垫向流出道腔内隆起,由流出道远端延伸至与右心室相接处,而紧密相邻的心肌细胞表达Ptch1、Ptch2、Smo,见图3D,E。气管与食管已完全分隔,气管腹侧Isl1阳性间充质细胞数量明显少于与流出道相邻的咽腹侧Isl1阳性细胞,见图3B与图3G。气管尾端连接左右肺芽,相邻间充质内Isl1阳性细胞群呈“C”型包绕肺芽两侧及腹侧;同时,部分Isl1阳性细胞经心背系膜延续至心背侧间充质突,见图3H。Gli3在呼吸内胚层表达,但在Isl1阳性锥形区表达并不明显;在流出道非心肌部、流出道心内膜垫内分布有丰富的Gli3阳性细胞,见图3I。弓动脉壁周围AP2α阳性细胞不再向动脉囊腹侧和流出道远端迁移,部分AP2α阳性细胞分布于Isl1阳性锥形区周围,二者紧密相邻,见图3J,K。 来自呼吸内胚层尾段的气管在胚龄10-11 d小鼠胚胎已形成,故胚龄12 d小鼠胚胎气管内胚层Ptch1、Ptch2、Smo表达仅局限于腹侧而背侧不表达,三者在流出道内的表达仍主要分布于心肌,见图4A-C。主肺动脉隔出现于第4,6弓动脉之间的主动脉囊后壁,并向腹侧延伸与与流出道心内膜垫远端逐渐融合,将流出道非心肌部分隔为升主动脉与肺动脉干。气管腹侧Isl1阳性细胞延续至两动脉壁,见图4D,E;动脉壁中膜内部分Isl1阳性第二生心区来源细胞共表达Gli3,见图4E。Gli3表达亦见于主肺动脉隔、弓动脉周围,见图4E,F,此两部位为心脏神经嵴细胞分布区域,故提示Gli3仍可表达于此类细胞。 对于胚龄13-15 d小鼠胚胎,Ptch1、Ptch2、Smo在气管内胚层绝大部分细胞失去表达,在心肌仍呈阳性表达,见图5A-C。主动脉和肺动脉干壁中膜第二生心区来源细胞正在分化为平滑肌细胞,此处未观察到Ptch1、Ptch2、Smo阳性细胞,但可见Gli3的广泛表达,见图5A-C与图5D。主、肺动脉瓣正在重塑,瓣膜以下,心内膜垫逐渐融合,仍含有丰富的Gli3阳性细胞,见图5D。气管腹侧Isl1阳性细胞数量减少,不再延伸入心脏动脉端与静脉端,见图5E。 2.2 Isl1、AP2α、Gli3和骨形态发生蛋白2的共表达 免疫组织化学染色显示,AP2α阳性心脏神经嵴细胞分布的区域包括弓动脉与动脉囊周围及流出道远端心胶质内均有Gli3的阳性表达,因此,选取二者进行免疫荧光双染,观察有无共表达。结果显示,对于胚龄10-10.5 d小鼠胚胎,AP2α和Gli3双阳性细胞主要位于弓动脉与动脉囊周围、流出道远端心胶质内,见图6A,说明正在迁移的心脏神经嵴细胞部分表达Gli3。 此外,由于Hedgehog和骨形态发生蛋白信号均对第二生心区、心脏神经嵴有调控作用,因此,此次实验选用Isl1与骨形态发生蛋白2、Gli3与骨形态发生蛋白2、Gli3与AP2α、骨形态发生蛋白2与AP2α分别进行免疫荧光双染。结果显示,对于胚龄10.5-12 d小鼠胚胎,Isl1和骨形态发生蛋白2双阳性细胞主要位于前肠内胚层及其腹侧间充质,见图6B,C,尤其在胚龄11-12 d小鼠胚胎,咽与呼吸内胚层及其腹侧间充质内可见大量双阳性细胞,见图6B,说明在第二生心区祖细胞数量急剧增加过程中骨形态发生蛋白2呈高表达。主动脉与肺动脉干管壁中膜亦可见Isl1和骨形态发生蛋白2双阳性细胞散在分布,见图6C。Gli3和骨形态发生蛋白2双阳性细胞除了分布于咽腹侧内胚层外,亦见于弓动脉周围、流出道远端壁的未分化心肌细胞及心胶质内的间充质细胞,见图6D,E,提示第二生心区来源的心肌细胞与神经嵴来源细胞均表达Gli3和骨形态发生蛋白2。AP2α和骨形态发生蛋白2双阳性细胞主要位于弓动脉、动脉囊周围及流出道远端心胶质内,见图6F,进一步提示正在迁移的心脏神经嵴细胞部分表达骨形态发生蛋白2。"
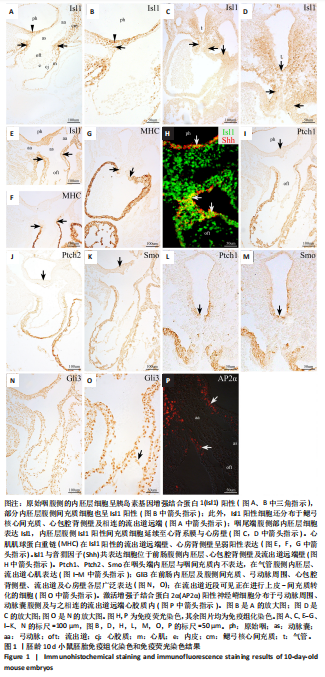
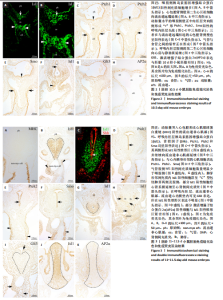
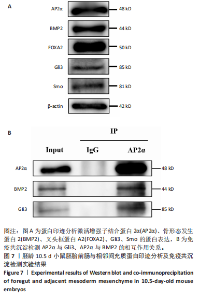
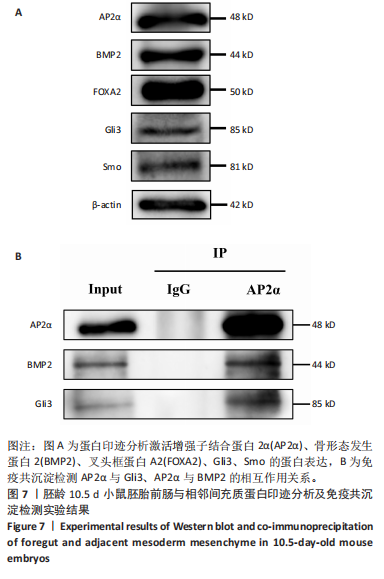
2.3 AP2α与Gli3、骨形态发生蛋白2的相互作用 免疫组化与免疫荧光双染结果显示,对于胚龄10.5-11 d小鼠胚胎,AP2α、Gli3分别与骨形态发生蛋白2在弓动脉、动脉囊周围及流出道远端心胶质内的部分细胞共表达。已有研究揭示骨形态发生蛋白2与促进神经嵴细胞的增殖、迁移和分化有关,Gli3与骨形态发生蛋白在许多位点共表达[22],因此,此次实验拟用免疫共沉淀探究AP2α与音猬因子信号通路及骨形态发生蛋白2之间是否存在相互作用,共同参与流出道的发育。蛋白印迹分析检测结果显示,对于胚龄10.5 d小鼠胚胎,AP2α、Gli3、骨形态发生蛋白2在前肠与相邻间充质均有较高的表达量,见图7A,可以进行下一步实验。 免疫共沉淀检测结果显示,Input组AP2α、Gli3、骨形态发生蛋白2均有表达,IP组AP2α的表达量明显高于Input 组,说明Protein G磁珠起到了富集作用;AP2α可将前肠周围组织中的Gli3、骨形态发生蛋白2沉淀下来,并且Gli3、骨形态发生蛋白2的表达量明显高于IgG组,说明AP2α特异性结合了Gli3、骨形态发生蛋白2,起到了沉淀作用;而阴性对照IgG组未见明显沉淀,见图7B,说明在心脏神经嵴细胞中AP2α与Gli3之间、AP2α与骨形态发生蛋白2之间存在相互作用关系。"

| [1] ZHAO K, YANG Z. The second heart field: the first 20 years. Mamm Genome. 2023;34(2):216-228. [2] Zaffran S, Kelly RG. New developments in the second heart field. Differentiation. 2012;84(1):17-24. [3] ALKOBTAWI M, RAY H, BARRIGA EH, et al. Characterization of Pax3 and Sox10 transgenic Xenopus laevis embryos as tools to study neural crest development. Dev Biol. 2018;444 Suppl 1(Suppl 1):S202-s208. [4] DE BONO C, LIU Y, FERRENA A, et al. Single-cell transcriptomics uncovers a non-autonomous Tbx1-dependent genetic program controlling cardiac neural crest cell development. Nat Commun. 2023;14(1):1551. [5] KIBALNYK Y, AFANASIEV E, NOBLE RMN, et al. The chromatin regulator Ankrd11 controls cardiac neural crest cell-mediated outflow tract remodeling and heart function. Nat Commun. 2024;15(1):4632. [6] ZHANG KK, XIANG M, ZHOU L, et al. Gene network and familial analyses uncover a gene network involving Tbx5/Osr1/Pcsk6 interaction in the second heart field for atrial septation. Hum Mol Genet. 2016; 25(6):1140-1151. [7] LI MR, LUO XJ, PENG J. Role of sonic hedgehog signaling pathway in the regulation of ion channels: focus on its association with cardio-cerebrovascular diseases. J Physiol Biochem. 2023;79(4):719-730. [8] YIN W, LIONTOS A, KOEPKE J, et al. An essential function for autocrine hedgehog signaling in epithelial proliferation and differentiation in the trachea. Development. 2022;149(3):dev199804. [9] GUZZETTA A, KOSKA M, ROWTON M, et al. Hedgehog-FGF signaling axis patterns anterior mesoderm during gastrulation. Proc Natl Acad Sci U S A. 2020;117(27):15712-15723. [10] LIM TB, FOO SYR, CHEN CK. The Role of Epigenetics in Congenital Heart Disease. Genes (Basel). 2021;12(3):390. [11] GILL E, BAMFORTH SD. Molecular Pathways and Animal Models of Truncus Arteriosus. Adv Exp Med Biol. 2024;1441:853-865. [12] WASHINGTON SMOAK I, BYRD NA, ABU-ISSA R, et al. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol. 2005;283(2):357-372. [13] CHRIST A, MARCZENKE M, WILLNOW TE. LRP2 controls sonic hedgehog-dependent differentiation of cardiac progenitor cells during outflow tract formation. Hum Mol Genet. 2020;29(19): 3183-3196. [14] CORTES C, FRANCOU A, DE BONO C, et al. Epithelial Properties of the Second Heart Field. Circ Res. 2018;122(1):142-154. [15] SCHUSSLER O, GHARIBEH L, MOOTOOSAMY P, et al. Cardiac Neural Crest Cells: Their Rhombomeric Specification, Migration, and Association with Heart and Great Vessel Anomalies . Cell Mol Neurobiol. 2021;41(3):403-429. [16] KODO K, UCHIDA K, YAMAGISHI H. Genetic and Cellular Interaction During Cardiovascular Development Implicated in Congenital Heart Diseases. Front Cardiovasc Med. 2021;8:653244. [17] TANG W, LI Y, LI A, et al. Clonal analysis and dynamic imaging identify multipotency of individual Gallus gallus caudal hindbrain neural crest cells toward cardiac and enteric fates. Nat Commun. 2021;12(1):1894. [18] ZHANG C, LI Y, CAO J, et al. Hedgehog signalling controls sinoatrial node development and atrioventricular cushion formation. Open Biol. 2021;11(6):210020. [19] SHEEHAN-ROONEY K, SWARTZ ME, LOVELY CB, et al. Bmp and Shh signaling mediate the expression of satb2 in the pharyngeal arches. PLoS One. 2013;8(3):e59533. [20] LIU C, GUO H, SHI C, et al. BMP signaling in the development and regeneration of tooth roots: from mechanisms to applications. Front Cell Dev Biol. 2023;11:1272201. [21] HE K, JIANG H, LI W, et al. Primary cilia mediate skeletogenic BMP and Hedgehog signaling in heterotopic ossification. Sci Transl Med. 2024;16(757):eabn3486. [22] MANZARI-TAVAKOLI A, BABAJANI A, FARJOO MH, et al. The Cross-Talks Among Bone Morphogenetic Protein (BMP) Signaling and Other Prominent Pathways Involved in Neural Differentiation. Front Mol Neurosci. 2022;15:827275. [23] LIANG S, LI HC, WANG YX, et al. Pulmonary endoderm, second heart field and the morphogenesis of distal outflow tract in mouse embryonic heart. Dev Growth Differ. 2014;56(4):276-292. [24] TAKIGAWA-IMAMURA H, FUMOTO K, TAKESUE H, et al. Exploiting mechanisms for hierarchical branching structure of lung airway. PLoS One. 2024;19(8):e0309464. [25] CARBALLO GB, HONORATO JR, DE LOPES GPF, et al. A highlight on Sonic hedgehog pathway. Cell Commun Signal. 2018;16(1):11. [26] CALLEJAS-MARIN A, MORENO-BRAVO JA, COMPANY V, et al. Gli2-Mediated Shh Signaling Is Required for Thalamocortical Projection Guidance. Front Neuroanat. 2022;16:830758. [27] NIEWIADOMSKI P, NIEDZIÓŁKA SM, MARKIEWICZ Ł, et al. Gli Proteins: Regulation in Development and Cancer. Cells. 2019;8(2):147. [28] PENG T, TIAN Y, BOOGERD CJ, et al. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500(7464):589-592. [29] YOSHIDA S, YOSHIDA K. Regulatory mechanisms governing GLI proteins in hedgehog signaling. Anat Sci Int. 2025;100(2):143-154. [30] HILGENDORF KI, MYERS BR, REITER JF. Emerging mechanistic understanding of cilia function in cellular signalling. Nat Rev Mol Cell Biol. 2024;25(7):555-573. [31] HASENPUSCH-THEIL K, WEST S, KELMAN A, et al. Gli3 controls the onset of cortical neurogenesis by regulating the radial glial cell cycle through Cdk6 expression. Development. 2018;145(17):dev163147. [32] YANG Z. The Principle of Cortical Development and Evolution. Neurosci Bull. 2025;41(3): 461-485. [33] PALMQUIST-GOMES P, MEILHAC SM. Shaping the mouse heart tube from the second heart field epithelium. Curr Opin Genet Dev. 2022; 73:101896. [34] MOTOYAMA J, MILENKOVIC L, IWAMA M, et al. Differential requirement for Gli2 and Gli3 in ventral neural cell fate specification. Dev Biol. 2003;259(1):150-161. [35] ROWTON M, PEREZ-CERVANTES C, HUR S, et al. Hedgehog signaling activates a mammalian heterochronic gene regulatory network controlling differentiation timing across lineages. Dev Cell. 2022;57(18):2181-2203.e2189. [36] ERHARDT S, ZHENG M, ZHAO X, et al. The Cardiac Neural Crest Cells in Heart Development and Congenital Heart Defects. J Cardiovasc Dev Dis. 2021;8(8):89. |
| [1] | Yu Lei, Zhang Wei, Qin Yi, Ge Gaoran, Bai Jiaxiang, Geng Dechun. Repair of femoral condyle defects using mesoporous bioactive glass grafted with bone morphogenetic protein 2 osteogenic peptide inspired by mussel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4629-4638. |
| [2] | Gao Feng, Wang Jiliang, Wang Hongbo, Yang Yongsheng, Liu Yuan, Fu Su. Passage-associated senescence decreases osteogenic activity of MC3T3-E1 cells via primary cilia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3741-3746. |
| [3] | Zheng Qian, Liu Pingping, Gu Yujie, Xie Lei. Effect of ursolic acid on osteogenic differentiation of human periodontal ligament stem cells#br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 80-86. |
| [4] | Yan Ru, Wang Kairu, Zhang Feiyan, Jia Shaobin, Cong Guangzhi. Endothelial cell-specific bone morphogenetic protein 2 affects angiogenesis: bioinformatics analysis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 103-110. |
| [5] | Zhao Yiting, Zhang Yuxiang, Ma Jie, He Xuejiao. Vascular endothelial growth factor 165/bone morphogenetic protein improves osteoblast injury under hypoxic and reoxygenated conditions [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5669-5674. |
| [6] | Shi Xian, Han Chunqing, Hu Anran, Kuang Shuyun, Ran Yimeng, Wu Yu. Atf7ip is a negative regulator of bone morphogenetic protein 2 promoting osteogenic differentiation in mouse embryonic adult cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 4931-4936. |
| [7] | Ma Zhanhua, Yan Xu, Jiang Yan, Cao Zhengming, Wang Yongkui, Li Dongzhe, Yang Tengyue, Jin Yikai, Fu Su, Zhang Chunlin. Primary cilia/intraflagellar transport mediates mechanics-responsive signaling pathway and promotes osteogenic differentiation of bone marrow stromal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 3937-3941. |
| [8] | Pei Jiansheng, Yang Wenjuan, He Jing, Yan Ru, Huang Hui, Jia Shaobin. Bone morphogenetic protein-2 mediated homocysteine promotes vascular calcification [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4027-4033. |
| [9] | Ding Zeyuan, Yan Yunan, Xie Jianshan, Shi Liang, Jing Ya, Yang Yanping. Expression pattern and signification of Cx43, beta-catenin and Smo in the second heart field [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 3042-3048. |
| [10] | Wang Buyu, Zhang Yong, Ruan Shiqiang, Deng Jiang. Preparation of collagen-binding domain-bone morphogenetic protein 2-collagen cartilage scaffold and its chondrogenic induction [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2378-2384. |
| [11] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [12] | Wang Jinling, Huang Xiarong, Qu Mengjian, Huang Fujin, Yin Lingwei, Zhong Peirui, Liu Jin, Sun Guanghua, Liao Yang, Zhou Jun. Effects of exercise training on bone mass and bone microstructure in aged osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 676-682. |
| [13] | Xie Yingchun, Xu Wenjuan, Li Yuwan. Schnurri3 regulates osteogenic differentiation of C3H10T1/2 cells induced by bone morphogenetic protein 2 [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5270-5276. |
| [14] | Tang Juan, Yu Donglin, Liu Guoqi, Song Jiaojiao, Zuo Jinhua, Fu Honghai. Osteogenic and angiogenic differentiation of dental pulp stem cells modified by hypoxia-inducible factor-1 alpha gene in vitro [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(24): 3865-3870. |
| [15] | Wang Buyu, Zhang Yong, Li Feifei, Dong Xiaoyu, Deng Jiang, Ruan Shiqiang. Role and application of bone morphogenetic protein 2 in the repair of osteochondral defects [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3259-3265. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||