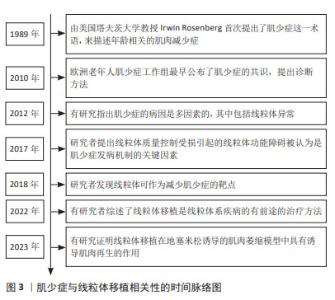
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (13): 2842-2848.doi: 10.12307/2025.053
Previous Articles Next Articles
Potential of mitochondrial transplantation in treatment of sarcopenia
Li Wei1, Yin Hongtao2, Sun Yongchen1, Xu Weijuan1, Sun Jinling1, Jin Xiaodong1
- 1Department of Geriatric Medicine, 2Department of Neurology, Zibo Central Hospital, Zibo 255036, Shandong Province, China
-
Received:2023-10-16Accepted:2024-04-02Online:2025-05-08Published:2024-09-12 -
Contact:Jin Xiaodong, Associate chief physician, Department of Geriatric Medicine, Zibo Central Hospital, Zibo 255036, Shandong Province, China -
About author:Li Wei, Master, Attending physician, Department of Geriatric Medicine, Zibo Central Hospital, Zibo 255036, Shandong Province, China
CLC Number:
Cite this article
Li Wei, Yin Hongtao, Sun Yongchen, Xu Weijuan, Sun Jinling, Jin Xiaodong. Potential of mitochondrial transplantation in treatment of sarcopenia[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2842-2848.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
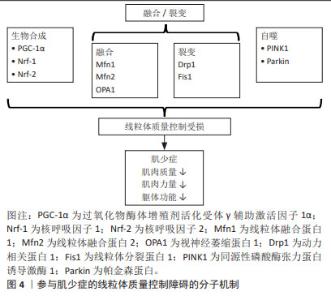
2.2 肌少症与线粒体功能障碍 虽然肌少症的确切机制尚未完全了解,但线粒体功能障碍已被认为是骨骼肌衰老的重要机制[2,6]。越来越多的证据表明,老年啮齿动物和人体骨骼肌中均存在受损和功能失调的线粒体[6]。通过31P磁共振波谱法测量发现老年人体内氧化磷酸化活性较成年对照者相比降低[7]。受损的氧化还原状态也与mtDNA突变的增加和受损线粒体的积累有关[8]。COEN 等[9]研究发现ATP合成/耗氧量比值与步速相关,而步速减慢正是亚洲肌少症工作组提出用于筛查肌少症的指标。另有研究发现了线粒体凋亡信号与老年人行走速度慢和肌肉体积减少相关[10]。这些发现表明骨骼肌线粒体的年龄相关性破坏可能与肌少症的进展有关。然而INCI等[11]在研究细胞衰老生物模型中发现裸鼹鼠寿命长达32年,骨骼肌线粒体的功能和结构维持到生命的晚期,并没有出现与年龄相关的疾病,这说明线粒体和肌少症之间相互作用的分子基础是复杂的。因此,了解线粒体激活的多种信号通路对于干预肌少症至关重要。 2.3 线粒体质量控制 线粒体质量控制涉及线粒体生物合成、线粒体动力学(融合-裂变)和线粒体自噬,并且多种细胞因子和分子信号参与其中,共同维持线粒体的完整性。线粒体质量控制对于调节线粒体的形态、质量和功能至关重要。如果线粒体质量控制失败,可能导致线粒体功能障碍和肌肉退化。 2.3.1 线粒体生物合成 过氧化物酶体增殖剂活化受体γ辅助激活因子1α(peroxisome pro-liferator activated receptor γ coactivator-1α,PGC-1α)是调节线粒体生物合成的主要因子,它与下游核转录辅因子合作,如核呼吸因子1,2(Nrf-1和Nrf-2)和雌激素相关受体α[12]。PGC-1α一旦被激活,Nrf-1和Nrf-2辅因子与靶核基因结合并促进核基因编码的线粒体蛋白和线粒体转录因子A(Tfam)的表达,其可以直接结合靶mtDNA并激活相应区域的复制和转录mtDNA[13]。最近基于啮齿动物模型的研究揭示了以PGC-1α为中心的生物合成相关基因表达与骨骼肌衰老之间的联系。在肌少症的发生和发展中,研究者发现快速老化模型SAMP8小鼠骨骼肌中PGC-1α、Nrf-1和Tfam的表达水平降低[14]。此外,还发现敲除 Nrf-2辅因子观察到肌肉质量和肌肉性能显著下降,表明敲除Nrf-2可能通过损害线粒体生物合成加剧骨骼肌脆性和肌少症[15]。有趣的是,YANG等[16-17]发现PGC-1α过表达可以通过增加线粒体蛋白质含量和抗氧化酶活性以及改变基因表达,使得老年小鼠骨骼肌横截面积和质量增加。基于这些发现,PGC-1α介导的线粒体生物合成可能是治疗肌少症新希望的靶点。 2.3.2 线粒体动力学(融合-裂变) 线粒体动力学的调控依赖融合与裂变间的动态平衡。它在线粒体功能和质量控制中起关键作用,并受到高度复杂的蛋白因子的严格调控。 线粒体融合过程主要受鸟苷三磷酸酶(GTPases)调控,包括线粒体外膜上的线粒体融合蛋白1(mitofusin 1,Mfn1)和Mfn2以及线粒体内膜上的视神经萎缩蛋白1(optic atrophy 1,OPA1)。这些融合机制启动了从受损线粒体到健康线粒体的组分分布,防止受损线粒体的积累[18]。在动物模型中,缺乏Mfn1和Mfn2的小鼠运动能力下降,这与线粒体生物能量受损有关[19],另发现OPA1缺失导致小鼠生长迟缓、肌纤维体积减小和炎症增加,导致骨骼肌萎缩[20]。相同的,在SAMP8小鼠中线粒体融合基因(Mfn2和OPA1)显著下降,并且在肌少症的整个进展期间表现出下降趋势[14]。在人类研究中发现髋部骨折伴肌少症患者的Mfn2蛋白表达也显著下降[21]。因此,线粒体融合失败会导致骨骼肌萎缩,身体功能下降和致死性增加。 另一方面,线粒体裂变主要受动力相关蛋白1(dynamin-related protein 1,Drp1)和线粒体分裂蛋白1(mitochondrial fission protein 1,Fis1)控制。DULAC等[22]证明在小鼠骨骼肌中Drp1低表达会导致线粒体功能障碍、自噬受损和失神经支配,诱导骨骼肌严重萎缩。肌肉特异性Drp1过度表达导致转基因小鼠mtDNA水平降低,蛋白质合成减弱[23]。这些数据表明线粒体裂变必须保持在生理范围内,并且稳定的Drp1水平对于维持线粒体功能是必需的。另有研究表明Fis1的丧失导致线粒体功能和肌肉蛋白质平衡受损,使得Fis1突变的果蝇飞行能力和寿命均降低[24]。除此之外,HUANG等[15]发现敲除老年肌少症小鼠Nrf-2因子,导致线粒体融合相关因子(Mfn1、Mfn2和Opa1)和线粒体裂变相关因子(Drp1)减少,提示Nrf-2的丢失可通过破坏平衡的线粒体动力学来加重肌少症,并且线粒体生物合成和动力学可能彼此相关。 2.3.3 线粒体自噬 线粒体自噬是通过结合自噬体和随后溶酶体的降解作用来选择性去除功能失调或受损线粒体,以维持线粒体稳态的一种自我调节过程[25]。自噬能力下降,体内功能失调或多余的细胞及细胞器增多,导致高水平的mtDNA突变,增加了活性氧的产生,进一步损伤线粒体。最近,PINK1/Parkin途径被认为是调节泛素依赖性线粒体自噬的最重要的信号通路之一[26]。简单地说,线粒体膜电位受损后,PINK1识别损伤的线粒体并聚集在线粒体外膜上,然后激活E3泛素连接酶Parkin蛋白,促进线粒体泛素化以启动线粒体自噬。 在动物研究中,缺乏Parkin基因的小鼠表现出肌肉收缩功能下降和骨骼肌线粒体受损[27]。另一方面,Parkin的过表达减轻了与衰老相关的肌肉质量和力量损失,并改善了线粒体生物发生和酶活性[28]。这些发现表明衰老过程中线粒体自噬减少与骨骼肌功能之间存在密切关系。有研究发现线粒体衍生囊泡也是一种消除受损线粒体或线粒体自噬的新方法,但它是不依赖Drp1的线粒体自噬途径[29]。值得注意的是,近年有研究指出线粒体囊泡衍生的泛醌氧化还原酶亚基S3(NDUFS3),可能是肌少症的新预测因子[30]。考虑到线粒体自噬在质量控制中的多重作用,Parkin或其他重要的线粒体自噬调节因子可能成为预防和减轻肌少症的新靶点。 总之,稳态线粒体质量控制的维持归因于线粒体生物发生、动力学(融合-裂变)和衰老中自噬的协同调节。质量控制稳态的破坏导致功能失调的线粒体积累,其对老年人的骨骼肌健康产生负面影响,甚至可能导致肌少症,见图4。"

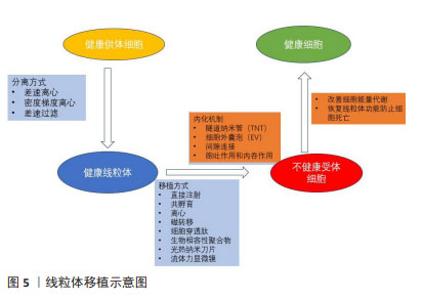
2.4 肌少症治疗与线粒体移植 运动、营养、药物是预防和治疗肌少症最有效的策略。运动已被证明可通过激活骨骼肌中的信号来调节线粒体网络并改善炎症反应,还可以通过调节线粒体融合、裂变和生物合成来改善线粒体的功能[31]。然而,部分老年人由于骨关节病、心脏病、脑卒中等慢性疾病,无法从运动中获益;优质蛋白质和氨基酸摄入对于老年人也是必要的,但尚不清楚其剂量、服用频率及持续时间是否会影响肌肉质量或功能[32]。研究证实选择性雄激素受体调节剂(SARM)可增强骨骼肌功能并增加瘦体质量,但药物可能有不良反应,迄今为止尚未获得FDA批准 [33]。以上是当前肌少症治疗方案的局限性。近年来线粒体移植作为治疗与线粒体或mtDNA功能障碍相关疾病的潜在治疗方法已引起许多科学家的关注。鉴于线粒体功能障碍在肌少症发生发展中的重要作用,线粒体移植有望成为肌少症潜在的治疗靶点。 以往多项小鼠动物模型研究表明,线粒体移植可以改善细胞能量代谢,恢复线粒体功能,预防细胞死亡。然而,将动物研究的结果转化至临床仍需要克服几个挑战,其中功能性线粒体的转移是主要挑战之一。细胞间线粒体转移以各种方式发生,包括隧道纳米管、细胞外囊泡、间隙连接以及游离线粒体的胞吐作用和内吞作用,这取决于细胞类型和条件。 隧道纳米管是相邻细胞之间的膜状管状突起,用于细胞成分交换和细胞间通讯,被认为是细胞之间的主要线粒体转移方式[34]。隧道纳米管已被证明与细胞损伤修复、免疫反应激活、细胞代谢重新编程和线粒体运输有关[35]。通过隧道纳米管进行线粒体运输的一种潜在机制是线粒体Rho GTP酶1(Miro1)的过表达,其将线粒体与细胞骨架运动蛋白连接起来。已有研究显示Miro1过表达促进线粒体从干细胞向隧道纳米管上的上皮细胞迁移,减少胶原沉积、炎性细胞浸润、上皮细胞凋亡和黏液分泌过多[36]。间隙连接通过将半通道插入专门的细胞间结构来促进两个相邻细胞之间的直接通信[37-38],间隙连接仅允许转移小离子和代谢物。细胞外囊泡可以在细胞之间转移整个线粒体颗粒以及mtDNA[39]。尽管在细胞外囊泡中检测到线粒体成分,但通过细胞外囊泡转移线粒体蛋白或mtDNA的机制仍然未知[40]。总体而言,线粒体的细胞间转移已被证明可通过用外源线粒体替代受损的线粒体来改善细胞功能并挽救线粒体缺陷。 线粒体移植的机制被认为是外源性线粒体与受体细胞中的内源性线粒体融合,取代或修复受损的线粒体,通过提高ATP含量,调节受损组织的细胞代谢,促进细胞和组织恢复。上述这些理论是基于线粒体进入细胞内产能提出的,称之为内化机制。然而同样是在实践研究中,MCCULLY团队[41]报道线粒体移植后10 min即可改善实验兔模型的心脏功能,而理论上线粒体进入心肌细胞却需要几个小时[42],用前面所描述的内化机制解释显然是不成立的。国内李震等[43]提出线粒体移植的非内化机制假说,即线粒体不需要进入细胞就能发挥功效,与细胞表面的信号转导或特定物质相互作用从而对器官组织有快速修复的作用。又或者是两种不同的机制共存?目前尚不清楚细胞是否可以同时通过多种途径转移线粒体。未来的治疗研究应侧重于线粒体移植机制的研究,了解线粒体移植反应中涉及的分子途径,以更好地了解线粒体移植在肌少症中的治疗效果。见图5。"

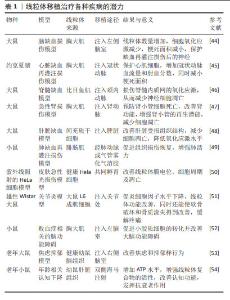
2.5 线粒体移植是肌少症治疗的潜在靶点 许多研究已经证实了线粒体移植治疗各种疾病的潜力[44-54],见表1。虽然目前还没有关于使用线粒体移植治疗肌少症的研究,但从机制的角度来看,线粒体移植具有通过恢复ATP产生、减少炎症和氧化应激、抑制蛋白质降解途径和细胞凋亡来促进组织健康的能力,通常在减缓肌少症进展中起核心作用。那么,线粒体移植是否会对肌少症进展中的骨骼肌健康产生积极影响?有限的研究表明线粒体移植在治疗肌肉萎缩,肌肉肌病和缺血性肌肉损伤等疾病中有良好获益,为后续在肌少症治疗研究中打下基础。 在地塞米松诱导的骨骼肌萎缩模型中,KIM等[4]发现线粒体移植增加了肌肉质量并提高了肌肉再生标志物结蛋白的表达。重要的是,线粒体移植通过Akt-FoxO(蛋白激酶B-叉头框蛋白O)信号传导途径显著降低肌肉特异性泛素E3连接酶MAFbx(肌肉萎缩凋亡蛋白)和MuRF-1(肌肉特异性环指蛋白1)的表达。基于这项结果表明线粒体移植在萎缩性肌肉疾病中具有治疗应用的潜力[4]。ORFANY等[55]在肢体缺血后2 h通过直接注射将不同比例的线粒体移植到肢体肌肉中,结果显示比目鱼肌、腓肠肌和股内侧肌的细胞凋亡和梗死面积减少。同样,ALWAY等[5]将线粒体注入尾静脉,发现可以促进肌肉再生并改善氯化钡诱导的肌肉损伤小鼠的肌肉功能,同时发现线粒体移植对不同类型骨骼肌纤维再生程度不同,其中Ⅱ型肌纤维的再生最为明显。LEE等[56]在肌腱病大鼠模型中,通过跟腱处注射大鼠成纤维细胞分离的线粒体,有较好的跟腱病治疗效果。虽然这些研究表明线粒体移植可能对肌少症有治疗效果,是治疗年龄相关疾病有希望的靶点,但仍需要进一步研究探索线粒体移植在动物模型和人体临床试验中治疗肌少症的潜力。"

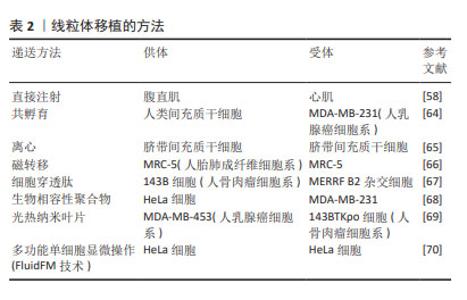
2.6 线粒体移植的挑战与展望 尽管线粒体移植是治疗肌少症的一种可能的治疗方法,在动物实验中也展现出巨大的前景,然而在推广应用前仍然有许多亟待解决的问题。 (1)移植的线粒体来源是临床应用中的关键因素。以往的线粒体移植研究大多使用自体组织分离的线粒体,这样能很好地避免线粒体移植后的免疫反应,但却限制了线粒体的来源,特别是对那些先天性功能障碍或老年多病共存的患者。研究发现,骨髓间充质干细胞作为线粒体来源进行移植,可以促进细胞增殖,抵抗氧化应激,防止细胞凋亡,并刺激线粒体的生物合成。在线粒体转移应用中很有前景,可能为治疗肌少症提供一种新的途径[57]。 (2)选取健康有活性而且数量充足的线粒体是线粒体移植的首要基础。几乎任何远离病变区域的正常组织或细胞都可以用作分离线粒体的来源。研究已证实自体骨骼肌如腹直肌[58]、胸大肌[59]、腓肠肌等是线粒体分离的良好来源[60],既保证了充足的数量,又减少免疫原性并发症。在线粒体活性方面,赵梓圳等[61]发现从4周龄青年小鼠肝脏提取的线粒体比从12月龄衰老小鼠提取的线粒体有更好的抗肿瘤作用。 (3)线粒体分离可以使用差速离心和密度梯度离心等技术获得。这两种方法分离线粒体可以通过消除具有较低密度的不良线粒体来选择完整的线粒体。然而,差速离心的收率较高,纯度较低,而密度梯度离心的收率较低,纯度较高。此外,这些线粒体分离方法需要耗时,重复的离心步骤,导致线粒体活力降低[62]。McCully团队提出的一种快速分离和纯化线粒体的方法,可以更好地满足介入时间小于30 min的临床应用要求[63],该方法的主要优点是使用差速过滤代替差速离心,其允许更快速地分离高度纯化、存活和完整的线粒体。 (4)线粒体移植的方法。可通过直接注射[58]、共孵育[64]、离心[65]、磁转移[66]、细胞穿透肽[67]、生物相容性聚合物[68]、光热纳米刀片和多功能单细胞显微操作(FluidFM技术)尝试将外源线粒体掺入受体细胞[69-70],见表2。在体内和体外模型中,可通过直接注射方法将分离的线粒体直接递送到受体细胞中[58]。在体内局部注射线粒体可用于局部损伤,而血管内注射对多器官线粒体疾病更有效。分离的线粒体与受体细胞共孵育已经成功地将外源线粒体体外递送到细胞中。共孵育很简单,但不适用于体内线粒体递送。目前已经开发了离心、磁转移、细胞穿透肽和生物相容性聚合物等方法进一步改善线粒体内化。"

| [1] YE C, ZHENG X, AIHEMAITIJIANG S, et al. Sarcopenia and catastrophic health expenditure by socio-economic groups in China: an analysis of household-based panel data. J Cachexia Sarcopenia Muscle. 2022;13(3):1938-1947. [2] WIEDMER P, JUNG T, CASTRO JP, et al. Sarcopenia - Molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200. [3] BELLANTI F, LO BUGLIO A, VENDEMIALE G. Mitochondrial Impairment in Sarcopenia. Biology (Basel). 2021;10(1):31. [4] KIM MJ, LEE JM, MIN K, et al. Xenogeneic transplantation of mitochondria induces muscle regeneration in an in vivo rat model of dexamethasone-induced atrophy. J Muscle Res Cell Motil. 2023. doi: 10.1007/s10974-023-09643-7. [5] ALWAY SE, PAEZ HG, PITZER CR, et al. Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J Cachexia Sarcopenia Muscle. 2023;14(1):493-507. [6] COEN PM, MUSCI RV, HINKLEY JM, et al. Mitochondria as a Target for Mitigating Sarcopenia. Front Physiol. 2019;9:1883. [7] TIAN Q, MITCHELL BA, ZAMPINO M, et al. Muscle mitochondrial energetics predicts mobility decline in well-functioning older adults: The baltimore longitudinal study of aging. Aging Cell. 2022;21(2):e13552. [8] FERRI E, MARZETTI E, CALVANI R, et al. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int J Mol Sci. 2020;21(15):5236. [9] COEN PM, JUBRIAS SA, DISTEFANO G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68(4):447-455. [10] MARZETTI E, LEES HA, MANINI TM, et al. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: an exploratory study. PLoS One. 2012;7(2):e32829. [11] INCI N, KAMALI D, AKYILDIZ EO, et al. Translation of Cellular Senescence to Novel Therapeutics: Insights From Alternative Tools and Models. Front Aging. 2022;3:828058. [12] PICCA A, LEZZA AM. Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions: Useful insights from aging and calorie restriction studies. Mitochondrion. 2015;25:67-75. [13] REBELO AP, DILLON LM, MORAES CT. Mitochondrial DNA transcription regulation and nucleoid organization. J Inherit Metab Dis. 2011;34(4): 941-951. [14] LIU HW, CHANG YC, CHAN YC, et al. Dysregulations of mitochondrial quality control and autophagic flux at an early age lead to progression of sarcopenia in SAMP8 mice. Biogerontology. 2020;21(3):367-380. [15] HUANG DD, FAN SD, CHEN XY, et al. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp Gerontol. 2019;119:61-73. [16] YANG S, LORO E, WADA S, et al. Functional effects of muscle PGC-1alpha in aged animals. Skelet Muscle. 2020;10(1):14. [17] GARCIA S, NISSANKA N, MARECO EA, et al. Overexpression of PGC-1α in aging muscle enhances a subset of young-like molecular patterns. Aging Cell. 2018;17(2):e12707. [18] ROMANELLO V, SANDRI M. The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass. Cell Mol Life Sci. 2021;78(4):1305-1328. [19] BELL MB, BUSH Z, MCGINNIS GR, et al. Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity. J Appl Physiol (1985). 2019;126(2):341-353. [20] RODRÍGUEZ-NUEVO A, DÍAZ-RAMOS A, NOGUERA E, et al. Mitochondrial DNA and TLR9 drive muscle inflammation upon Opa1 deficiency. EMBO J. 2018;37(10):e96553. [21] MARZETTI E, CALVANI R, LORENZI M, et al. Association between myocyte quality control signaling and sarcopenia in old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp Gerontol. 2016;80:1-5. [22] DULAC M, LEDUC-GAUDET JP, REYNAUD O, et al. Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation. J Physiol. 2020;598(17):3691-3710. [23] TOUVIER T, DE PALMA C, RIGAMONTI E, et al. Muscle-specific Drp1 overexpression impairs skeletal muscle growth via translational attenuation. Cell Death Dis. 2015;6(2):e1663. [24] LEE TT, CHEN PL, SU MP, et al. Loss of Fis1 impairs proteostasis during skeletal muscle aging in Drosophila. Aging Cell. 2021;20(6):e13379. [25] 侯国珍,郭琪,韩佩佩.肌少症自噬激活和线粒体质量控制信号途径的研究进展[J].中国医学科学院学报,2022,44(4):709-716. [26] CHEN G, KROEMER G, KEPP O. Mitophagy: An Emerging Role in Aging and Age-Associated Diseases. Front Cell Dev Biol. 2020;8:200. [27] GOUSPILLOU G, GODIN R, PIQUEREAU J, et al. Protective role of Parkin in skeletal muscle contractile and mitochondrial function. J Physiol. 2018; 596(13):2565-2579. [28] LEDUC-GAUDET JP, REYNAUD O, HUSSAIN SN, et al. Parkin overexpression protects from ageing-related loss of muscle mass and strength. J Physiol. 2019;597(7):1975-1991. [29] SUGIURA A, MCLELLAND GL, FON EA, et al. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33(19):2142-2156. [30] MARZETTI E, GUERRA F, CALVANI R, et al. Circulating Mitochondrial-Derived Vesicles, Inflammatory Biomarkers and Amino Acids in Older Adults With Physical Frailty and Sarcopenia: A Preliminary BIOSPHERE Multi-Marker Study Using Sequential and Orthogonalized Covariance Selection - Linear Discriminant Analysis. Front Cell Dev Biol. 2020;8:564417. [31] ALIZADEH PAHLAVANI H, LAHER I, et al. Exercise and mitochondrial mechanisms in patients with sarcopenia. Front Physiol. 2022;13:1040381. [32] GANAPATHY A, NIEVES JW. Nutrition and Sarcopenia-What Do We Know? Nutrients. 2020;12(6):1755. [33] CHRISTIANSEN AR, LIPSHULTZ LI, HOTALING JM, et al. Selective androgen receptor modulators: the future of androgen therapy? Transl Androl Urol. 2020;9(Suppl 2):S135-S148. [34] QIN Y, JIANG X, YANG Q, et al. The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells. Front Oncol. 2021;11:672781. [35] JACKSON MV, MORRISON TJ, DOHERTY DF, et al. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells. 2016;34(8):2210-2223. [36] AHMAD T, MUKHERJEE S, PATTNAIK B, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994-1010. [37] LIU Z, SUN Y, QI Z, et al. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci. 2022;12(1):66. [38] SHANMUGHAPRIYA S, LANGFORD D, NATARAJASEENIVASAN K. Inter and Intracellular mitochondrial trafficking in health and disease. Ageing Res Rev. 2020;62:101128. [39] PHINNEY DG, DI GIUSEPPE M, NJAH J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. [40] VALENTI D, VACCA RA, MORO L, et al. Mitochondria Can Cross Cell Boundaries: An Overview of the Biological Relevance, Pathophysiological Implications and Therapeutic Perspectives of Intercellular Mitochondrial Transfer. Int J Mol Sci. 2021;22(15):8312. [41] MCCULLY JD, COWAN DB, PACAK CA, et al. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol. 2009;296(1):H94-H105. [42] SHIN B, SAEED MY, ESCH JJ, et al. A Novel Biological Strategy for Myocardial Protection by Intracoronary Delivery of Mitochondria: Safety and Efficacy. JACC Basic Transl Sci. 2019;4(8):871-888. [43] 李震,曹新惠,王春明.线粒体移植疗法[J].中国细胞生物学学报,2023, 45(7):1089-1096. [44] ZHANG Z, MA Z, YAN C, et al. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury. Behav Brain Res. 2019;356:322-331. [45] GUARIENTO A, BLITZER D, DOULAMIS I, et al. Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection. J Thorac Cardiovasc Surg. 2020;160(2):e15-e29. [46] FANG SY, ROAN JN, LEE JS, et al. Transplantation of viable mitochondria attenuates neurologic injury after spinal cord ischemia. J Thorac Cardiovasc Surg. 2021;161(5):e337-e347. [47] JABBARI H, ROUSHANDEH AM, ROSTAMI MK, et al. Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat. Biochim Biophys Acta Mol Basis Dis. 2020;1866(8):165809. [48] ULGER O, KUBAT GB, CICEK Z, et al. The effects of mitochondrial transplantation in acetaminophen-induced liver toxicity in rats. Life Sci. 2021;279:119669. [49] MOSKOWITZOVA K, ORFANY A, LIU K, et al. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2020;318(1):L78-L88. [50] HU SS, LI RY, CAO XH, et al. Structural integrity is essential for the protective effect of mitochondrial transplantation against UV-induced cell death. J Photochem Photobiol B. 2022;234:112534. [51] LEE AR, WOO JS, LEE SY, et al. Mitochondrial Transplantation Ameliorates the Development and Progression of Osteoarthritis. Immune Netw. 2022; 22(2):e14. [52] YAN C, MA Z, MA H, et al. Mitochondrial Transplantation Attenuates Brain Dysfunction in Sepsis by Driving Microglial M2 Polarization. Mol Neurobiol. 2020;57(9):3875-3890. [53] JAVANI G, BABRI S, FARAJDOKHT F, et al. Mitochondrial transplantation improves anxiety- and depression-like behaviors in aged stress-exposed rats. Mech Ageing Dev. 2022;202:111632. [54] ZHANG Z, WEI D, LI Z, et al. Hippocampal Mitochondrial Transplantation Alleviates Age-Associated Cognitive Decline via Enhancing Wnt Signaling and Neurogenesis. Comput Intell Neurosci. 2022;2022:9325302. [55] ORFANY A, ARRIOLA CG, DOULAMIS IP, et al. Mitochondrial transplantation ameliorates acute limb ischemia. J Vasc Surg. 2020;71(3):1014-1026. [56] LEE JM, HWANG JW, KIM MJ, et al. Mitochondrial Transplantation Modulates Inflammation and Apoptosis, Alleviating Tendinopathy Both In Vivo and In Vitro. Antioxidants (Basel). 2021;10(5):696. [57] TIAN X, PAN M, ZHOU M, et al. Mitochondria Transplantation from Stem Cells for Mitigating Sarcopenia. Aging Dis. 2023;14(5):1700-1713. [58] GUARIENTO A, PIEKARSKI BL, DOULAMIS IP, et al. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2021;162(3):992-1001. [59] KAZA AK, WAMALA I, FRIEHS I, et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg. 2017;153(4):934-943. [60] MOSKOWITZOVA K, SHIN B, LIU K, et al. Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J Heart Lung Transplant. 2019;38(1):92-99. [61] 赵梓圳,付爱玲.线粒体治疗:一种新型的线粒体相关疾病的生物疗法[J].生物工程学报,2021,37(4):1168-1177. [62] MCCULLY JD, COWAN DB, EMANI SM, et al. Mitochondrial transplantation: From animal models to clinical use in humans. Mitochondrion. 2017;34: 127-134. [63] PREBLE JM, PACAK CA, KONDO H, et al. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. J Vis Exp. 2014;(91):e51682. [64] CAICEDO A, FRITZ V, BRONDELLO JM, et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep. 2015;5:9073. [65] KIM MJ, HWANG JW, YUN CK, et al. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci Rep. 2018; 8(1):3330. [66] MACHEINER T, FENGLER VH, AGREITER M, et al. Magnetomitotransfer: An efficient way for direct mitochondria transfer into cultured human cells. Sci Rep. 2016;6:35571. [67] CHANG JC, LIU KH, LI YC, et al. Functional recovery of human cells harbouring the mitochondrial DNA mutation MERRF A8344G via peptide-mediated mitochondrial delivery. Neurosignals. 2013;21(3-4):160-173. [68] WU S, ZHANG A, LI S, et al. Polymer Functionalization of Isolated Mitochondria for Cellular Transplantation and Metabolic Phenotype Alteration. Adv Sci (Weinh). 2018;5(3):1700530. [69] WU TH, SAGULLO E, CASE D, et al. Mitochondrial Transfer by Photothermal Nanoblade Restores Metabolite Profile in Mammalian Cells. Cell Metab. 2016;23(5):921-929. [70] GÄBELEIN CG, FENG Q, SARAJLIC E, et al. Mitochondria transplantation between living cells. PLoS Biol. 2022;20(3):e3001576. [71] MAEDA H, KAMI D, MAEDA R, et al. TAT-dextran-mediated mitochondrial transfer enhances recovery from models of reperfusion injury in cultured cardiomyocytes. J Cell Mol Med. 2020;24(9):5007-5020. [72] ZHU Z, LI X, WANG X, et al. Photobiomodulation augments the effects of mitochondrial transplantation in the treatment of spinal cord injury in rats by facilitating mitochondrial transfer to neurons via Connexin 36. Bioeng Transl Med. 2022;8(3):e10473. |
| [1] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei, Zhou Jiatan. Based on Mendelian randomization, the causal relationship between 1400 metabolites and sarcopenia and the correlation analysis of cardiovascular disease were investigated [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-11. |
| [2] | Li Jiatong, Jin Yue, Liu Runjia, Song Bowen, Zhu Xiaoqian, Li Nianhu . Association between thyroid function levels and phenotypes associated with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1312-1320. |
| [3] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [4] | Jiang Siqi, Huang Huanhuan, Yu Xinyu, Peng Ying, Zhou Wei, Zhao Qinghua. Meta-analysis of dose-effect of exercise on improving muscle health in community-dwelling older adults with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6295-6304. |
| [5] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei. Causal association between metabolites and sarcopenia: a big data analysis of genome-wide association studies in the European population [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6369-6380. |
| [6] | Sun Yahui, Wang Yufeng, Guo Chao, Yao Junjie, Ji Yuanyuan, Li Zhongxu, Lou Huijuan, Jiang Jinglei, Sun Yiping, Xu Jing, Cong Deyu. Effect of massage on extracellular matrix collagen deposition in skeletal muscle of type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5549-5555. |
| [7] | Nan Songhua, Peng Chaojie, Cui Yinglin. Mitochondrial dysfunction and brain aging: a bibliometrics analysis based on the Web of Science Core Collection database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5642-5651. |
| [8] | Zhang Xingyu, Wu Dou, Zhao Enzhe, Song Xubin, Zhang Xiaolun. Treatment and repair of musculoskeletal degenerative diseases and injuries from the perspective of muscle-bone crosstalk mechanism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5179-5186. |
| [9] | Yang Shuo, Zhang Zhen, Bai Shuo, Sheng Li, Shen Liang, Sun Qingfeng, Gao Beiyao, Ge Ruidong, Jiang Shan. Mitochondrial dysfunction in tendinopathy: possibility of mitochondria-targeting therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4276-4285. |
| [10] | Jiang Qiang, Yu Jie, Geng Zixiang, Wang Ning, Guo Jia, Yang Guangyue, Wang Peige, Zhao Yongfang. Comparison of phenotypes and mechanistic characteristics in two mouse models of sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 2922-2929. |
| [11] | Chen Jiayi, Li Huijing, Nong Yuxuan, Yin Yunfang, Liu Xiaobo, Chen Yue, Hu Xiaoshen, Zhong Dongling, Li Juan, Liu Tianyu, Jin Rongjiang. Meta-analysis of the correlation between phase angle and sarcopenia and its diagnostic indexes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(12): 2575-2589. |
| [12] | Lu Donglei, Feng Zhanpeng, Cao Liquan, Tang Yi, Tan Sijie, Yu Zhongtao. Exercise intervention methods for senile sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5723-5731. |
| [13] | Xu Rui, Li Yanyan, Xu Hong. Effect and mechanism of short-chain fatty acids in aged rats with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5172-5176. |
| [14] | Chen Tianxin, Dong Tingting, Li Yan, Zhang Sheng, Zhang Lei. Causal relationship between blood metabolites and sarcopenia-related traits: a Mendelian randomization study [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4288-4292. |
| [15] | Guo Hui, Kong Jianda, Tian Chunlan. The role of mitochondrial autophagy-related receptor proteins and signaling pathways in the prevention and treatment of sarcopenia through exercise [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4397-4404. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||