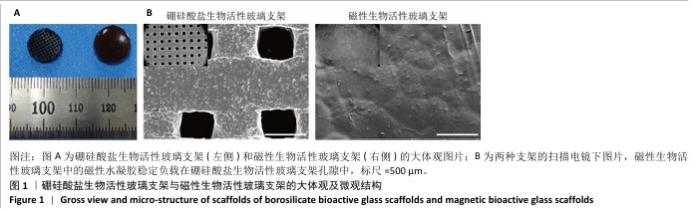
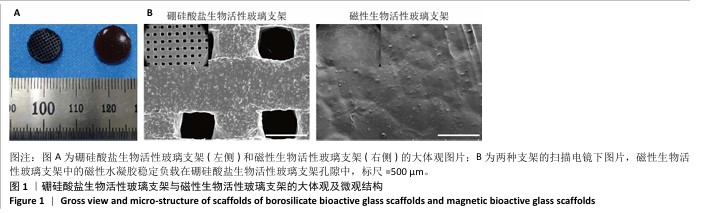
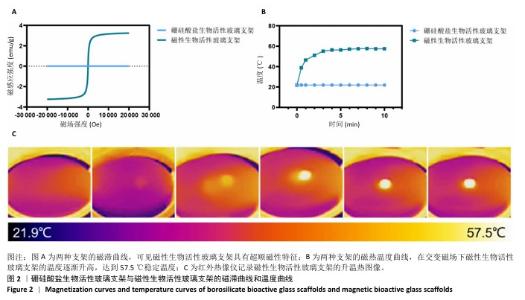
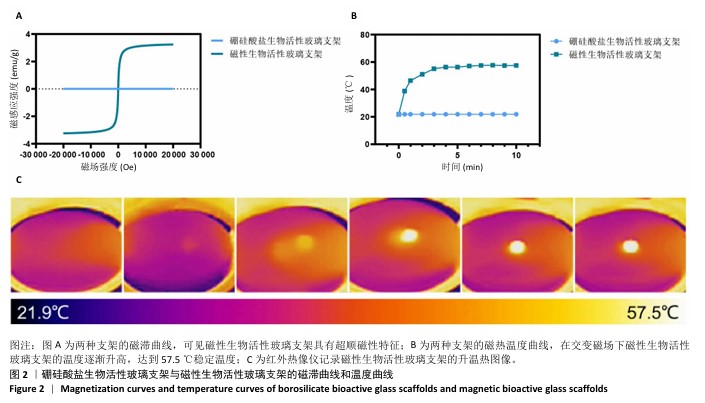
Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (14): 3494-3503.doi: 10.12307/2026.630
Previous Articles Next Articles
Magnetocaloric antitumor and osteogenic properties of magnetic bioactive glass scaffolds
Li Qingshan1, 2, Li Runmeng2, Gao Yuyang2, Han Gang1, 3, Chen Jiying1, Guo Quanyi1, 2
- 1Chinese People's Liberation Army Medical School, Beijing 100853, China; 2Institute of Orthopedics, Fourth Medical Center of Chinese PLA General Hospital, National Clinical Research Center for Orthopedics and Sports Rehabilitation, Beijing Key Laboratory of Orthopedic Regenerative Medicine, Key Laboratory of Musculoskeletal Trauma & War Injuries, Beijing 100853, China; 3Department of Bone Tumors, Fourth Medical Center of Chinese PLA General Hospital, Beijing 100853, China
-
Received:2025-03-21Accepted:2025-06-12Online:2026-05-18Published:2025-09-06 -
Contact:Guo Quanyi, MD, Chief physician, Professor, Chinese People's Liberation Army Medical School, Beijing 100853, China; Institute of Orthopedics, Fourth Medical Center of Chinese PLA General Hospital, National Clinical Research Center for Orthopedics and Sports Rehabilitation, Beijing Key Laboratory of Orthopedic Regenerative Medicine, Key Laboratory of Musculoskeletal Trauma & War Injuries, Beijing 100853, China Chen Jiying, MD, Chief physician, Professor, Chinese People's Liberation Army Medical School, Beijing 100853, China -
About author:Li Qingshan, Master candidate, Chinese People's Liberation Army Medical School, Beijing 100853, China; Institute of Orthopedics, Fourth Medical Center of Chinese PLA General Hospital, National Clinical Research Center for Orthopedics and Sports Rehabilitation, Beijing Key Laboratory of Orthopedic Regenerative Medicine, Key Laboratory of Musculoskeletal Trauma & War Injuries, Beijing 100853, China -
Supported by:National Key Research & Development Program, No. 2021YFC2400504 (to HG)
CLC Number:
Cite this article
Li Qingshan, Li Runmeng, Gao Yuyang, Han Gang, Chen Jiying, Guo Quanyi. Magnetocaloric antitumor and osteogenic properties of magnetic bioactive glass scaffolds[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3494-3503.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
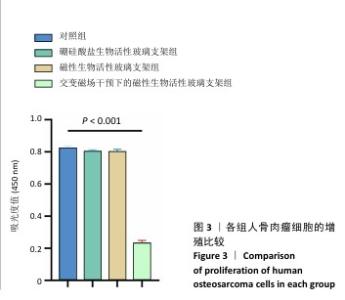
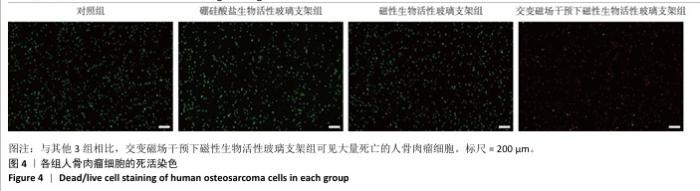
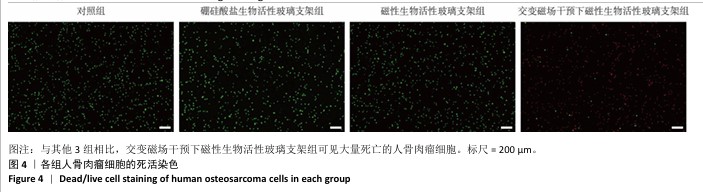
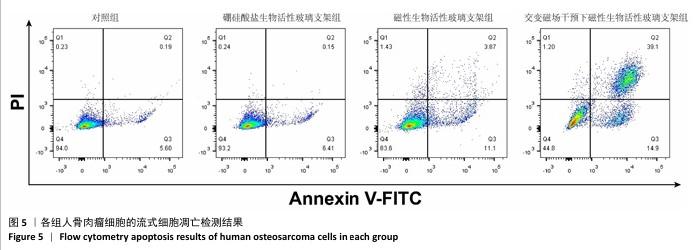
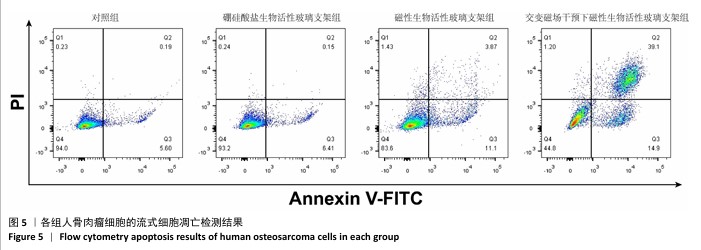
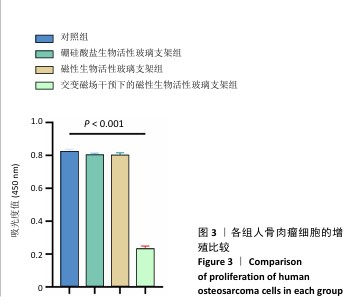
2.3 支架的磁热抗肿瘤能力 2.3.1 CCK-8实验结果 培养24 h后,交变磁场干预下磁性生物活性玻璃支架组细胞吸光度值低于对照组、硼硅酸盐生物活性玻璃支架组和磁性生物活性玻璃支架组(P < 0.05),对照组、硼硅酸盐生物活性玻璃支架组和磁性生物活性玻璃支架组细胞吸光度值比较差异无显著性意义(P > 0.05),见图3,证明磁性生物活性玻璃支架在交感磁场作用下具备抑制肿瘤细胞增殖的作用。 2.3.2 死活染色实验结果 活死染色中,绿色荧光标记有活性的人骨肉瘤细胞,红色荧光标记死亡的人骨肉瘤细胞。与对照组、硼硅酸盐生物活性玻璃支架组和磁性生物活性玻璃支架组相比,交变磁场干预下磁性生物活性玻璃支架组可见大量死亡的人骨肉瘤细胞,显示出良好的抗肿瘤能力,见图4,表明磁热疗法是一种有效的杀死肿瘤的方式。 2.3.3 流式细胞凋亡实验结果 流式细胞凋亡实验结果如图5所示。定量分析结果显示,交变磁场干预下磁性生物活性玻璃支架组(39.10%)细胞凋亡率高于对照组(0.19%)、硼硅酸盐生物活性玻璃支架组(0.15%)和磁性生物活性玻璃支架组(3.87%),进一步证明了磁性生物活性玻璃支架通过磁热效应具备优秀的抗肿瘤能力。 "
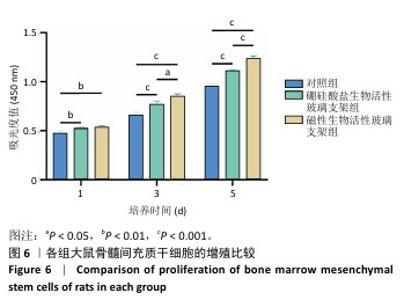
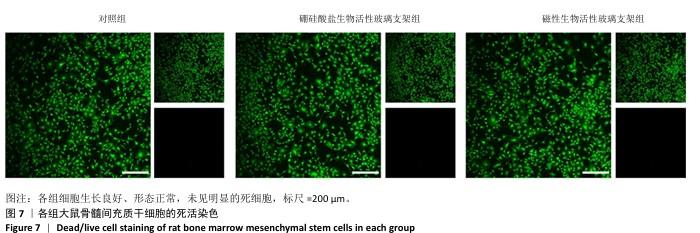
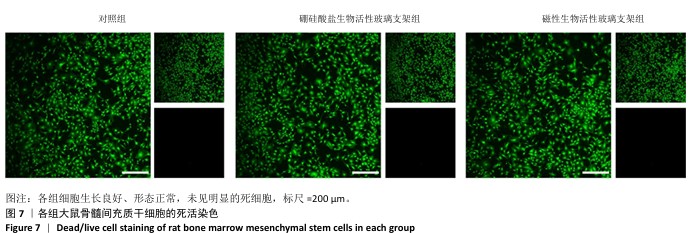
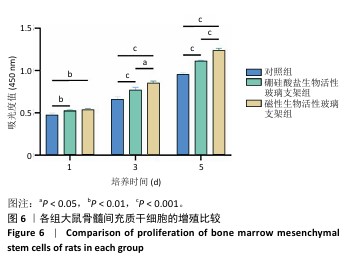
2.4 支架的生物相容性 2.4.1 CCK-8实验结果 培养1,3,5 d后,各组细胞吸光度值逐渐升高,硼硅酸盐生物活性玻璃支架组、磁性生物活性玻璃支架组培养1,3,5 d的细胞吸光度值均高于对照组(P < 0.01或P < 0.001),磁性生物活性玻璃支架组培养3,5 d的细胞吸光度值高于硼硅酸盐生物活性玻璃支架组(P < 0.05或P < 0.001),见图6。证明两组支架释放出适当的生物活性离子可促进大鼠骨髓间充质干细胞的增殖。 2.4.2 死活染色实验结果 死活染色结果显示,各组大鼠骨髓间充质干细胞生长良好、形态正常,未见明显的死细胞,见图7,说明两种支架具有良好的生物相容性。 "
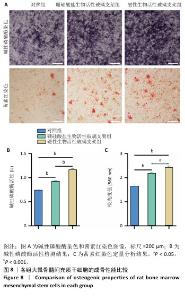
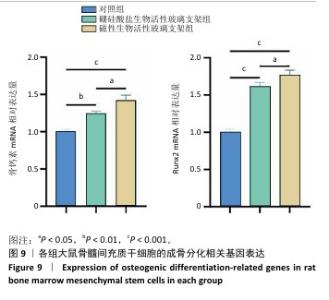
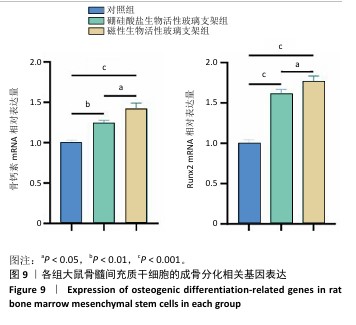
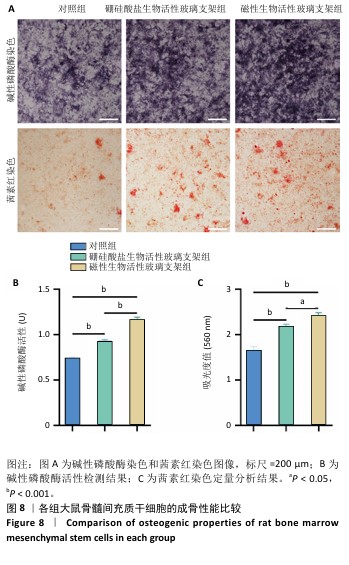
2.5 支架的促成骨性能 2.5.1 碱性磷酸酶染色与活性检测结果 碱性磷酸酶染色显示,相较于对照组,两支架组蓝紫色染色面积更大、染色程度更深,并且以磁性生物活性玻璃支架组染色面积和染色程度优于硼硅酸盐生物活性玻璃支架组,如图8A显示。碱性磷酸酶活性定量分析结果显示,两支架组碱性磷酸酶活性高于对照组(P < 0.001),磁性生物活性玻璃支架组碱性磷酸酶活性高于硼硅酸盐生物活性玻璃支架组(P < 0.05或P < 0.001),见图8B,证明磁性生物活性玻璃支架具有更优秀的体外促成骨能力。磁性生物活性玻璃支架组碱性磷酸酶染色与活性检测结果优于硼硅酸盐生物活性玻璃支架组,这是由于引入了了磁性纳米颗粒,磁性纳米颗粒的降解促进了铁离子的释放,而适量铁离子进一步增强大鼠骨髓间充质干细胞的成骨分化能力。 2.5.2 茜素红染色结果 茜素红染色显示,两支架组矿化结节形成数量明显多于对照组,磁性生物活性玻璃支架组矿化结节形成数量多于硼硅酸盐生物活性玻璃支架组,见图8A。茜素红染色钙结节定量分析显示,磁性生物活性玻璃支架组吸光度值明显高于硼硅酸盐生物活性玻璃支架组和对照组,见图8C。结果表明,磁性生物活性玻璃支架展现出更出色的促进大鼠骨髓间充质干细胞成骨分化性能。 2.5.3 成骨基因表达 qRT-PCR检测结果 磁性生物活性玻璃支架组、硼硅酸盐生物活性玻璃支架组骨钙素和Runx2 mRNA表达均高于对照组,磁性生物活性玻璃支架组骨钙素和Runx2 mRNA表达高于硼硅酸盐生物活性玻璃支架组,见图9,从基因角度验证了磁性生物活性玻璃支架具备良好的促进大鼠骨髓间充质干细胞成骨分化的作用。值得注意的是,磁性生物活性玻璃支架组成骨基因表达水平较硼硅酸盐生物活性玻璃支架组要高,这是因为磁性纳米颗粒降解释放的铁离子可激活相关信号通路,协同生物活性玻璃支架释放的硅、钙等离子共同促进成骨相关基因的表达,从而增强干细胞的成骨分化能力。"
| [1] HARRISON DJ, GELLER DS, GILL JD, et al. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18(1):39-50. [2] CHEN C, XIE L, REN T, et al. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1-10. [3] PAPAKONSTANTINOU E, STAMATOPOULOS A, I ATHANASIADIS D, et al. Limb-salvage surgery offers better five-year survival rate than amputation in patients with limb osteosarcoma treated with neoadjuvant chemotherapy. A systematic review and meta-analysis. J Bone Oncol. 2020;25:100319. [4] JANEWAY KA, GRIER HE. Sequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effects. Lancet Oncol. 2010; 11(7):670-678. [5] ZHAO X, WU Q, GONG X, et al. Osteosarcoma: a review of current and future therapeutic approaches. Biomed Eng Online. 2021;20(1):24. [6] TANG Z, YU D, BAO S, et al. Porous Titanium Scaffolds with Mechanoelectrical Conversion and Photothermal Function: A Win-Win Strategy for Bone Reconstruction of Tumor-Resected Defects. Adv Healthc Mater. 2024;13(7):e2302901. [7] LI C, ZHANG W, NIE Y, et al. Time-Sequential and Multi-Functional 3D Printed MgO2/PLGA Scaffold Developed as a Novel Biodegradable and Bioactive Bone Substitute for Challenging Postsurgical Osteosarcoma Treatment. Adv Mater. 2024;36(34):e2308875. [8] LI L, HUANG Y, QIN J, et al. Development of a borosilicate bioactive glass scaffold incorporating calcitonin gene-related peptide for tissue engineering. Biomater Adv. 2022;138:212949. [9] RAVANBAKHSH M, LABBAF S, KARIMZADEH F, et al. Mesoporous bioactive glasses for the combined application of osteosarcoma treatment and bone regeneration. Mater Sci Eng C Mater Biol Appl. 2019;104:109994. [10] TIAN P, ZHAO L, KIM J, et al. Dual stimulus responsive borosilicate glass (BSG) scaffolds promote diabetic alveolar bone defectsrepair by modulating macrophage phenotype. Bioact Mater. 2023;26:231-248. [11] JIN Y, LIU H, CHU L, et al. Initial therapeutic evidence of a borosilicate bioactive glass (BSG) and Fe3O4 magnetic nanoparticle scaffold on implant-associated Staphylococcal aureus bone infection. Bioact Mater. 2024;40:148-167. [12] FAN M, REN Y, ZHU Y, et al. Borosilicate bioactive glass synergizing low-dose antibiotic loaded implants to combat bacteria through ATP disruption and oxidative stress to sequentially achieve osseointegration. Bioact Mater. 2024;44:184-204. [13] CIRALDO FE, BOCCARDI E, MELLI V, et al. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018;75:3-10. [14] FANG CH, TSAI PI, HUANG SW, et al. Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis. BMC Infect Dis. 2017;17(1):516. [15] ALMUTAIRI LA, YU B, DYNE E, et al. Mild magnetic hyperthermia is synergistic with an antibiotic treatment against dual species biofilms consisting of S. aureus and P. aeruginosa by enhancing metabolic activity. Int J Hyperthermia. 2023;40(1):2226845. [16] CAZARES-CORTES E, CABANA S, BOITARD C, et al. Recent insights in magnetic hyperthermia: From the “hot-spot” effect for local delivery to combined magneto-photo-thermia using magneto-plasmonic hybrids. Adv Drug Deliv Rev. 2019;138:233-246. [17] LUKÁCSI S, MUNKÁCSY G, GYŐRFFY B. Harnessing Hyperthermia: Molecular, Cellular, and Immunological Insights for Enhanced Anticancer Therapies. Integr Cancer Ther. 2024;23:15347354241242094. [18] AHMED K, ZAIDI SF, MATI-UR-REHMAN, et al. Hyperthermia and protein homeostasis: Cytoprotection and cell death. J Therm Biol. 2020;91:102615. [19] 袁勋,丁振罡,付力伟,等.甲基丙烯酰化透明质酸/脱细胞华通胶水凝胶支架的制备与表征[J]. 中国组织工程研究,2024,28(22): 3517-3523. [20] 赵红霞,孙政伟,韩阳,等.富血小板纤维蛋白复合甲基丙烯酰化明胶水凝胶的促成骨性能[J]. 中国组织工程研究,2025,29(4): 809-817. [21] ZHOU B, JIANG X, ZHOU X, et al. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: therapeutic strategies and recent advances. Biomater Res. 2023;27(1):86. [22] JONES JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9(1):4457-4486. [23] MIGUEZ-PACHECO V, HENCH LL, BOCCACCINI AR. Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater. 2015;13:1-15. [24] 丁晶鑫,王会,崔旭,等.硼硅酸盐生物活性玻璃的研究现状与发展趋势[J].硅酸盐学报,2022,50(4):1074-1084. [25] ŁUCZAK JW, PALUSIŃSKA M, MATAK D, et al. The Future of Bone Repair: Emerging Technologies and Biomaterials in Bone Regeneration. Int J Mol Sci. 2024;25(23):12766. [26] FAUSKE L, BONDEVIK H, BRULAND ØS, et al. Negative and Positive Consequences of Cancer Treatment Experienced by Long-term Osteosarcoma Survivors: A Qualitative Study. Anticancer Res. 2015; 35(11):6081-6090. [27] ZHANG X, WEI H, DONG C, et al. 3D printed hydrogel/bioceramics core/shell scaffold with NIR-II triggered drug release for chemo-photothermal therapy of bone tumors and enhanced bone repair. Chem Eng J. 2023;461:141855. [28] MA Y, ZHANG B, SUN H, et al. The Dual Effect of 3D-Printed Biological Scaffolds Composed of Diverse Biomaterials in the Treatment of Bone Tumors. Int J Nanomedicine. 2023;18:293-305. [29] VILAS-BOAS V, CARVALHO F, ESPIÑA B. Magnetic Hyperthermia for Cancer Treatment: Main Parameters Affecting the Outcome of In Vitro and In Vivo Studies. Molecules. 2020;25(12):2874. [30] HAZARIKA KP, BORAH JP. Study of biopolymer encapsulated Eu doped Fe3O4 nanoparticles for magnetic hyperthermia application. Sci Rep. 2024;14(1):9768. [31] CRISTOFOLINI L, SZCZEPANOWICZ K, ORSI D, et al. Hybrid Polyelectrolyte/Fe3O4 Nanocapsules for Hyperthermia Applications. ACS Appl Mater Interfaces. 2016;8(38):25043-25050. [32] Vigata M, Meinert C, Hutmacher DW, et al. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics. 2020;12(12):1188. [33] NARAYANASWAMY R, TORCHILIN VP. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules. 2019;24(3):603. [34] ZHU M, WANG Y, FERRACCI G, et al. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci Rep. 2019;9(1):6863. [35] KONG Y, ZHANG X, MA X, et al. Silicon-substituted calcium phosphate promotes osteogenic-angiogenic coupling by activating the TLR4/PI3K/AKT signaling axis. J Biomater Appl. 2022;37(3):459-473. [36] WANG C, LIN K, CHANG J, et al. Osteogenesis and angiogenesis induced by porous β-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34(1):64-77. |
| [1] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [2] | Guo Yuchao, Ni Qianwei, Yin Chen, Jigeer·Saiyilihan, Gao Zhan . Quaternized chitosan hemostatic materials: synthesis, mechanism, and application [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2091-2100. |
| [3] | Sun Lei, Zhang Qi, Zhang Yu. Pro-osteoblastic effect of chlorogenic acid protein microsphere/polycaprolactone electrospinning membrane [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1877-1884. |
| [4] | Xu Wenhe, Li Xiaobing, Liu Fang. Functionalized biomimetic mineralized collagen modified orthopedic implants [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 516-527. |
| [5] | Liu Xiaohong, Zhao Tian, Mu Yunping, Feng Wenjin, Lyu Cunsheng, Zhang Zhiyong, Zhao Zijian, Li Fanghong. Acellular dermal matrix hydrogel promotes skin wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 395-403. |
| [6] | Wang Hao, He Qin, Wang Pingxi, Zhang Jun, Wu Zhilin. Deferoxamine-loaded strontium alginate hydrogel promotes the repair of skull injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3609-3617. |
| [7] | Kong Xiaojuan, Tan Zhenyu, Lei Lei. Repair of rat endometrial injury by using miR-424-5p modified exosome/Poloxam 407 hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3626-3635. |
| [8] | Yang Biao, , Wu Zhonghuan, , Jiang Fugui, , He Chenglong, , Li Tingdong, . Conductive hydrogel with cell-free fat extract repairs spinal cord injuries in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3652-3662. |
| [9] | Gong Yukang, Ye Gaoqi, Wang Chenhao, Chen Dejin, Gao Wenshan. Effects and mechanisms of natural polyphenol-based hydrogels in promoting bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3675-3686. |
| [10] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [11] | Cheng Xinqi, Shao Longhui, Shen Huaqiao, Liu Hongwei. Osteogenic and antibacterial effects of titanium alloy modified with copper-strontium binary doped calcium silicate coating [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4639-4646. |
| [12] | Dumanbieke·Amantai, He Huiyu, Han Xiangzhen. Hydroxyapatite-graphene oxide composite coating promotes bone defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2030-2037. |
| [13] | Zhang Shuang, Xu Xiaomei, Zeng Yang, Yuan Xiaoping, Lin Fuwei. Rev-erbα’s effect on osteoblastogenesis of mouse bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(31): 4921-4926. |
| [14] | Chen Jia, Yang Yiqiang, Hu Chen, Chen Qi, Zhao Tian, Yong Min, Ma Dongyang, Ren Liling. Fabrication of prevascularized osteogenic differentiated cell sheet based on human bone marrow mesenchymal stem cells and human umbilical vein endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(31): 4934-4940. |
| [15] | Cui Xintao, Zhang Zhenyu. Physical factors and mechanisms affecting osteogenic ability of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(31): 5064-5070. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||