Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (11): 2243-2251.doi: 10.12307/2025.350
Previous Articles Next Articles
Mechanism by which exogenous basic fibroblast growth factor promotes wound healing in rats
Li Zhenchao1, Du Xiling2, Han Zhixin1, Niu Dawei1, Fan Changwei1
- 1Department of Orthopedics and Burn, Anyang People’s Hospital, Anyang 455000, Henan Province, China; 2Hebi Polytechnic, Hebi 458000, Henan Province, China
-
Received:2024-01-11Accepted:2024-02-22Online:2025-04-18Published:2024-08-10 -
Contact:Han Zhixin, Attending physician, Department of Orthopedics and Burn, Anyang People’s Hospital, Anyang 455000, Henan Province, China -
About author:Li Zhenchao, Associate chief physician, Department of Orthopedics and Burn, Anyang People’s Hospital, Anyang 455000, Henan Province, China -
Supported by:Henan Provincial Medical Science and Technology Tackling Program Joint Construction Project, No. LHGJ20230855 (to HZX); Anyang Science and Technology Tackling Program, No. 2023C01SF149 (to HZX)
CLC Number:
Cite this article
Li Zhenchao, Du Xiling, Han Zhixin, Niu Dawei, Fan Changwei. Mechanism by which exogenous basic fibroblast growth factor promotes wound healing in rats[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(11): 2243-2251.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

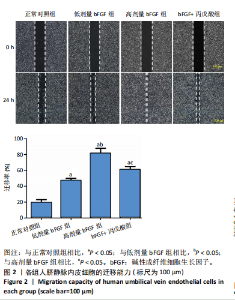
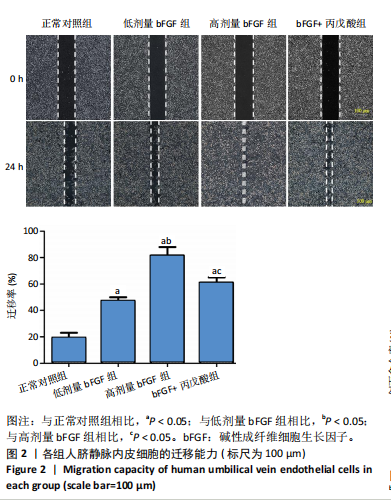
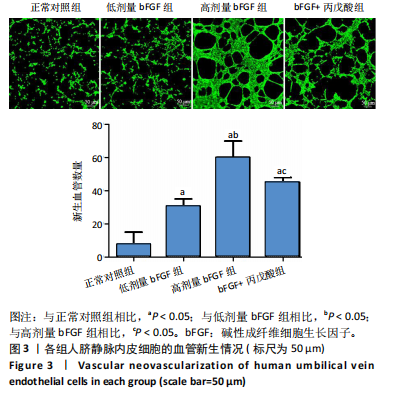
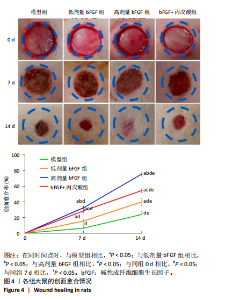
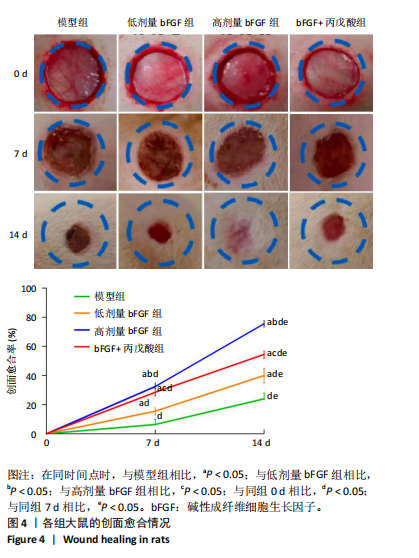
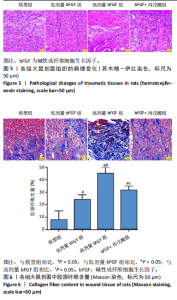
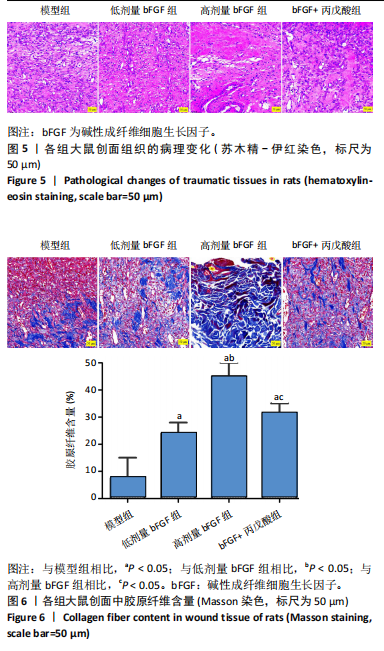
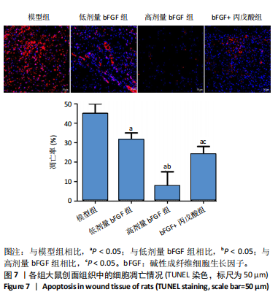
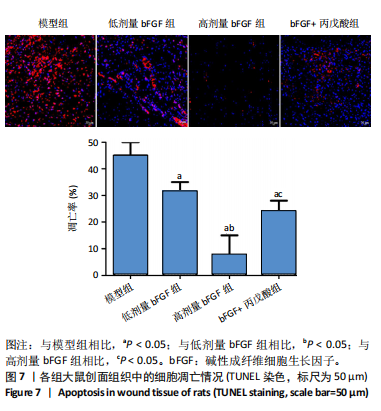
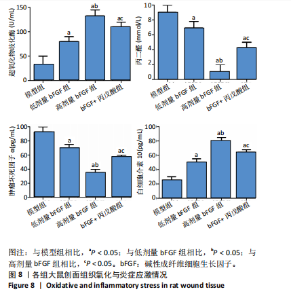
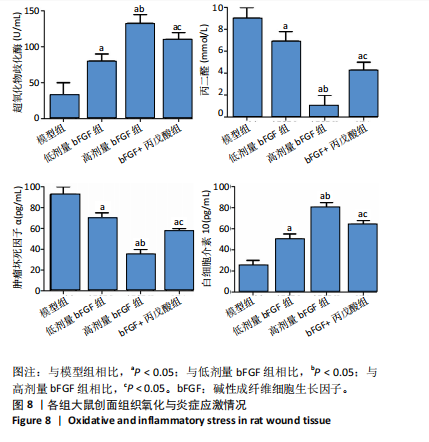
2.1 各组人脐静脉内皮细胞的增殖能力 与正常对照组相比,低剂量bFGF组、高剂量bFGF组以及bFGF+丙戊酸组EdU阳性细胞比例明显升高;与低剂量bFGF组相比,高剂量bFGF组EdU阳性细胞比例明显升高;与高剂量bFGF组相比,bFGF+丙戊酸组EdU阳性细胞比例明显下降,差异均有显著性意义(均P < 0.05),见图1。 2.2 各组人脐静脉内皮细胞的迁移能力 划痕实验结果显示,与正常对照组相比,低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组细胞迁移率明显升高;与低剂量bFGF组相比,高剂量bFGF组细胞迁移率明显升高;与高剂量bFGF组相比,bFGF+丙戊酸组细胞迁移率明显下降,差异均有显著性意义(均P < 0.05),见图2。 2.3 各组人脐静脉内皮细胞的血管生成能力 小管生成实验结果显示,与正常对照组相比,低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组的血管新生数量明显升高;与低剂量bFGF组相比,高剂量bFGF组的血管新生数量明显升高;与高剂量bFGF组相比,bFGF+丙戊酸组的血管新生数量明显下降,差异均有显著性意义(均P < 0.05),见图3。 2.4 实验动物数量分析 60只SD大鼠在实验过程中未发生因造模而死亡的现象。 2.5 各组大鼠创面的愈合率 在造模0 d时,模型组、低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组大鼠创面大体观察未见明显异常;造模7 d时,模型组大鼠创面边缘出现脓性分泌物,水肿严重;低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组创面明显缩小,水肿明显减轻,尤其是高剂量bFGF组创面可见生长良好的肉芽组织;造模14 d时,高剂量bFGF组创面面积进一步缩小,创面四周的新生皮肤与正常皮肤组织未见明显差异,新生的皮毛将愈合的创面覆盖;而模型组创面面积仍比较大,创面边缘仍有脓性分泌物排出。统计结果显示,在同一时间点时,与模型组相比,低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组大鼠创面愈合率明显升高;与低剂量bFGF组相比,高剂量bFGF组大鼠创面愈合率明显升高;与高剂量bFGF组相比,bFGF+丙戊酸组大鼠创面愈合率明显降低,差异均有显著性意义(均P < 0.05)。在同组内,与0 d时相比,造模7,14 d时大鼠创面的愈合率明显升高;与7 d时相比,14 d时大鼠创面的愈合率明显升高,差异均有显著性意义(均P < 0.05),见图4。 2.6 各组大鼠创面组织的病理改变 苏木精-伊红染色结果显示,造模14 d时,模型组大鼠创面组织中仍能检测到炎性递质分布,成纤维细胞的排列比较紊乱,低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组大鼠创面组织中炎性细胞分布明显减少,新生的毛细血管数量明显增加,高剂量bFGF组大鼠创面出现明显的皮脂腺等皮肤附属器,见图5。 2.7 各组大鼠创面组织中的纤维含量 Masson染色结果显示,造模14 d时,与模型组相比,低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组大鼠创面组织中胶原纤维含量明显升高;与低剂量bFGF组相比,高剂量bFGF组大鼠创面组织中胶原纤维含量明显升高;与高剂量bFGF组相比,bFGF+丙戊酸组大鼠创面组织中胶原纤维含量明显降低,差异均有显著性意义(均P < 0.05),见图6。 2.8 各组大鼠创面组织中的细胞凋亡率 TUNEL染色结果显示,造模14 d时,与模型组相比,低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组大鼠创面组织中的细胞凋亡率明显下降;与低剂量bFGF组相比,高剂量bFGF组大鼠创面组织中的细胞凋亡率明显下降;与高剂量bFGF组相比,bFGF+丙戊酸组大鼠创面组织中的细胞凋亡率明显升高,差异均有显著性意义(均P < 0.05),见图7。 2.9 各组大鼠创面组织的氧化应激和炎症应激情况 试剂盒检测结果显示,与模型组相比,低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组大鼠血清中丙二醛和肿瘤坏死因子α水平明显下降,超氧化物歧化酶和白细胞介素10水平明显升高;与低剂量bFGF组相比,高剂量bFGF组血清中丙二醛和肿瘤坏死因子α水平明显下降,超氧化物歧化酶和白细胞介素10水平明显升高;与高剂量bFGF组相比,bFGF+丙戊酸组大鼠血清中丙二醛和肿瘤坏死因子α的水平明显升高,超氧化物歧化酶和白细胞介素10水平明显下降,差异均有显著性意义(均P < 0.05),见图8。"

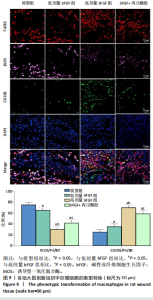
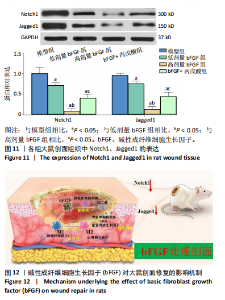
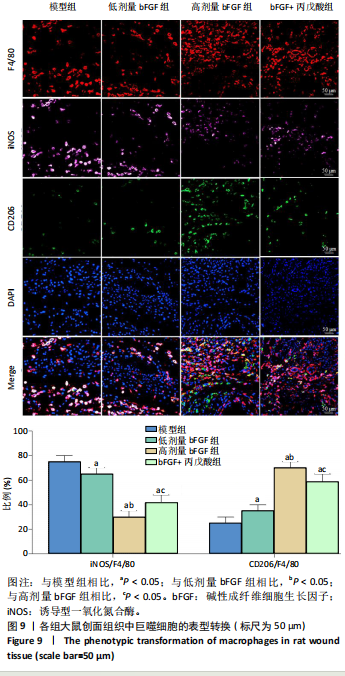
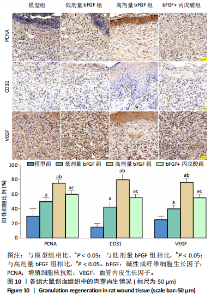
的比值明显升高,M2型巨噬细胞比例明显升高;与高剂量bFGF组相比,bFGF+丙戊酸组大鼠创面组织中诱导型一氧化氮合酶与F4/80的比值明显升高,M1型巨噬细胞比例明显升高,CD206与F4/80的比值明显降低,M2型巨噬细胞比例明显降低,差异均有显著性意义(均P < 0.05),见图9。 2.11 各组大鼠中创面组织中的肉芽再生 免疫组化结果显示,与模型组相比,低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组大鼠创面组织中增殖细胞核抗原、CD31和血管内皮生长因子的表达明显升高;与低剂量bFGF组相比,高剂量bFGF组创面组织中增殖细胞核抗原、CD31和血管内皮生长因子的表达明显升高;与高剂量bFGF组相比,bFGF+丙戊酸组大鼠创面组织中增殖细胞核抗原、CD31和血管内皮生长因子的表达明显下降,差异均有显著性意义(均P < 0.05),见图10。 2.12 各组大鼠中创面组织中Notch1、Jagged1的表达 Western blot结果显示,与模型组相比,低剂量bFGF组、高剂量bFGF组、bFGF+丙戊酸组大鼠创面组织中Notch1、Jagged1的表达明显下降;与低剂量bFGF组相比,高剂量bFGF组创面组织中Notch1、Jagged1的表达明显下降;与高剂量bFGF组相比,bFGF+丙戊酸组大鼠创面组织中Notch1、Jagged1的表达明显升高,差异均有显著性意义(均P < 0.05),见图11。 2.13 bFGF对大鼠创面修复的影响机制 当大鼠的皮肤出现创面后,Notch1/Jagged1信号被激活,Notch1、Jagged1的表达随之升高,创面组织处的氧化与炎性应激加剧,丙"
二醛水平升高,超氧化物歧化酶水平降低,创面组织中的巨噬细胞以M1型为主,炎症性递质肿瘤坏死因子α的合成与释放升高,抑炎性递质白细胞介素10的释放下降,大鼠创面组织中增殖细胞核抗原、CD31和血管内皮生长因子的表达水平较低,创面愈合较慢;当以bFGF对大鼠创面进行干预后,能明显抑制Notch1/Jagged1信号的激活,Notch1、Jagged1的表达随之下降;创面组织处的氧化与炎性应激被抑制,大鼠创面中的巨噬细胞从M1型向M2型发生转换,增殖细胞核抗原、CD31和血管内皮生长因子的表达水平升高,促进创面组织处的血管再生和肉芽再生,促进创面愈合,以Notch1/Jagged1信号激活剂丙戊酸进行功能挽救实验,结果显示,丙戊酸能部分逆转bFGF对创面愈合的促进作用,见图12。"

| [1] AFSHAR A, KHORADMEHR A, NOWZARI F, et al. Tissue Extract from Brittle Star Undergoing Arm Regeneration Promotes Wound Healing in Rat. Mar Drugs. 2023;21(7):381. [2] OTAKE H, MANO Y, DEGUCHI S, et al. Effect of Ointment Base on the Skin Wound-Healing Deficits in Streptozotocin-Induced Diabetic Rat. Biol Pharm Bull. 2023;46(5):707-712. [3] LI D, ZHANG C, GAO Z, et al. Curcumin-Loaded Macrophage-Derived Exosomes Effectively Improve Wound Healing. Mol Pharm. 2023;20(9): 4453-4467. [4] ALI S, ISHTEYAQUE S, KHAN F, et al. Accelerative Wound-Healing Effect of Aqueous Anthocephalus Cadamba Leaf Extract in a Diabetic Rat Model. Int J Low Extrem Wounds. 2023;22(2):409-417. [5] 李凤菊,朱玉霞,侯堆鹏,等.德莫林皮肤创面无机诱导活性敷料联合重组牛碱性成纤维细胞生长因子治疗放射性皮肤损伤的临床效果分析[J].中国医药,2023,18(10):1537-1541. [6] MOUNIR R, ALSHAREEF WA, EL GEBALY EA, et al. Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals (Basel). 2023;16(10):1379. [7] DU P, DIAO L, LU Y, et al. Heparin-based sericin hydrogel-encapsulated basic fibroblast growth factor for in vitro and in vivo skin repair. Heliyon. 2023;9(3):e13554. [8] 李锐,邹双,高征征,等.碱性成纤维细胞生长因子团聚体对糖尿病大鼠外周神经病变的治疗作用[J].中国药理学与毒理学杂志, 2017,31(4):295-302. [9] 李官虎,郎庆旭,刘纯岩,等.丙戊酸联合X射线照射对乳腺癌MDA-MB-231细胞增殖的抑制作用及其机制[J].吉林大学学报(医学版),2022,48(3):622-629. [10] VOLKOVA MV, BOYARINTSEV VV, TROFIMENKO AV, et al. Local injection of bone-marrow derived mesenchymal stromal cells alters a molecular expression profile of a contact frostbite injury wound and improves healing in a rat model. Burns. 2023;49(2):432-443. [11] ZHAO Q, XU J, HAN X, et al. Growth differentiation factor 10 induces angiogenesis to promote wound healing in rats with diabetic foot ulcers by activating TGF-β1/Smad3 signaling pathway. Front Endocrinol (Lausanne). 2023;13:1013018. [12] KHAN A, ANDLEEB A, AZAM M, et al. Aloe vera and ofloxacin incorporated chitosan hydrogels show antibacterial activity, stimulate angiogenesis and accelerate wound healing in full thickness rat model. J Biomed Mater Res B Appl Biomater. 2023;111(2):331-342. [13] LUO L, AN Y, GENG K, et al. High glucose-induced endothelial STING activation inhibits diabetic wound healing through impairment of angiogenesis. Biochem Biophys Res Commun. 2023;668:82-89. [14] EL-AASR M, NOHARA T, IKEDA T, et al. LC-MS/MS metabolomics profiling of Glechoma hederacea L. methanolic extract; in vitro antimicrobial and in vivo with in silico wound healing studies on Staphylococcus aureus infected rat skin wound. Nat Prod Res. 2023; 37(10):1730-1734. [15] TINGTING S, XINYUE F, TIANTIAN Y, et al. Comparison of the effects of negative pressure wound therapy and negative pressure wound therapy with instillation on wound healing in a porcine model. Front Surg. 2023;10:1080838. [16] XU TY, QING SL, ZHAO JX, et al. Metrnl deficiency retards skin wound healing in mice by inhibiting AKT/eNOS signaling and angiogenesis. Acta Pharmacol Sin. 2023;44(9):1790-1800. [17] SULIMAN MAASHI M, FELEMBAN SG, ALMASMOUM HA, et al. Nicaraven-loaded electrospun wound dressings promote diabetic wound healing via proangiogenic and immunomodulatory functions: a preclinical investigation. Drug Deliv Transl Res. 2023;13(1):222-236. [18] ZHOU J, XIA K, LI Y, et al. Zwitterionic nanocapsule-based wound dressing with the function of gradient release of multi-drugs for efficient wound healing. J Mater Chem B. 2023;11(30):7197-7208. [19] 徐捷,金利泰,王涛,等.紫草素通过上调C-X-C基序趋化因子受体4促进细胞自噬加快糖尿病创面愈合的机制研究[J].中华糖尿病杂志,2024,16(1):84-91. [20] 田淑芳,顾帅鹏,赵炎,等.高压电烧伤致大面积头皮缺损、颅骨外露创面修复1例[J].中华整形外科杂志,2023,39(5):576-579. [21] 韦积华,罗富强,谢康麒,等.舒洛地特对糖尿病足溃疡大鼠HIF-1α/GPER/VEGF通路及创面愈合的影响[J].中国老年学杂志,2023, 43(5):1151-1155. [22] 殷东京,沈国良.自体富血小板凝胶结合封闭负压引流对深Ⅱ度烧伤患者创面愈合进程及EGF、bFGF水平的影响[J].中国现代医学杂志,2023,33(8):87-92. [23] 刘亚坤,李刚,颜娟,等.紫檀芪对糖尿病性皮肤溃疡模型大鼠创面愈合的影响及机制[J].中国药房,2023,34(16):1967-1971. [24] 高喜翔,高明杰,谷涌泉,等.诱导多能干细胞分化的平滑肌细胞促进糖尿病鼠创面愈合的研究[J].中国实验动物学报,2023,31(7): 880-887. [25] ZARE R, ABDOLSAMADI H, SOLEIMANI ASL S, et al. The bFGF Can Improve Angiogenesis in Oral Mucosa and Accelerate Wound Healing. Rep Biochem Mol Biol. 2023;11(4):547-552. [26] WANG B, CHEN J, ZHANG C, et al. Biomimetic nanoparticles of platelet membranes carrying bFGF and VEGFA genes promote deep burn wound healing. Int Immunopharmacol. 2023;125(Pt A):111164. [27] 李晓辉,黄象艳.自体富血小板血浆在慢性难愈合创面治疗中的应用进展[J].山东医药,2023,63(4):107-110. [28] 郝治,龚海峰,刘丽梅,等.中药油剂愈溃油对大鼠糖尿病皮肤溃疡模型创面愈合及Wnt、notch通路的影响[J].四川中医,2022, 40(8):51-56. [29] 王义,郑旸,董孟杰,等.人脂肪干细胞通过Notch信号通路促进皮肤创伤愈合的初步研究[J].口腔生物医学,2022,13(1):9-14. [30] 宋承骏,应乐园,马柏强.姜黄素对大鼠皮肤创面愈合及血管新生的影响和作用机制[J].中国医师杂志,2023,25(2):226-231. |
| [1] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [2] | Dong Meilin, Du Haiyu, Liu Yuan. Quercetin-loaded carboxymethyl chitosan hydrogel promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 692-699. |
| [3] | Yan Rui, Wang Yiyu, Liu Xue, Jiang Yourong, Cheng Huanzhi, Ma Zhe. Application of exosome-loaded hydrogel in nerve injury regeneration and wound healing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7439-7446. |
| [4] | Zuo Chaoqi, Zhang Zhiqiang, Cao Nan, Guo Xuan, Xie Kai, Wang Haixia, Zhang Guangliang. Application of concentrated growth factor in treatment of chronic wounds [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6971-6978. |
| [5] | Li Meiyun, Liu Sen, Chen Kaiyuan, Shi Ling, Song Meichen, Cao Jiahong, Wu Yan, Yu Jing. MXene nanoparticles Ti3C2Tx and photothermal effect promote wound healing in diabetic mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6052-6060. |
| [6] | Cao Dayong, Zheng Junjie, Wang Lei, Yang Yang, Guo Haina, Xing Peipeng, Xia Chengde, Di Haiping. Autologous scalp repair of wounds in the medium-thickness skin donor area: safety and effectiveness [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3804-3810. |
| [7] | He Rui, Li Chongyi, Wang Ruiyao, Zeng Dan, Fan Daidi. Application of MXene-based hydrogels in wound repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3486-3493. |
| [8] | Lin Li, Jiao Linxi, Yu Fangning, Ma Yichao, Zhang Bo, Xu Xuying. Preclinical study of platelet-rich plasma combined with adipose stem cell transplantation in accelerating wound healing: a systematic evaluation and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2753-2763. |
| [9] | Long Chenyan, Cheng Biao, Tian Ju. Cellular and molecular mechanisms of platelet-rich plasma in promoting wound healing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2793-2801. |
| [10] | Wu Zhixin, Jiang Wenwen, Zhan Jianhui, Li Yangshurun, Ren Wenyan, Wang Yiyu. Hydrogels: role and problems in the repair of oral and maxillofacial defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2178-2188. |
| [11] | Chen Zan, Lei Fei, Ye Fei, Zhou Qingzhong, Yuan Hao, Zheng Lipeng, Zha Xian, Feng Daxiong. Relationship between drainage time and early efficacy after short-segment lumbar fusion [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 927-933. |
| [12] | Zhang Ya, Mu Qiuju, Wang Zilin, Liu Hongjie, Zhu Lili. Hydrogel loaded with platelet-rich plasma promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 690-696. |
| [13] | Xing Hao, Meng Qingfeng, Chang Zhengqi. Mechanism of negative pressure wound therapy in the auxiliary treatment of bone and soft tissue infection [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 621-626. |
| [14] | Liu Chunli, Yan Yujuan, Mo Liwen, Wu Zhijie, Zhang Li. Puerarin inhibits the differentiation of Raw264.7 cells into osteoclasts through the Notch signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5636-5641. |
| [15] | Tang Lulu, Pan Xiaojia, Lai Yingtao, Wang Li. Regulatory mechanism of ferroptosis on pressure ulcers: bioinformatics analysis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5656-5661. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||