Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (13): 3350-3358.doi: 10.12307/2026.307
Previous Articles Next Articles
Osteogenic-adipogenic differentiation imbalance of bone marrow mesenchymal stem cells and osteonecrosis of the femoral head: from molecular mechanisms to therapeutic strategies
Zhang Shilei1, 2, Qin Chuanhong1, 2, Wang Jianxu1, 2, Sun Shui1, 2
- 1Department of Joint Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, Shandong Province, China; 2Orthopedic Research Laboratory, Medical Science and Technology Innovation Center, Shandong First Medical University (Shandong Academy of Medical Sciences), Jinan 250117, Shandong Province, China
-
Accepted:2025-08-09Online:2026-05-08Published:2025-12-26 -
Contact:Sun Shui, MD, PhD, Professor, Department of Joint Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, Shandong Province, China; Orthopedic Research Laboratory, Medical Science and Technology Innovation Center, Shandong First Medical University (Shandong Academy of Medical Sciences), Jinan 250117, Shandong Province, China -
About author:Zhang Shilei, MS, Department of Joint Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, Shandong Province, China; Orthopedic Research Laboratory, Medical Science and Technology Innovation Center, Shandong First Medical University (Shandong Academy of Medical Sciences), Jinan 250117, Shandong Province, China
CLC Number:
Cite this article
Zhang Shilei, Qin Chuanhong, Wang Jianxu, Sun Shui. Osteogenic-adipogenic differentiation imbalance of bone marrow mesenchymal stem cells and osteonecrosis of the femoral head: from molecular mechanisms to therapeutic strategies[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(13): 3350-3358.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
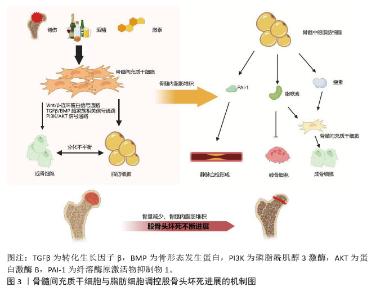
2.2 骨髓间充质干细胞在股骨头坏死发病过程中成骨与成脂分化平衡的转变 2.2.1 骨髓间充质干细胞成骨和成脂分化失衡导致股骨头坏死 骨髓脂肪细胞和成骨细胞主要来源于骨髓间充质干细胞,成脂分化增加的同时会抑制成骨分化[17]。骨髓间充质干细胞成骨分化能力的降低和成脂分化能力的增强与股骨头坏死的疾病进程息息相关[10]。骨髓间充质干细胞中的脂肪细胞分化增强会增加脂肪堆积,导致股骨头骨内压力升高,最终阻碍血液循环,从而加速股骨头坏死进展[5,20]。多种信号通路和脂肪因子参与了骨髓间充质干细胞成骨-成脂分化之间的平衡,从而导致成脂标志物[脂联素和过氧化物酶体增殖物激活受体γ(peroxisome proliferators-activated receptor-gamma,PPARγ)]和成骨细胞标志物[碱性磷酸酶、Runt相关转录因子2(Runt-relatedtranscription factor2,Runx2)和骨钙素]的表达差异[10]。骨髓间充质干细胞、脂肪细胞参与股骨头坏死的简要机制见图3。"

2.2.2 调节骨髓间充质干细胞成脂和成骨分化平衡的关键因子 (1)Runx2:Runx2是调控成骨分化早期与晚期的关键转录因子,促进间充质祖细胞向成骨谱系分化[33]。Runx2基因敲除(Runx2?/?)小鼠实验提供了重要证据:纯合突变小鼠出生后即死亡,且骨骼呈现完全未矿化状态[34-35]。值得注意的是,激素性股骨头坏死患者骨组织中Runx2表达显著下调,涉及Wnt/β-连环蛋白信号、BMP超家族、PI3K/AKT通路等多种相关信号通路的调控[15,36-38]。研究显示,糖皮质激素通过诱导Runx2/Cbfal丝氨酸位点磷酸化,抑制骨髓间充质干细胞的成骨分化[39]。在骨髓间充质干细胞中激活Runx2可显著加速成骨分化并抑制成脂分化,且外源性Runx2可拮抗地塞米松对脂肪分化的促进作用,提示精准调控Runx2或其下游效应分子表达可能缓解长期糖皮质激素治疗的严重不良反应[33]。此外,过表达P-糖蛋白可通过增加Runx2与碱性磷酸酶的表达,促进由地塞米松诱导而抑制的骨髓间充质干细胞成骨分化。已有研究证实,PPARγ可通过抑制Runx2表达抑制成骨分化[12]。 (2)PPARγ和C/EBPs:PPARγ作为成脂分化关键转录因子,属于核激素受体亚家族,其活性受配体调控。研究发现,PPARγ表达上调与股骨头坏死的发生密切相关,而抑制骨髓间充质干细胞中PPARγ的表达可阻断激素诱导的成脂分化,这为股骨头坏死的防治提供了新思路[37]。体内外实验证实,糖皮质激素可激活PPARγ基因表达,乙醇亦可上调骨髓间充质干细胞中PPARγ mRNA水平[16,39]。以3T3-L1和3T3-F442A细胞模型为例,在脂肪分化过程中,PPARγ mRNA的激活常早于其他脂肪细胞基因[40]。研究发现,富血小板血浆能显著降低PPARγ表达水平,并下调血清三酰甘油和总胆固醇水平[1]。此外,C/EBPs转录因子家族(C/EBPα、β、δ)在脂肪生成中发挥重要作用,其通过调控脂肪细胞基因表达及葡萄糖摄取参与代谢调节。其中C/EBPβ与C/EBPδ在脂肪分化早期启动脂生成信号后迅速下降,而C/EBPα则持续存在。特别值得注意的是,C/EBPα能直接增强PPARγ启动子区的转录活性,C/EBPα与PPARγ的协同作用已在多项研究中被证实是成脂分化的核心调控机制。 (3)MicroRNAs(miRNAs):miRNAs 是一种小的单链非编码RNA分子,通常包含大约22个核苷酸。这些分子在骨重建过程、股骨头坏死的发病机制和治疗中有着重要作用[41-42]。以下是研究最多的与股骨头坏死相关的miRNAs,见表2。"

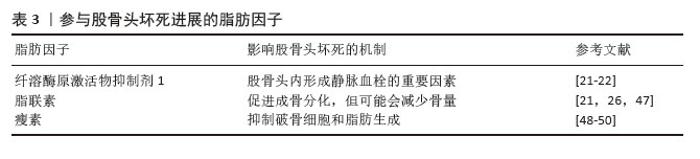
创伤性股骨头坏死患者外周血中高表达miR-93-5p,其可能通过骨形态发生蛋白2信号通路阻碍成骨细胞的分化,导致细胞活性显著降低及钙结节形成减少。miR-93-5p已被证实能在体外促进人骨髓间充质干细胞的增殖[43]。此外,miR-320a表达也出现明显上调,但Runx2表达明显下调;miR-320a过表达抑制了成骨相关蛋白的表达,包括Runx2、Ⅰ型胶原和骨钙素,也阻碍了骨髓间充质干细胞的成骨分化能力[42]。 miR-224-5p在糖皮质激素处理的骨髓间充质干细胞中上调,可以抑制成骨分化,同时促进成脂分化。Smad4是细胞内转化生长因子β信号通路的核心信号转导分子,是miR-224-5p的靶基因。此外,Smad4和TAZ共同参与调节细胞命运和维持干细胞自我更新。miR-224-5p可能通过Smad4靶向介导骨髓间充质干细胞的成脂-成骨分化。因此,miR-224-5p-Smad4-TAZ轴是一种有效的信号传导机制,抑制miR-224-5p表达可能促进成骨和减少脂肪生成,从而延缓股骨头坏死的发病进展。这一研究为治疗类固醇导致的股骨头坏死提供了一个新的治疗靶点[44]。 外泌体作为细胞间信号传递的新递质,已被报道与骨关节炎、肩袖损伤和骨质疏松症等许多骨和关节疾病密切相关。股骨头坏死患者外泌体中miR-100-5p表达上调,通过靶向骨形态发生蛋白受体2并使BMPR2/Smad1/5/9信号通路失活来抑制人骨髓间充质干细胞成骨分化[45]。 miR-27a通过调节骨髓间充质干细胞的成骨分化,在激素性股骨头坏死的骨保护机制中发挥重要作用。研究发现,miR-27a可增加碱性磷酸酶活性和成骨相关标志物Runx2的表达,同时下调脂肪生成标志物PPARγ的表达[36]。 2.2.3 脂肪细胞释放多种脂肪因子参与股骨头坏死过程 研究表明,内脏脂肪细胞分泌各种生理活性物质,称为脂肪因子。骨髓间隙富含成熟脂肪细胞,它们是骨髓中脂肪因子分泌的主要来源[46],各种脂肪因子的具体作用机制见表3。"
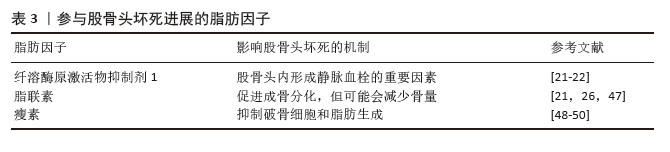
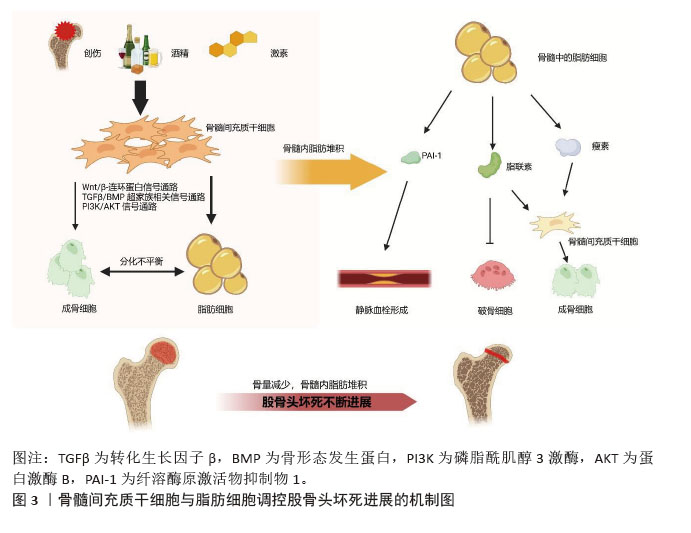
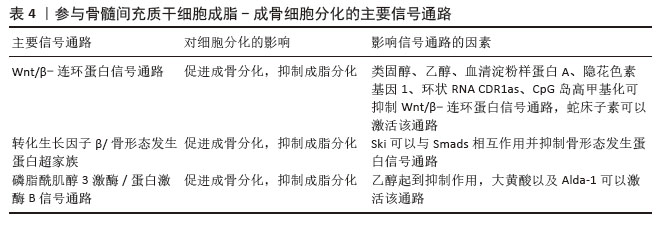
骨髓脂肪组织是骨髓微环境的一个组成部分[46]。骨髓脂肪组织作为一个重要的内分泌器官,释放出大量的激素和脂肪因子,并具有不同的作用。而且,骨髓脂肪组织分泌的脂联素显著高于白色脂肪组织。由于脂联素的产生,骨髓脂肪组织可能会影响骨稳态、免疫反应、血管活动等全身效应[18,22]。 纤溶酶原激活物抑制剂1属于脂肪因子之一,其通过结合组织型纤溶酶原激活物抑制纤维蛋白溶解,这种相互作用表明纤溶酶原激活物抑制剂1与血栓形成或高凝状态之间存在关联[22]。骨髓脂肪细胞中纤溶酶原激活物抑制剂1的显著表达可能通过细胞间的旁分泌作用诱导静脉血栓形成。地塞米松诱导骨髓脂肪细胞产生纤溶酶原激活物抑制剂1也可能是骨坏死的发病机制之一[21]。 脂联素以其抗动脉粥样硬化和抗炎特性而闻名,在脂质代谢调节中起重要作用。研究表明它可以抑制破骨细胞并激活成骨分化[47]。然而,脂联素也会对骨量产生负面影响,增加骨折的风险[21]。在一项临床试验中,与健康对照者以及创伤性股骨头坏死或髋关节炎患者相比,非创伤性股骨头坏死患者血浆脂联素水平明显降低[26]。 瘦素可以调节成骨细胞的合成代谢,能够促进间充质干细胞向成骨细胞分化,同时限制脂肪的形成。瘦素抑制核因子κB配体受体激活因子并促进骨保护素表达,从而限制破骨细胞的生成[48]。瘦素表达增加可抑制脂肪细胞的合成代谢,从而使成骨细胞增多。正常情况下,瘦素可激活JAK3/STAT3通路,抑制脂肪生成[49]。最近一项研究显示,瘦素还可以产生神经生长因子促进骨髓腔内受损的神经纤维再生[50]。 2.3 骨髓间充质干细胞成脂-成骨细胞分化的主要信号通路 2.3.1 Wnt/β-连环蛋白信号通路 Wnt信号通路(包括Wnt1、Wnt3a、Wnt5b、Wnt7a、Wnt10b等家族成员)通过与膜结合卷曲受体及LRP5/6共受体相互作用,启动级联信号传导并促使β-连环蛋白在细胞核内聚集,这种结合可阻止β-连环蛋白在胞浆中降解,并促进其核转位,β-连环蛋白与T细胞因子/淋巴增强因子转录因子家族结合并协同激活靶基因转录[51-52]。研究表明Wnt/β-连环蛋白信号通路可抑制成脂分化[53],同时促进成骨分化[34]。 类固醇可以通过抑制β-连环蛋白活性及调控成骨细胞中Wnt信号相关分子表达,干扰Wnt信号通路正常功能,从而减少骨形成。普伐他汀可能通过抑制PPARγ表达并激活Wnt信号通路,发挥预防激素性股骨头坏死的作用,这一发现凸显了普伐他汀在病程中对成脂与成骨过程的双向调控效应[37]。Wnt/β-连环蛋白通路在骨髓间充质干细胞成骨分化中起关键作用。YU等[54]最新研究发现乙醇可显著降低β-连环蛋白水平,而蛇床子素能以剂量依赖性方式恢复β-连环蛋白表达并增强成骨能力。最近一项研究显示,在激素性股骨头坏死大鼠模型中,β-连环蛋白信号通路受到显著抑制,而通过给予Wnt激动剂1激活该通路后,可减轻激素性股骨头坏死模型大鼠股骨头内脂滴堆积和骨小梁稀疏现象[55]。此外,有研究表明,甘草酸通过激活Wnt/β-连环蛋白通路发挥作用,可降低糖皮质激素过量引发的氧化应激水平,从而增强间充质干细胞的成骨分化、抑制其成脂分化,最终维持成骨-成脂稳态[8]。 值得注意的是,股骨头坏死的危险因素与血清淀粉样蛋白A升高的诱因存在高度相似性。乙醇、激素或创伤诱导的血清淀粉样蛋白A表达增加,其分子机制均显示血清淀粉样蛋白A可通过抑制Wnt/β-连环蛋白信号并激活MAPK信号通路下游PPARγ来影响骨代谢[3]。CRY1基因沉默时,在成脂刺激条件下总β-连环蛋白及核内β-连环蛋白水平升高,同时GSK-3β表达降低,结果表明CRY1下调可能激活经典Wnt/β-连环蛋白信号通路,因此CRY1可作为新型成脂分化调控因子抑制体外实验中的成脂分化过程[56]。 此外,环状RNA CDR1as通过miR-7-5p/Wnt5b通路调控骨髓间充质干细胞成骨与成脂分化。激素性股骨头坏死患者骨髓间充质干细胞中CDR1as表达升高,通过竞争性结合miR-7-5p促进Wnt5b表达。研究显示,升高的Wnt5b可抑制Wnt/β-连环蛋白信号通路关键分子β-连环蛋白,且二者表达呈显著负相关[57]。作为Wnt信号受体,卷曲蛋白1(Frizzled1,FZD1)同样参与了成骨细胞矿化过程,其启动子受Sp1、E2F1、AP2等多种转录因子调控。研究发现,股骨头坏死患者FZD1基因存在异常CpG岛高甲基化,导致Wnt/β-连环蛋白信号失活及后续细胞功能障碍。研究证实,适当浓度的DNA甲基转移酶抑制剂可通过诱导FZD1基因重新表达,改善激素性股骨头坏死患者的间充质干细胞功能[58]。 2.3.2 转化生长因子β/骨形态发生蛋白超家族相关信号通路 骨形态发生蛋白在骨折修复中起着不可或缺的作用。骨形态发生蛋白属于酸性糖蛋白家族,主要由成骨细胞合成和分泌,广泛存在于骨基质中。目前骨形态发生蛋白家族成员超过20个,是一组能够刺激骨间充质祖细胞向成熟成骨细胞分化的强大成骨因子。骨形态发生蛋白2是最关键的细胞外信号分子,不仅促进骨形成、诱导骨细胞分化,还能调控Runx2等多种成骨转录因子的基因表达[38]。骨形态发生蛋白受体2可直接磷酸化并激活BMPR1,BMPR1磷酸化SMAD1/5/9,促进SMAD1/5/9和SMAD4的内聚和核易位,从而促进成骨和血管生成,同时抑制脂肪生成[45]。Smads蛋白在转化生长因子β/骨形态发生蛋白信号通路中具有核心地位。研究表明Ski(一种多功能转录调节因子)可以与Smads相互作用并抑制骨形态发生蛋白信号传导;同时,Ski还通过与Smad蛋白的相互作用,作为转化生长因子β信号传导的负调控因子。因此,任何导致Ski激增的因素都可以负向调节转化生长因子β/骨形态发生蛋白2信号通路。此外,Ski基因敲降可减少骨髓间充质干细胞的成脂分化,这表明Ski作为一种新分子,可能在激素性股骨头坏死条件下调节脂肪形成过程[59]。SIRT2是一种典型的NAD+依赖性去乙酰化酶,可以作为骨形态发生蛋白2的上游抑制因子。下调SIRT2可显著提高骨形态发生蛋白2的表达水平,从而促进骨髓间充质干细胞成骨分化,减轻糖皮质激素诱导的氧化应激和细胞凋亡[60]。 2.3.3 PI3K/AKT信号通路 大量证据表明,PI3K/AKT通路参与了骨髓间充质干细胞成骨分化过程。该通路与乙醇抑制细胞成骨分化导致骨流失有关。体外研究表明,乙醇显著抑制骨髓间充质干细胞的增殖和成骨分化,同时刺激成脂分化;而大黄酸可以通过PI3K/AKT通路部分抵消对成骨的负面影响[15]。最近的研究证实,单酰基甘油脂肪酶参与骨髓间充质干细胞分化的调控,抑制单酰基甘油脂肪酶可通过激活PI3K/AKT/GSK3β信号通路,有效逆转糖皮质激素对骨髓间充质干细胞分化的影响[61]。此外,醛脱氢酶1的高选择性激动剂(Alda-1)可以通过PI3K/AKT信号通路减轻乙醇对骨髓间充质干细胞成骨的抑制作用。因此,PI3K/AKT信号也是骨髓间充质干细胞成骨分化的途径之一[62]。 信号通路对间充质干细胞分化的影响见表4。"
| [1] XU HH, LI SM, FANG L, et al. Platelet-rich plasma promotes bone formation, restrains adipogenesis and accelerates vascularization to relieve steroids-induced osteonecrosis of the femoral head. Platelets. 2021;32: 950-959. [2] ZHANG W, ZHENG C, YU T, et al. The therapeutic effect of adipose-derived lipoaspirate cells in femoral head necrosis by improving angiogenesis. Front Cell Dev Biol. 2022;10:1014789. [3] PENG X, MA Y, WANG Q, et al. Serum Amyloid A Correlates With the Osteonecrosis of Femoral Head by Affecting Bone Metabolism. Front Pharmacol. 2021; 12:767243. [4] WANG Y, LI Y, MAO K, et al. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003;(410):213-224. [5] MIYANISHI K, YAMAMOTO T, IRISA T, et al. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30(1):185-190. [6] DUAN P, WANG H, YI X, et al. C/EBPα regulates the fate of bone marrow mesenchymal stem cells and steroid-induced avascular necrosis of the femoral head by targeting the PPARγ signalling pathway. Stem Cell Res Ther. 2022;13(1):342. [7] SUH KT, KIM SW, ROH HL, et al. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2005; (431):220-225. [8] XU H, FANG L, ZENG Q, et al. Glycyrrhizic acid alters the hyperoxidative stress-induced differentiation commitment of MSCs by activating the Wnt/β-catenin pathway to prevent SONFH. Food Funct. 2023;14(2):946-960. [9] WANG T, TENG S, ZHANG Y, et al. Role of mesenchymal stem cells on differentiation in steroid-induced avascular necrosis of the femoral head. Exp Ther Med. 2017; 13(2):669-675. [10] DUAN DY, TANG J, TIAN HT, et al. Adipocyte-secreted microvesicle-derived miR-148a regulates adipogenic and osteogenic differentiation by targeting Wnt5a/Ror2 pathway. Life Sci. 2021;278:119548. [11] VANDE BERG BC, GILON R, MALGHEM J, et al. Correlation between baseline femoral neck marrow status and the development of femoral head osteonecrosis in corticosteroid-treated patients: a longitudinal study by MR imaging. Eur J Radiol. 2006;58(3):444-449. [12] HAN N, LI Z, CAI Z, et al. P-glycoprotein overexpression in bone marrow-derived multipotent stromal cells decreases the risk of steroid-induced osteonecrosis in the femoral head. J Cell Mol Med. 2016; 20(11):2173-2182. [13] GONG Y, LI Z, ZOU S, et al. Vangl2 limits chaperone-mediated autophagy to balance osteogenic differentiation in mesenchymal stem cells. Dev Cell. 2021;56(14):2103-2120.e9. [14] CHEN Q, SHOU P, ZHENG C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128-1139. [15] YU H, LIU P, ZHU D, et al. Chrysophanic acid shifts the differentiation tendency of BMSCs to prevent alcohol-induced osteonecrosis of the femoral head. Cell Prolif. 2020;53(8):e12871. [16] LI J, LI Y, WANG Y, et al. Preventive effects of siRNA targeting PPARγ gene on steroid-induced osteonecrosis in rabbits. Connect Tissue Res. 2014;55(5-6):322-330. [17] KONG X, LI X, ZHANG C, et al. Ethyl acetate fraction of Huogu formula inhibits adipogenic differentiation of bone marrow stromal cells via the BMP and Wnt signaling pathways. Int J Biol Sci. 2017; 13(4):480-491. [18] CAWTHORN WP, SCHELLER EL, LEARMAN BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2): 368-375. [19] LABUSCA L. Adipose tissue in bone regeneration - stem cell source and beyond. World J Stem Cells. 2022;14(6): 372-392. [20] HINES JT, JO WL, CUI Q, et al. Osteonecrosis of the Femoral Head: an Updated Review of ARCO on Pathogenesis, Staging and Treatment. J Korean Med Sci. 2021;36(24): e177. [21] HOZUMI A, OSAKI M, SAKAMOTO K, et al. Dexamethasone-induced plasminogen activator inhibitor-1 expression in human primary bone marrow adipocytes. Biomed Res. 2010;31(5):281-286. [22] FUKUSHIMA T, HOZUMI A, TOMITA M, et al. Steroid changes adipokine concentration in the blood and bone marrow fluid. Biomed Res. 2016;37(3): 215-220. [23] YU W, ZHONG L, YAO L, et al. Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J Clin Invest. 2021;131(2):e140214. [24] SAKAMOTO K, OSAKI M, HOZUMI A, et al. Simvastatin suppresses dexamethasone-induced secretion of plasminogen activator inhibitor-1 in human bone marrow adipocytes. BMC Musculoskelet Disord. 2011;12(1):82. [25] WANG H, YUAN T, WANG Y, et al. Osteoclasts and osteoarthritis: Novel intervention targets and therapeutic potentials during aging. Aging Cell. 2024; 23(4):e14092. [26] SHUAI B, SHEN L, YANG YP, et al. Low plasma adiponectin as a potential biomarker for osteonecrosis of the femoral head. J Rheumatol. 2010;37(10): 2151-2155. [27] LIU Y, WU J, ZHU Y, et al. Therapeutic application of mesenchymal stem cells in bone and joint diseases. Clin Exp Med. 2014;14(1):13-24. [28] HERNIGOU P. Bone transplantation and tissue engineering, part IV. Mesenchymal stem cells: history in orthopedic surgery from Cohnheim and Goujon to the Nobel Prize of Yamanaka. Int Orthop. 2015; 39(4):807-817. [29] EDER C, SCHMIDT-BLEEK K, GEISSLER S, et al. Mesenchymal stromal cell and bone marrow concentrate therapies for musculoskeletal indications: a concise review of current literature. Mol Biol Rep. 2020;47(6):4789-4814. [30] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [31] QIU Y, LUO Y, GUO G, et al. BMSCs-derived exosomes carrying miR-668-3p promote progression of osteoblasts in osteonecrosis of the femoral head: Expression of proteins CD63 and CD9. Int J Biol Macromol. 2024; 280(Pt 4):136177. [32] SUN T, MAN Z, PENG C, et al. A specific affinity cyclic peptide enhances the adhesion, expansion and proliferation of rat bone mesenchymal stem cells on β‑tricalcium phosphate scaffolds. Mol Med Rep. 2019;20(2):1157-1166. [33] LIN L, DAI SD, FAN GY. Glucocorticoid-induced differentiation of primary cultured bone marrow mesenchymal cells into adipocytes is antagonized by exogenous Runx2. APMIS. 2010;118(8):595-605. [34] HAN L, WANG B, WANG R, et al. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res Ther. 2019;10(1):377. [35] KOMORI T, YAGI H, NOMURA S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755-764. [36] CUI Y, HUANG T, ZHANG Z, et al. The potential effect of BMSCs with miR-27a in improving steroid-induced osteonecrosis of the femoral head. Sci Rep. 2022;12(1):21051. [37] JIANG Y, ZHANG Y, ZHANG H, et al. Pravastatin prevents steroid-induced osteonecrosis in rats by suppressing PPARγ expression and activating Wnt signaling pathway. Exp Biol Med (Maywood). 2014; 239(3):347-355. [38] CHEN G, DENG C, LI YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2): 272-288. [39] LI J, WANG Y, LI Y, et al. The effect of combined regulation of the expression of peroxisome proliferator-activated receptor-γ and calcitonin gene-related peptide on alcohol-induced adipogenic differentiation of bone marrow mesenchymal stem cells. Mol Cell Biochem. 2014;392(1-2):39-48. [40] WANG Y, YIN L, LI Y, et al. Preventive effects of puerarin on alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2008; 466(5):1059-1067. [41] NAN K, ZHANG Y, ZHANG X, et al. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu). Stem Cell Res Ther. 2021;12(1):331. [42] ZHANG Y, ZHANG N, WEI Q, et al. MiRNA-320a-5p contributes to the homeostasis of osteogenesis and adipogenesis in bone marrow mesenchymal stem cell. Regen Ther. 2022;20:32-40. [43] ZHANG Y, WEI QS, DING WB, et al. Increased microRNA-93-5p inhibits osteogenic differentiation by targeting bone morphogenetic protein-2. PLoS One. 2017;12(8):e0182678. [44] CAO Y, JIANG C, WANG X, et al. Reciprocal effect of microRNA-224 on osteogenesis and adipogenesis in steroid-induced osteonecrosis of the femoral head. Bone. 2021;145:115844. [45] YANG W, ZHU W, YANG Y, et al. Exosomal miR-100-5p inhibits osteogenesis of hBMSCs and angiogenesis of HUVECs by suppressing the BMPR2/Smad1/5/9 signalling pathway. Stem Cell Res Ther. 2021;12(1):390. [46] LI Y, MENG Y, YU X. The Unique Metabolic Characteristics of Bone Marrow Adipose Tissue. Front Endocrinol (Lausanne). 2019;10:69. [47] XIE Y, MA Q, WANG J, et al. Effects of Modified Messenger RNA of Adiponectin Delivered by Lipid Nanoparticles on Adipogenesis and Bone Metabolism In Vitro and In Vivo. Cells. 2025;14(12):891. [48] KARSENTY G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4(5): 341-348. [49] NOH M. Interleukin-17A increases leptin production in human bone marrow mesenchymal stem cells. Biochem Pharmacol. 2012;83(5):661-670. [50] GAO X, MURPHY MM, PEYER JG, et al. Leptin receptor+ cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor. Nat Cell Biol. 2023;25(12):1746-1757. [51] ZHU S, CHEN W, MASSON A, et al. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024;10(1):71. [52] NELSON WJ, NUSSE R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483-1487. [53] LI Y, JIN D, XIE W, et al. PPAR-γ and Wnt Regulate the Differentiation of MSCs into Adipocytes and Osteoblasts Respectively. Curr Stem Cell Res Ther. 2018;13(3):185-192. [54] YU H, ZHU D, LIU P, et al. Osthole stimulates bone formation, drives vascularization and retards adipogenesis to alleviate alcohol-induced osteonecrosis of the femoral head. J Cell Mol Med. 2020;24(8):4439-4451. [55] XIA C, XU H, FANG L, et al. β-catenin inhibition disrupts the homeostasis of osteogenic/adipogenic differentiation leading to the development of glucocorticoid-induced osteonecrosis of the femoral head. Elife. 2024;12:RP92469. [56] SUN S, ZHOU L, YU Y, et al. Knocking down clock control gene CRY1 decreases adipogenesis via canonical Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun. 2018;506(3):746-753. [57] CHEN G, WANG Q, LI Z, et al. Circular RNA CDR1as promotes adipogenic and suppresses osteogenic differentiation of BMSCs in steroid-induced osteonecrosis of the femoral head. Bone. 2020;133:115258. [58] SMITH AJ, DIEPPE P, HOWARD PW, et al. Failure rates of metal-on-metal hip resurfacings: analysis of data from the National Joint Registry for England and Wales. Lancet. 2012;380(9855):1759-1766. [59] ZHAO X, WEI Z, LI D, et al. Glucocorticoid Enhanced the Expression of Ski in Osteonecrosis of Femoral Head: The Effect on Adipogenesis of Rabbit BMSCs. Calcif Tissue Int. 2019;105(5):506-517. [60] FANG S, HE T, YOU M, et al. Glucocorticoids promote steroid-induced osteonecrosis of the femoral head by down-regulating serum alpha-2-macroglobulin to induce oxidative stress and facilitate SIRT2-mediated BMP2 deacetylation. Free Radic Biol Med. 2024;213:208-221. [61] YANG N, LI M, LI X, et al. MAGL blockade alleviates steroid-induced femoral head osteonecrosis by reprogramming BMSC fate in rat. Cell Mol Life Sci. 2024;81(1):418. [62] LIN X, ZHU D, WANG K, et al. Activation of aldehyde dehydrogenase 2 protects ethanol-induced osteonecrosis of the femoral head in rat model. Cell Prolif. 2022; 55(6):e13252. [63] KAROICHAN A, BAUDEQUIN T, AL-JALLAD H, et al. Encapsulation and differentiation of adipose-derived mesenchymal stem cells in a biomimetic purine cross-linked chitosan sponge. J Biomed Mater Res A. 2022;110(3):585-594. [64] YE Y, LIU Q, LI C, et al. miR-125a-5p Regulates Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells under Oxidative Stress. Biomed Res Int. 2021;2021:6684709. [65] YOON PW, KANG JY, KIM CH, et al. Culture-Expanded Autologous Adipose-Derived Mesenchymal Stem Cell Treatment for Osteonecrosis of the Femoral Head. Clin Orthop Surg. 2021;13(1):37-46. [66] YAMAGAMI R, TERAO T, KASAI T, et al. Baseline magnetic resonance imaging findings associated with short-term clinical outcomes after intraarticular administration of autologous adipose-derived stem cells for knee osteoarthritis. Regen Ther. 2024;28: 227-234. [67] WU T, SHU T, KANG L, et al. Icaritin, a novel plant-derived osteoinductive agent, enhances the osteogenic differentiation of human bone marrow- and human adipose tissue-derived mesenchymal stem cells. Int J Mol Med. 2017;39(4): 984-992. [68] XIE Q, WANG Z, ZHOU H, et al. The role of miR-135-modified adipose-derived mesenchymal stem cells in bone regeneration. Biomaterials. 2016;75: 279-294. [69] CRUZ AC, CAON T, MENIN Á, et al. Adipose-derived stem cells incorporated into platelet-rich plasma improved bone regeneration and maturation in vivo. Dent Traumatol. 2015;31(1):42-48. [70] LAU CS, PARK SY, ETHIRAJ LP, et al. Role of Adipose-Derived Mesenchymal Stem Cells in Bone Regeneration. Int J Mol Sci. 2024;25(12):6805. [71] DA SILVA D, CROUS A, ABRAHAMSE H. Synergistic Effects of Photobiomodulation and Differentiation Inducers on Osteogenic Differentiation of Adipose-Derived Stem Cells in Three-Dimensional Culture. Int J Mol Sci. 2024;25(24):13350. [72] LI R, CHEN C, ZHENG RQ, et al. Influences of hucMSC-exosomes on VEGF and BMP-2 expression in SNFH rats. Eur Rev Med Pharmacol Sci. 2019;23(7):2935-2943. [73] WANG C, STÖCKL S, LI S, et al. Effects of Extracellular Vesicles from Osteogenic Differentiated Human BMSCs on Osteogenic and Adipogenic Differentiation Capacity of Naïve Human BMSCs. Cells. 2022;11(16):2491. [74] CHEN XJ, SHEN YS, HE MC, et al. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2019;112:108746. [75] XU H, WANG L, ZHU X, et al. Jintiange capsule ameliorates glucocorticoid-induced osteonecrosis of the femoral head in rats by regulating the activity and differentiation of BMSCs. J Tradit Complement Med. 2024; 14(5):568-580. [76] CHEN C, WANG B, ZHAO X, et al. Lithium Promotes Osteogenesis via Rab11a-Facilitated Exosomal Wnt10a Secretion and β-Catenin Signaling Activation. ACS Appl Mater Interfaces. 2024;16(24):30793-30809. [77] XU H, WANG C, LIU C, et al. Cotransplantation of mesenchymal stem cells and endothelial progenitor cells for treating steroid-induced osteonecrosis of the femoral head. Stem Cells Transl Med. 2021;10(5):781-796. [78] WU J, CAO L, LIU Y, et al. Functionalization of Silk Fibroin Electrospun Scaffolds via BMSC Affinity Peptide Grafting through Oxidative Self-Polymerization of Dopamine for Bone Regeneration. ACS Appl Mater Interfaces. 2019;11(9):8878-8895. [79] FU Z, LAI Y, ZHUANG Y, et al. Injectable heat-sensitive nanocomposite hydrogel for regulating gene expression in the treatment of alcohol-induced osteonecrosis of the femoral head. APL Bioeng. 2023;7(1):016107. [80] ZHU ZH, SONG WQ, ZHANG CQ, et al. Dimethyloxaloylglycine increases bone repair capacity of adipose-derived stem cells in the treatment of osteonecrosis of the femoral head. Exp Ther Med. 2016; 12(5):2843-2850. [81] WANG Y, MA X, CHAI W, et al. Multiscale Stem Cell Technologies for Osteonecrosis of the Femoral Head. Stem Cells Int. 2019; 2019:8914569. |
| [1] | Haonan Yang, Zhengwei Yuan, Junpeng Xu, Zhiqi Mao, Jianning Zhang. Preliminary study on the mechanisms and efficacy of deep brain stimulation in treating depression [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-9. |
| [2] | Jiang Xinghai, Song Yulin, Li Dejin, Shao Jianmin, Xu Junzhi, Liu Huakai, Wu Yingguo, Shen Yuehui, Feng Sicheng. Vascular endothelial growth factor 165 genes transfected into bone marrow mesenchymal stem cells to construct a vascularized amphiphilic peptide gel module [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1903-1911. |
| [3] | Chen Haojie, Wang Dai, Shen Shan. Immune inflammatory microenvironment mechanisms in peri-implantitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2054-2062. |
| [4] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [5] | Hu Xiongke, Liu Shaohua, Tan Qian, Liu Kun, Zhu Guanghui. Shikonin intervention with bone marrow mesenchymal stem cells improves microstructure of femur in aged mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1609-1615. |
| [6] | Yuan Xiaoshuang, Yang Xu, Yang Bo, Chen Xiaoxu, Tian Ting, Wang Feiqing, Li Yanju, Liu Yang, Yang Wenxiu. Effect of conditioned medium of diffuse large B-cell lymphoma cells on proliferation and apoptosis of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1632-1640. |
| [7] | Li Zhenyu, Zhang Siming, Bai Jiaxiang, Zhu Chen. Osthole improves osteogenic differentiation function of bone marrow mesenchymal stem cells under high-glucose conditions [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1641-1648. |
| [8] | Han Nianrong, Huang Yifei, Akram · Osman, Liu Yanlu, Hu Wei . Programmed cell death receptor-1 suppresses osteogenic differentiation of rat bone marrow mesenchymal stem cells in a high-glucose microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1649-1657. |
| [9] | Jin Dongsheng, Zhao Zhanghong, Zhu Ziyin, Zhang Sen, Sun Zuyan, Deng Jiang. Effects of icariin-loaded microsphere-three-dimensional scaffold on osteogenic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1658-1668. |
| [10] | Zou Yulian, Chen Chaopei, Huang Haixia, Lan Yuyan, Liu Min, Huang Ting. Resveratrol promotes osteogenic differentiation of bone marrow mesenchymal stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1669-1678. |
| [11] | Liu Anting, Lu Jiangtao, Zhang Wenjie, He Ling, Tang Zongsheng, Chen Xiaoling. Regulation of AMP-activated protein kinase by platelet lysate inhibits cadmium-induced neuronal apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1800-1807. |
| [12] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [13] | Chen Ju, Zheng Jinchang, Liang Zhen, Huang Chengshuo, Lin Hao, Zeng Li. Effect and mechanism of beta-caryophyllene in mice with osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1341-1347. |
| [14] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [15] | Zhu Kuicheng, Du Chunyan, Zhang Jintao. Mechanism by which hairless gene mutation promotes white adipose tissue browning in hairless mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1424-1430. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

