Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (13): 2753-2763.doi: 10.12307/2025.041
Previous Articles Next Articles
Preclinical study of platelet-rich plasma combined with adipose stem cell transplantation in accelerating wound healing: a systematic evaluation and meta-analysis
Lin Li, Jiao Linxi, Yu Fangning, Ma Yichao, Zhang Bo, Xu Xuying
- Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing 100010, China
-
Received:2023-12-18Accepted:2024-03-10Online:2025-05-08Published:2024-09-11 -
Contact:Xu Xuying, MD, Chief physician, Doctoral supervisor, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing 100010, China -
About author:Lin Li, MD, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing 100010, China -
Supported by:National Natural Science Foundation of China, No. 82174388 (to XXY); Beijing Hundred Million Talents Project Training Fund, No. 2019A30 (to XXY); The Fifth Batch of National Clinical Outstanding Talents of Traditional Chinese Medicine, No. [2021]271 (to XXY); Beijing Hospital Management Center “Peak” Talent Training Program, No. DFL20220801 (to XXY)
CLC Number:
Cite this article
Lin Li, Jiao Linxi, Yu Fangning, Ma Yichao, Zhang Bo, Xu Xuying. Preclinical study of platelet-rich plasma combined with adipose stem cell transplantation in accelerating wound healing: a systematic evaluation and meta-analysis[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2753-2763.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
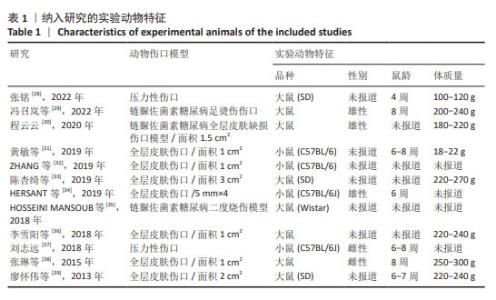
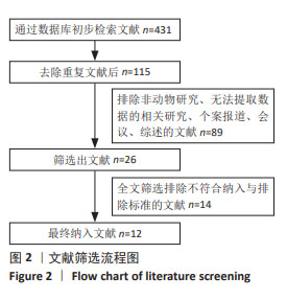
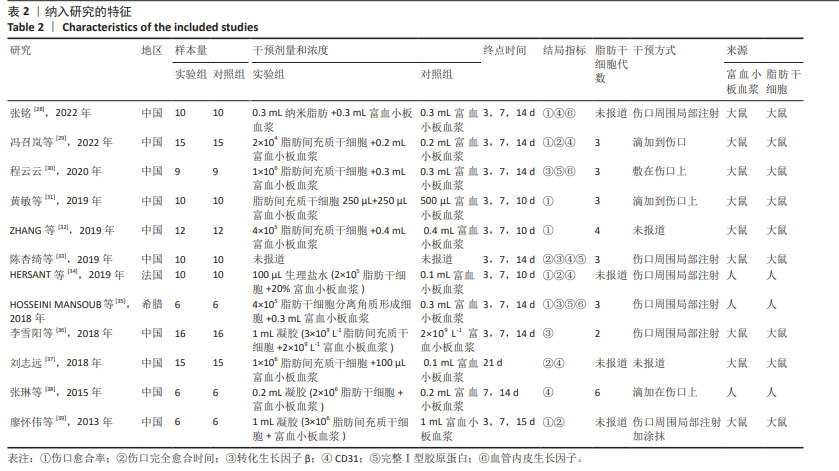
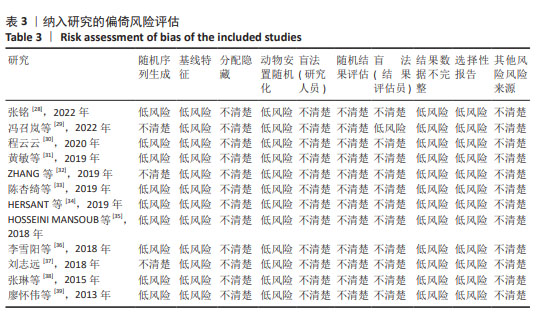
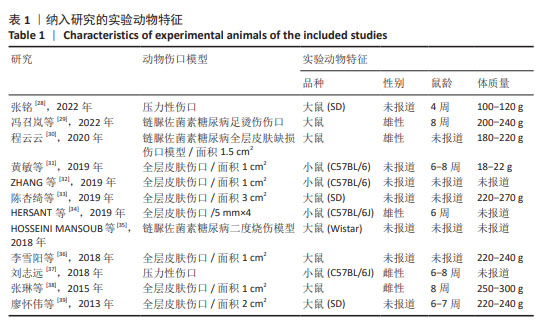
2.2 纳入研究的特征 见表1,2。 纳入的12项研究中除了1篇发表于2013年、1篇发表于2015年,其余均发表于2018年后;其中1项于法国进行,1项于希腊进行,其余10项均在中国进行。除去每个实验中的空白对照组,总样本量为250只动物,其中125只接受富血小板血浆联合脂肪干细胞移植,其余仅接受富血小板血浆治疗。在所有研究中,3项研究中富血小板血浆和脂肪干细胞来源于人类[34-35,38],其余来源于小鼠。12项研究中的8项为大鼠模型[28-30,33,35-36,38-39],4项为小鼠模型[31-32,34,37]。3项研究中实验动物的性别为雄性[29-30,34],2项研究为雌性[37-38],其余未报告实验动物的具体性别。所有研究中有2项为压力损伤模型[28,37],3项为糖尿病伤口模型[29-30,35],其余为全层皮肤伤口。"
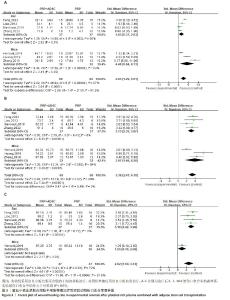
2.4 Meta分析结果 2.4.1 伤口愈合情况 伤口愈合率分析:7项研究进行了第3,7,10天以上的伤口愈合率对比[28-29,31-32,34-35,39],治疗后第3天,对纳入的7项研究进行异质性检验,结果显示异质性明显(P < 0.000 01,I2=87%),采用随机效应模型合并效应量,结果显示实验组伤口愈合率大于对照组[SMD=2.65,95%CI(1.29,4.01),Z=3.81,P=0.000 1],见图3A。亚组分析对4项大鼠研究进行异质性检验[28-29,35,39],结果显示异质性明显(P=0.002,I2=80%),采用随机效应模型合并效应量,结果显示实验组伤口愈合率大于对照组[SMD=1.65,95%CI(0.37,2.93),Z=2.52,P=0.01]。对3项小鼠研究进行异质性检验[31-32,34],结果显示异质性明显(P < 0.000 01,I2=93%),采用随机效应模型合并效应量,结果显示实验组伤口愈合率大于对照组[SMD=4.48,95%CI(0.84,8.13),Z=2.41,P=0.02]。使用Stata 15.0软件进行敏感性分析后,剔除任一文献置信区间并无明显改变,提示研究结果比较稳定。 治疗后第7天,对7项研究进行异质性检验[28-29,31-32,34-35,39],结果显示异质性明显(P=0.02,I2=60%),采用随机效应模型合并效应量,结果显示实验组伤口愈合率大于对照组[SMD=3.38,95%CI(2.47,4.30),Z=7.24,P < 0.000 01],见图3B。亚组分析对4项大鼠研究进行异质性检验[28-29,35,39],结果显示无明显异质性(P=0.43,I2=0%),采用随机效应模型(虽然大鼠研究异质性较小,但是依据所有研究绘制的森林图,I2=60%,故采用了随机效应模型)合并效应量,结果显示实验组伤口愈合率大于对照组[SMD=3.13,95%CI(2.39,3.87),Z=8.33,P < 0.000 01]。对纳入的3项小鼠研究进行异质性检验[31-32,34], 结果显示异质性明显(P=0.002,I2=84%),采用随机效应模型合并效应量,结果显示伤口愈合率大于对照组[SMD=3.97,95%CI(1.68,6.26),Z=3.40,P=0.000 7]。使用Stata 15.0软件进行敏感性分析后,剔除任一文献置信区间并无明显改变,提示研究结果比较稳定。 治疗10 d后,对5项研究进行异质性检验[28-29,34-35,39],结果显示异质性明显(P=0.01,I2=70%),采用随机效应模型合并效应量,结果显示实验组伤口愈合率大于对照组[SMD=2.62,95%CI(1.50,3.73),Z=4.61,P < 0.000 01],见图3C。亚组分析对4项大鼠研究进行异质性检验[28-29,35,39],结果显示无明显异质性(P=0.72,I2=0%),采用随机效应模型合并效应量(虽然大鼠研究异质性较小,但是依据所有研究绘制的森林图,I2=70%,故采用了随机效应模型),结果显示实验组伤口愈合率大于对照组[SMD=3.16,95%CI(2.42,3.90),Z=8.41,P < 0.000 01]。 仅1项小鼠研究报道了10 d后的伤口愈合率[34],使用Stata 15.0软件进行敏感性分析后,剔除任一文献置信区间并无明显改变,提示研究结果比较稳定。"
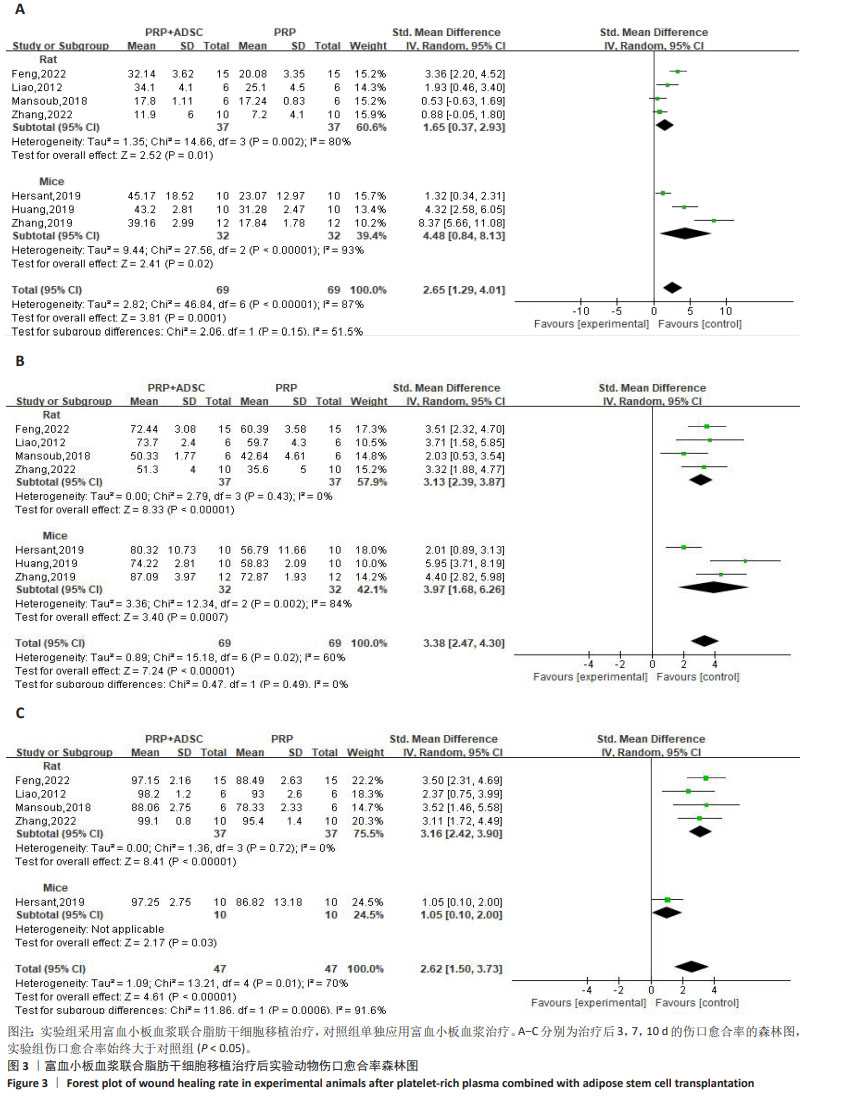

伤口完全愈合时间:对2项小鼠研究[34,37]、3项大鼠研究进行异质性分析[29,33,39],由于异质性高(P < 0.000 01,I2=87%),采用随机效应模型合并效应量,结果显示实验组伤口完全愈合时间短于对照组[SMD=-2.12,95%CI(-3.50,-0.74),P=0.003]。敏感性分析表明高异质性源于刘志远[37]的研究,排除后异质性水平下降(P=0.40,I2=0%),见图4A。 对实验动物种属进行亚组分析,对纳入的3项大鼠研究进行异质性检验,结果显示异质性不明显(P=0.27,I2=24%),但由于5项研究整体异质性较高,故采用随机效应模型合并效应量,结果显示实验组伤口完全愈合时间短于对照组[SMD=-1.16,95%CI (-1.81,-0.52),Z=3.52,P=0.000 4],见图4B。对纳入的2项小鼠研究进行异质性检验,结果显示异质性明显(P < 0.000 01,I2=95%),采用随机效应模型合并效应量,结果显示两组伤口完全愈合时间无明显差异[SMD=-4.05,95%CI(-9.18,1.07),Z=1.55,P=0.12],见图4B。但由于数据量少,需要更多的研究来得出结论。"

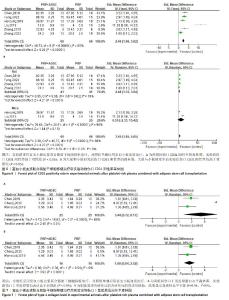
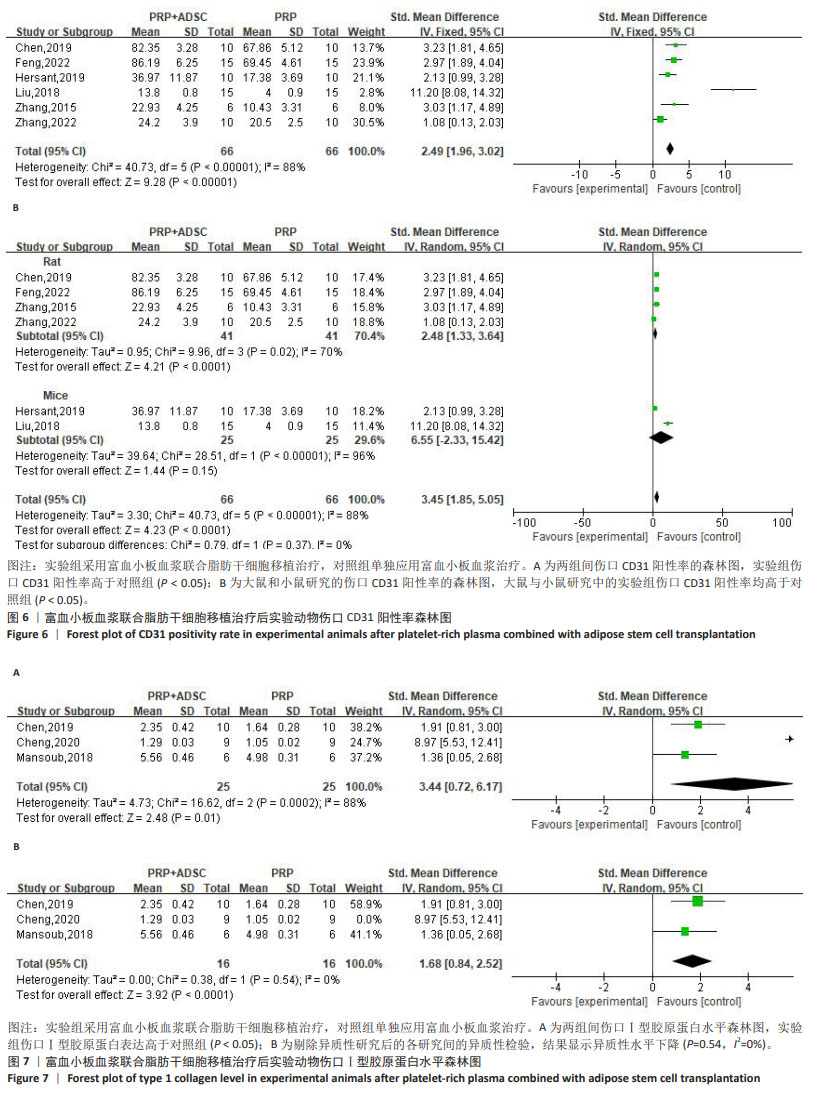
2.4.2 伤口转化生长因子β表达 对4项大鼠研究进行异质性检验[30,33,35-36],结果显示异质性明显(P < 0.000 01,I2=94%),采用随机效应模型合并效应量,结果显示实验组伤口转化生长因子β表达高于对照组[SMD=5.65,95%CI(1.22,10.08),Z=2.50,P=0.01],见图5。使用Stata 15.0软件进行敏感性分析后,剔除任一文献置信区间并无明显改变,提示研究结果比较稳定。 2.4.3 伤口CD31阳性率 对4项大鼠研究[28-29,33,38]、2项小鼠研究进行异质性检验[34,37],结果显示异质性明显(P < 0.000 01,I2=88%),采用随机效应模型合并效应量,结果显示实验组伤口CD31阳性率高于对照组[SMD=2.49,95%CI(1.96,3.02),Z=9.28,P < 0.000 01],见图6A。 对实验动物种属进行亚组分析,纳入的4项大鼠研究异质性明显(P=0.02,I2=70%),采用随机效应模型合并效应量,结果显示实验组伤口CD31阳性率高于对照组[SMD=2.48,95%CI(1.33,3.64),Z=4.21,P < 0.000 1],见图6B。2项小鼠研究的异质性检验明显(P < 0.000 01,I2=96%),采用随机效应模型合并效应量,结果显示实验组伤口CD31阳性率高于对照组[SMD=6.55,95%CI(-2.33,15.42),Z=1.44,P=0.15],见图6B。但纳入研究均存在较大异质性,在亚组分析后仍有显著异质性,使用Stata 15.0软件进行敏感性分析后,剔除任一文献置信区间并无明显改变,提示研究结果比较稳定。 2.4.4 伤口Ⅰ型胶原蛋白表达 对3项大鼠研究进行异质性检验[30,33,35],结果显示异质性明显(P=0.000 2,I2=88%),采用随机效应模型合并效应量,结果显示实验组伤口Ⅰ型胶原蛋白表达高于对照组[SMD=3.44,95%CI(0.72,6.17),Z=2.48,P=0.01], 见图7A。使用Stata 15.0软件进行敏感性分析后,发现高异质性来源于程云云[30]的研究,排除异质性研究后各研究间的异质性水平下降(P=0.54,I2=0%),见图7B,但由于数据量少,需要更多的研究来得出结论。"

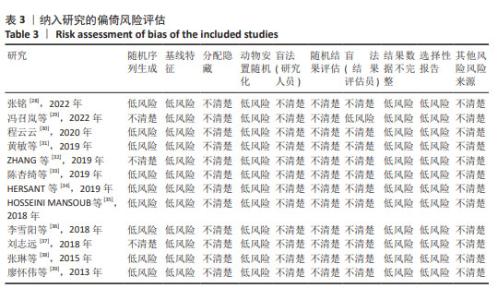
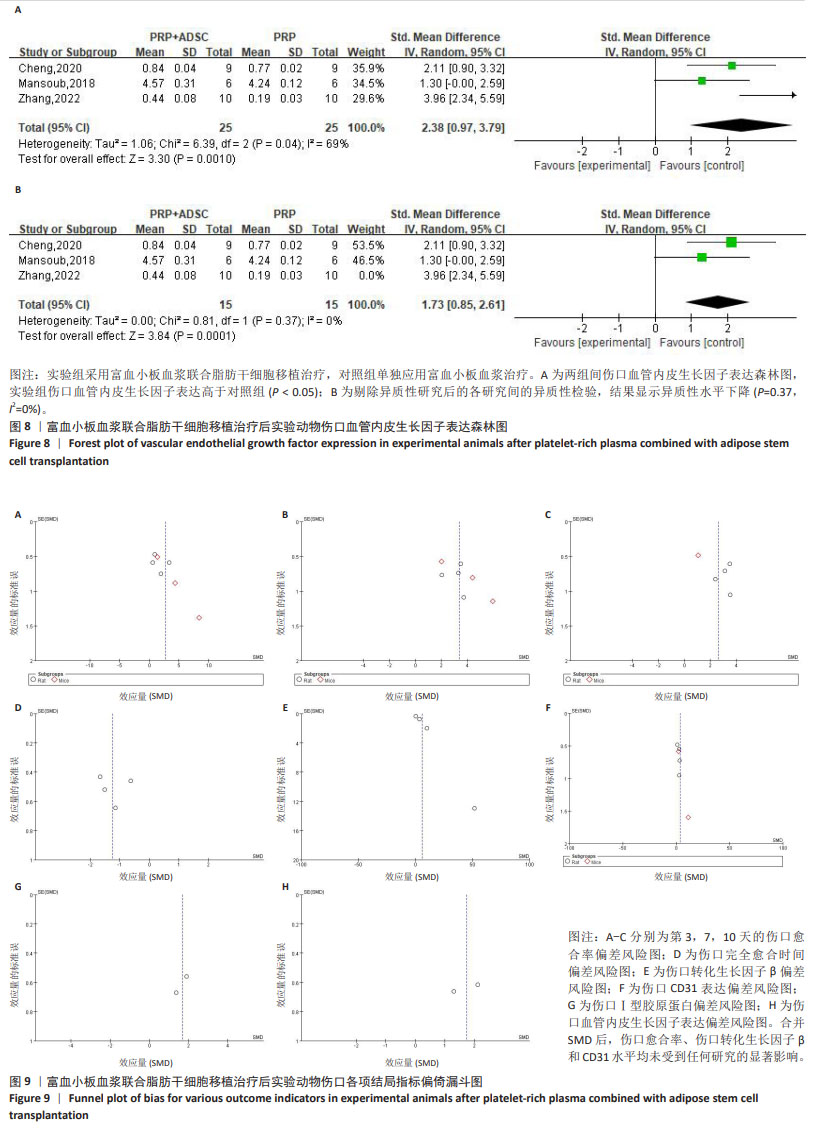
2.4.5 伤口血管内皮生长因子表达 对3项大鼠研究进行异质性检验[28,30,35],结果显示异质性明显(P=0.04,I2=69%),采用随机效应模型合并效应量,结果显示实验组伤口血管内皮生长因子表达高于对照组[SMD=2.38,95%CI(0.97,3.79),Z=3.30,P=0.001 0],见图8A。使用Stata 15.0软件进行敏感性分析后,发现高异质性来源于张铭[28]的研究,排除异质性研究后异质性水平下降(P=0.37,I2=0%),见图8B所示,但由于数据量少,需要更多的研究来得出结论。 2.5 偏倚风险分析 为了评估结果的稳定性,依据每项结局指标对每项研究进行了敏感性分析,合并SMD后,伤口愈合率、伤口转化生长因子β和CD31水平均未受到任何研究的显著影响,见图9。"
| [1] GRAVES N, PHILLIPS CJ, HARDING K. A narrative review of the epidemiology and economics of chronic wounds. Br J Dermatol. 2022;187(2):141-148. [2] AL ABOUD AM, MANNA B. Wound Pressure Injury Management. 2020 Jun 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2023. [3] TEJADA S, BATLE JM, FERRER MD, et al. Therapeutic Effects of Hyperbaric Oxygen in the Process of Wound Healing. Curr Pharm Des. 2019;25(15):1682-1693. [4] ZHAO R, LIANG H, CLARKE E, et al. Inflammation in Chronic Wounds. Int J Mol Sci. 2016;17(12):2085. [5] SUN H, PULAKAT L, ANDERSON DW. Challenges and New Therapeutic Approaches in the Management of Chronic Wounds. Curr Drug Targets. 2020;21(12):1264-1275. [6] DUSCHER D, BARRERA J, WONG VW, et al. Stem Cells in Wound Healing: The Future of Regenerative Medicine? A Mini-Review. Gerontology. 2016;62(2):216-225. [7] BRAZIL JC, QUIROS M, NUSRAT A, et al. Innate immune cell-epithelial crosstalk during wound repair. J Clin Invest. 2019; 129(8):2983-2993. [8] SHOJAEI F, RAHMATI S, BANITALEBI DM. A review on different methods to increase the efficiency of mesenchymal stem cell-based wound therapy. Wound Repair Regen. 2019; 27(6):661-671. [9] MARANDA EL, RODRIGUEZ-MENOCAL L, BADIAVAS EV. Role of Mesenchymal Stem Cells in Dermal Repair in Burns and Diabetic Wounds. Curr Stem Cell Res Ther. 2017;12(1):61-70. [10] KIM WS, PARK BS, SUNG JH. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009;9(7):879-887. [11] ROUSSELLE P, MONTMASSON M, GARNIER C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019;75-76:12-26. [12] MA T, FU B, YANG X, et al. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120(6):10847-10854. [13] ZHANG Y, BAI X, SHEN K, et al. Exosomes Derived from Adipose Mesenchymal Stem Cells Promote Diabetic Chronic Wound Healing through SIRT3/SOD2. Cells. 2022; 11(16):2568. [14] HASSAN WU, GREISER U, WANG W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22(3): 313-325. [15] HU N, CAI Z, JIANG X, et al. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023;157:175-186. [16] OUYANG L, QIU D, FU X, et al. Overexpressing HPGDS in adipose-derived mesenchymal stem cells reduces inflammatory state and improves wound healing in type 2 diabetic mice. Stem Cell Res Ther. 2022;13(1):395. [17] NOLAN GS, SMITH OJ, JELL G, et al. Fat grafting and platelet-rich plasma in wound healing: a review of histology from animal studies. Adipocyte. 2021;10(1):80-90. [18] JAMES IB, COLEMAN SR, RUBIN JP. Fat, Stem Cells, and Platelet-Rich Plasma. Clin Plast Surg. 2016;43(3):473-488. [19] KUFFLER DP. Platelet-Rich Plasma Promotes Axon Regeneration, Wound Healing, and Pain Reduction: Fact or Fiction. Mol Neurobiol. 2015;52(2):990-1014. [20] REN ZQ, DU B, DONG HJ, et al. Autologous Platelet-Rich Plasma Repairs Burn Wound and Reduces Burn Pain in Rats. J Burn Care Res. 2022;43(1):263-268. [21] KOBAYASHI E, FUJIOKA-KOBAYASHI M, SCULEAN A, et al. Effects of platelet rich plasma (PRP) on human gingival fibroblast, osteoblast and periodontal ligament cell behaviour. BMC Oral Health. 2017;17(1):91. [22] LUBKOWSKA A, DOLEGOWSKA B, BANFI G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 Suppl 1): 3S-22S. [23] NAKAMURA S, ISHIHARA M, TAKIKAWA M, et al. Platelet-rich plasma (PRP) promotes survival of fat-grafts in rats. Ann Plast Surg. 2010;65(1):101-106. [24] BLUMENSCHEIN AR, FREITAS-JUNIOR R, MOREIRA MA, et al. Is the combination of fat grafts and platelet rich plasma effective in rats? Acta Cir Bras. 2016;31(10): 668-674. [25] SMITH OJ, LEIGH R, KANAPATHY M, et al. Fat grafting and platelet-rich plasma for the treatment of diabetic foot ulcers: A feasibility-randomised controlled trial. Int Wound J. 2020;17(6):1578-1594. [26] RANGASWAMY M. Regenerative Wound Healing by Open Grafting of Autologous Fat and PRP-Gel - A New Concept and Potential Alternative to Flaps. Plast Reconstr Surg Glob Open. 2021;9(1):e3349. [27] HOOIJMANS CR, ROVERS MM, DE VRIES RB, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [28] 张铭.纳米脂肪联合富血小板血浆修复压力性损伤创面的基础研究[D].潍坊:潍坊医学院,2022. [29] 冯召岚,盛健健,董爱武,等.自体富血小板凝胶联合脂肪干细胞促进糖尿病足大鼠创面修复的机制研究[J].中国医学创新,2022,19(23):24-30. [30] 程云云.ADSCs-PRP对糖尿病大鼠创面愈合的影响及机制研究[D].沈阳:中国医科大学,2020. [31] 黄敏,颜洪.脂肪间充质干细胞条件培养液联合富血小板纤维蛋白修复小鼠皮肤损伤[J].中国组织工程研究,2019, 23(1):18-23. [32] ZHANG L, ZHANG B, LIAO B, et al. Platelet-rich plasma in combination with adipose-derived stem cells promotes skin wound healing through activating Rho GTPase-mediated signaling pathway. Am J Transl Res. 2019;11(7):4100-4112. [33] 陈杏绮,罗志军,殷安柱,等.同种异体脂肪干细胞联合自体富血小板血浆凝胶修复大鼠皮肤软组织创面的临床效果[J].中国药物经济学,2019,14(10):84-87+93. [34] HERSANT B, SID-AHMED M, BRAUD L, et al. Platelet-Rich Plasma Improves the Wound Healing Potential of Mesenchymal Stem Cells through Paracrine and Metabolism Alterations. Stem Cells Int. 2019;2019:1234263. [35] HOSSEINI MANSOUB N, GÜRDAL M, KARADADAŞ E, et al. The role of PRP and adipose tissue-derived keratinocytes on burn wound healing in diabetic rats. Bioimpacts. 2018;8(1):5-12. [36] 李雪阳,杨超,沈才齐,等.TGFβ1参与的脂肪干细胞-富血小板血浆凝胶促进大鼠创面修复的机制研究[J].临床和实验医学杂志,2018,17(22):2385-2389. [37] 刘志远.富血小板血浆负载人脂肪来源干细胞促进小鼠压疮愈合的实验研究[D].遵义:遵义医学院,2018. [38] 张琳,富泽龙,冯锐.人脂肪干细胞复合富血小板血浆治疗裸鼠放射性皮肤损伤的实验研究[J].医学研究杂志,2015, 44(7):57-61. [39] 廖怀伟,韩超,刘丽忠,等.富血小板血浆凝胶对脂肪干细胞修复皮肤软组织缺损创面的影响[J].重庆医学,2013,42(32): 3859-3862. [40] 蒋宇江,黄志群,唐强.烧伤残余创面发病机制及治疗研究进展[J].中国烧伤创疡杂志,2023,35(6):439-442+455. [41] ZHANG L, HU C, XU W, et al. Advances in wound repair and regeneration: Systematic comparison of cell free fat extract and platelet rich plasma. Front Chem. 2022;10: 1089277. [42] VINISH M, CUI W, STAFFORD E, et al. Dendritic cells modulate burn wound healing by enhancing early proliferation. Wound Repair Regen. 2016;24(1):6-13. [43] LIU W, WANG DR, CAO YL. TGF-beta: a fibrotic factor in wound scarring and a potential target for anti-scarring gene therapy. Curr Gene Ther. 2004;4(1): 123-136. [44] DOUGLAS HE. TGF-ß in wound healing: a review. J Wound Care. 2010;19(9): 403-406. [45] WEBER CE, LI NY, WAI PY, et al. Epithelial-mesenchymal transition, TGF-β, and osteopontin in wound healing and tissue remodeling after injury. J Burn Care Res. 2012;33(3):311-318. [46] BRANTON MH, KOPP JB. TGF-beta and fibrosis. Microbes Infect. 1999;1(15): 1349-1365. [47] DELISSER HM, NEWMAN PJ, ALBELDA SM. Platelet endothelial cell adhesion molecule (CD31). Curr Top Microbiol Immunol. 1993;184:37-45. [48] LIU L, ZHENG CX, ZHAO N, et al. Mesenchymal Stem Cell Aggregation-Released Extracellular Vesicles Induce CD31(+) EMCN(+) Vessels in Skin Regeneration and Improve Diabetic Wound Healing. Adv Healthc Mater. 2023;12(20):e2300019. [49] KOLOKOLCHIKOVA EG, ZHIRKOVA EA, GOLOVATENKO-ABRAMOV PK, et al. Morphofunctional evaluation of the effect of collagen-1-based dressing on skin regeneration after burn trauma in mice of two genetic strains. Bull Exp Biol Med. 2010;149(1):154-160. [50] GAN L, FAGERHOLM P, PALMBLAD J. Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand. 2004; 82(5):557-563. [51] GOSWAMI AG, BASU S, HUDA F, et al. An appraisal of vascular endothelial growth factor (VEGF): the dynamic molecule of wound healing and its current clinical applications. Growth Factors. 2022;40(3-4): 73-88. [52] LI L, LIU H, XU C, et al. VEGF promotes endothelial progenitor cell differentiation and vascular repair through connexin 43. Stem Cell Res Ther. 2017;8(1):237. |
| [1] | Zhang Xinxin, Gao Ke, Xie Shidong, Tuo Haowen, Jing Feiyue, Liu Weiguo. Network meta-analysis of non-surgical treatments for foot and ankle ability and dynamic balance in patients with chronic ankle instability [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1931-1944. |
| [2] | Sun Yundi, Cheng Lulu, Wan Haili, Chang Ying, Xiong Wenjuan, Xia Yuan. Effect of neuromuscular exercise for knee osteoarthritis pain and function: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1945-1952. |
| [3] | Wang Yida, Liu Jun, Wang Xiaoling, Wang Liyan, Yang Chengru, Zhang Xuexiao. Effects of wearable electronic device-based interventions on physical activity and sedentary behavior in healthy adolescents: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1693-1704. |
| [4] | Zhang Zixian, Xu Youliang, Wu Shaokui, Wang Xiangying. Effects of blood flow restriction training combined with resistance training on muscle indicators in college athletes: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1705-1713. |
| [5] | Wang Juan, Wang Guanglan, Zuo Huiwu. Efficacy of exercise therapy in the treatment of anterior cruciate ligament reconstruction patients: #br# a network meta-analysis #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1714-1726. |
| [6] | Zheng Huakun, Yin Mingyue, Liu Qian. Effects of interval and continuous training on the quality of life in physically inactive adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1727-1740. |
| [7] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [8] | Dong Meilin, Du Haiyu, Liu Yuan. Quercetin-loaded carboxymethyl chitosan hydrogel promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 692-699. |
| [9] | Wang Zilin, Mu Qiuju, Liu Hongjie, Shen Yuxue, Zhu Lili. Protective effects of platelet-rich plasma hydrogel on oxidative damage in L929 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 771-779. |
| [10] | Li Zhe, Li Ping, Zhang Chao, Guo Guangling. A network meta-analysis of efficacy of mesenchymal stem cells from different sources in treatment of premature ovarian failure animal models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7898-7908. |
| [11] | Wang Feng, Cao Chunfeng, He Chao, Zhang Tao, Zhou Zixian, Zhu Fengchen. All-inside versus traditional techniques of anterior cruciate ligament reconstruction: meta-analysis of therapeutic efficacy and radiological outcomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7629-7638. |
| [12] | Tian Jinxin, Zhao Yuxin, Hu Tong, Cui Tiantian, Ma Lihong. Effects of different transcranial magnetic stimulation modes on refractory depression in adults: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7639-7648. |
| [13] | Yan Rui, Wang Yiyu, Liu Xue, Jiang Yourong, Cheng Huanzhi, Ma Zhe. Application of exosome-loaded hydrogel in nerve injury regeneration and wound healing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7439-7446. |
| [14] | Wang Kaigang, Hao Dongsheng, Ma Pei, Zhou Shuo, Li Ruimin. Comparison of efficacy of different biological scaffolds for pulp regeneration therapy in immature permanent teeth: a Bayesian network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7447-7460. |
| [15] | Que Meng, Gao Fengqing, He Yang, Ruan Changgeng. Patent technology analysis of platelet-rich plasma preparation devices [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7461-7469. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||