Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (25): 4075-4081.doi: 10.3969/j.issn.2095-4344.1783
Previous Articles Next Articles
Response of bone marrow mesenchymal stem cells to mechanical microenvironment: viewpoint, actuality, thinking and future
- First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China
-
Revised:2019-04-08Online:2019-09-08Published:2019-09-08 -
Contact:He Fei, MD, Chief physician, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China -
About author:Sun Bin, Master candidate, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China -
Supported by:the National Natural Science Foundation of China, No. 31460244 (to HF); the Applied Basic Research Project of Yunnan Provincial Science and Technology Plan, No. 2015FA002 (to HF)
CLC Number:
Cite this article
Sun Bin, Duan Hao, Zhong Zongyu, Liu Zheng, Li Xiaozhuang, Tang Zhihong, He Fei. Response of bone marrow mesenchymal stem cells to mechanical microenvironment: viewpoint, actuality, thinking and future[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(25): 4075-4081.
share this article

2.1 不同机械力对骨髓间充质干细胞调控的影响 近年来研究发现生物化学刺激和外部机械力学刺激均可影响骨髓间充质干细胞的增殖分化[5]。外部不同机械力学信号包括牵张力、压缩力、流体剪切力、静水压力等[6]。不同的机械力对骨髓间充质干细胞的分化方向有显著影响,研究发现机械力学刺激可能通过激活Wnt/β-连环蛋白通路,降低SOD1酶、DAC1蛋白的表达来诱导骨髓间充质干细胞成骨分化[7-9]。下面将综述骨髓间充质干细胞对不同外部力学机械信号的反应。 2.1.1 牵张力 牵张力是骨代谢过程中的一个重要的机械调节因素[10],其对骨髓间充质干细胞起到拉伸应力作用,可促进细胞成纤维化、成软骨化、成骨化相关基因的表达[11-13]。通过使用细胞应力加载系统给予脐带间充质干细胞循环单轴机械刺激(拉伸、松弛),在0%,5%,10%的应变下刺激10 d,成骨标记物骨钙蛋白、骨桥蛋白、Ⅰ型胶原等表达水平显著增加,验证了循环机械信号刺激会促进脐带间充质干细胞向成骨方向分化[14]。Khetan等[15]将骨髓间充质干细胞培养在含有基质金属蛋白酶可降解肽的共价交联的3D水凝胶中,模拟细胞所受牵引力环境,使用平行板压缩实验验证了骨髓间充质干细胞受到牵引力作用下产生成骨作用的模量变化。在骨髓间充质干细胞增殖过程中施加循环拉力,使内源性骨形成蛋白2、成骨相关基因、钙沉积的表达增加[13,16]。还有研究者使用光刻法和深度反应离子刻蚀法制作基底材料,然后用单轴拉伸应力单元系统以震级3%、10%和0.26 Hz频率给予兔源骨髓间充质干细胞循环张力,发现高张力组趋向成肌分化,低张力组趋向成骨分化[17]。最近国外一些最新研究也验证了通过对骨髓间充质干细胞施加机械拉伸力,可抑制NF-κB通路传导,而NF-κB传导通路对骨髓间充质干细胞成骨分化有抑制作用,因此机械拉力可诱导骨髓间充质干细胞向成骨方向分化[18]。另一相似研究中,对大鼠骨髓间充质干细胞给予每天2次10%延伸率、0.5 Hz频率循环拉伸刺激,在1,3,5,7 d分别检测成骨标记物的表达,然后通过干扰RNA敲低Cbfa1基因,成骨标记物表达出现下降,证明了牵张力刺激可能通过ERK1/2激活的Cbfa1信号通路促进大鼠骨髓间充质干细胞成骨分化[19]。Wu等[20]也发现IncRNA H19是人骨髓间充质干细胞张力诱导成骨分化中的一个关键启动子,机械张力可以提高H19的表达从而促进骨髓间充质干细胞向成骨分化,而干扰H19表达可使骨髓间充质干细胞成骨分化受到抑制。 2.1.2 压缩力 压缩力刺激已经被证明能够增强骨髓间充质干细胞向软骨方向分化。2004年Huang等[21]已经发现压缩力会影响转化生长因子β1的表达,进而影响骨髓间充质细胞成软骨分化。其他学者也验证了在没有外源性生长因子的诱导干预下,通过对其所受压缩力进行调控,可促进骨髓间充质干细胞中软骨形成基因表达上调,表明了压缩力是调控骨髓间充质干细胞分化的重要因素[22-23]。为确定动态压缩力对成软骨相关基因表达的影响,在0,7,14,21 d分别在动态压缩反应器中使用1 Hz的频率进行动态加载,并在不同时间段测定转化生长因子β3的变化,验证了压缩力可以增强软骨细胞外基质合成从而使骨髓间充质干细胞向软骨分化[24]。赵永亮等[25]将小鼠骨髓间充质干细胞在海藻酸钠凝胶中进行培养,并使用基底压缩加载系统给予正弦波、0.5 Hz、20 kPa的周期性压缩力干预,检测发现压缩力干预组成软骨基因表达增加,验证了周期性压缩力可促进骨髓间充质干细胞成软骨分化。最近一些研究也通过寻找细胞响应压力的方式,从而了解骨髓间充质干细胞自身对压力的响应机制。如Xiao等[26]给予骨髓间充质干细胞压力,证明了TRPM7可独立感知机械刺激信号,并调节控制Ca2+流入,从而促进人骨髓间充质干细胞成骨分化。Miysshita等[27]构建了具有机械压缩力仿生支架,在不同的细胞密度下分别使用17.0,19.6,22.0 kPa的压缩力和0.83 Hz频率刺激6,9,12 h,其中细胞密度为4.0×105在19.6 kPa加载9 h实验组分化程度最优,验证了机械压缩力可促进间充质干细胞向牙本质细胞分化。 2.1.3 流体剪切力 已知在骨骼中通过机械诱导可以调控干细胞分化,如Arnsdorf等[28]研究初步提示Rho依赖性收缩蛋白与流体剪切力可影响细胞的成骨分化。为系统研究流体剪切力和细胞分化之间的关系,Liu等[29]对接受流体剪切力的细胞形态变化进行了定量研究,使用1.2,1.6,1.9 Pa的流体剪切力刺激细胞1 h,结果发现1.2-1.9 Pa的流体剪切力对细胞成骨产生刺激,适宜的流体剪切力能够影响细胞形态的伸展。Stavenschi等[30]研究在0.5,1,2 Hz频率下,将间充质干细胞在封闭环境中分别给予1,2,5 Pa剪切力,持续1,2,4 h刺激,可以促进成骨标志物Cox2、Runx2和Opn的mRNA表达上调,确定了施加2 Pa和2 Hz频率组表达上调最明显。最近国外一些研究将流体剪切力和纳米形貌组合,发现可以更好地影响间充质干细胞黏附、增殖和迁移[31]。还有研究表明在不同形貌基质上通过控制流体剪切力可以改变细胞的收缩力从而影响间充质干细胞的分化[32]。 2.1.4 静水压力 静水压力也是间充质干细胞分化中的一种关键调节因子。很多研究表明静水压力可以促进间充质干细胞中软骨基因的表达[33]。Steward等[34]通过构建不同刚度水凝胶培养间充质干细胞,并给予1 Hz频率和10 MPa的静水压力,每天刺激4 h,持续21 d,发现间充质干细胞可以响应静水压激活刚性水凝胶中的机械转导途径,从而增强了软骨的形成。在琼脂糖和纤维蛋白的培养基中,周期性给予静水压降低了碱性磷酸酶的活性,表明静水压在维持软骨分化中起着关键作用[35]。Tang等[36]制作聚氨酯支架,使用可产生灌注和流体动力的反应器对其培养的细胞进行刺激,在60 mm Hg(7.98 kPa),0.5 Hz的静水压力下支架内的骨髓间充质干细胞成骨分化基因表达增加。最近一些研究中,对大鼠骨髓间充质干细胞给予持续性与周期性静水压,检测大鼠骨髓间充质干细胞中成软骨分化基因Sox9、Aggrecan和Col-Ⅱ表达上调,静水压可以促进大鼠骨髓间充质干细胞向软骨方向分化[37]。Stavenschi等[38]也发现在骨内低至10 kPa静水压力水平就可以驱动人骨髓间充质干细胞成骨分化,循环静水压在骨的力学微环境中起到重要作用。 2.2 基底刚度机械力学信号对间充质干细胞的影响 人体不同组织细胞所感应的刚度信号不同,例如脑组织或骨组织所感受的刚度就有很大的差异,在不同的组织刚度培养基底上培养间充质干细胞,细胞形态学表现、蛋白表达方面均与组织培养基相对应[39]。国内学者也通过构建神经组织、软骨组织、骨组织等类似弹性模量的聚丙烯酸酰胺基底培养大鼠骨髓间充质干细胞,结果显示在0.5 kPa基底上神经分化的相关蛋白如巢蛋白、微管蛋白等表达增多,在10 kPa基底上软骨分化标记物COL-Ⅱ表达上调,但40 kPa基底上成骨标记物未出现明显差异,说明在低硬度基底上更趋向神经和软骨分化,在高硬度基底上成骨分化趋势不明显[40]。作者所在课题组前期在不同刚度的聚二甲基硅氧烷基底上培养大鼠骨髓间充质干细胞,也验证了在较硬的基底上细胞面积大,细胞骨架排列紧密,较软基底上细胞呈圆球形且胞内骨架蛋白较少[41]。另一项相似的实验中,在较软的基质(1 kPa)上接种间充质干细胞更趋向于成脂、成软骨分化,但在较硬的基质(15 kPa)上表现出向成骨和成肌分化[42]。Parekh等[43]也发现随着基底刚度从0.2-59 kPa变化,骨髓间充质干细胞的成骨分化能力会随之增强。国外一些研究也证实了基底刚度和骨髓间充质干细胞分化间的关系。Burke等[44]通过建立血管生成的晶格模型,预测了基质刚度与可用性氧对间充质干细胞分化发挥了调节作用。Srinivasan等[45]发现外胚层间充质干细胞在较低刚度底物上通过Rho-ROCK信号依赖性方式增加其标记物CD44的表达,CD44的高表达导致外胚层间充质干细胞向脂肪和软骨方向分化潜能增加,但如果换成刚度较高的底物时其向脂肪、软骨分化的效果会减弱。 间充质干细胞能感受不同模式的基底刚度从而直接或间接调控自身增殖分化,见表1。有学者制作了可调控刚度的圆柱形聚乙烯醇水凝胶进行人骨髓间充质干细胞体外培养,发现在低刚度时呈现神经分化,高刚度时呈现成骨分化[46],见图2。国外一些最新的研究通过构建可控制刚度的不同基底来探寻与间充质干细胞分化之间的关系,如Wang等[47]通过建立不同梯度刚度的Ti支架系统培养间充质干细胞,细胞的黏附、生长、活性等有明显差异。还有研究构建了具有热响应的弹性纳米杂化支架,通过不同温度条件控制支架的刚度,支架刚度通过松弛和软化的改变验证了人骨髓间充质干细胞对刚度的响应,从而影响其成骨和成软骨的分化作用[48]。Zhao等[49]制作了硫醇化明胶(Gtn-SH)和硫醇化透明质酸(HA-SH)化学修饰的高度生物相容性水凝胶,通过聚乙二醇四丙烯酸酯交联密度控制水凝胶的刚度,在不同刚度水凝胶上培养人骨髓间充质干细胞,证明了人骨髓间充质干细胞倾向其内微环境相似刚度的方向分化。Chen等[50]建立了一种可以进行震荡灌注的3D支架,并通过该装置来研究基质硬度对骨髓间充质干细胞存活、分布和成骨分化的影响,发现震荡灌注与基质硬度在合适的参数范围内可协同促进骨髓间充质干细胞成骨分化。"
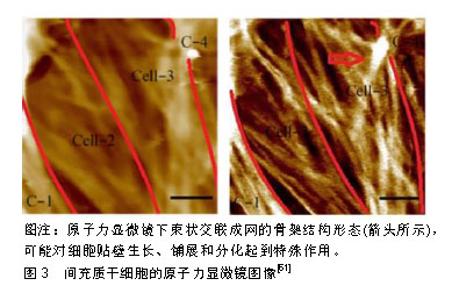
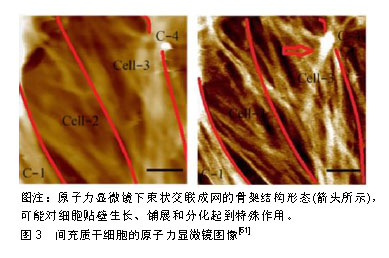
基底刚度虽然会影响间充质干细胞分化,但并不是基底刚度越大其分化效果越好,还要符合骨髓间充质干细胞生物学特性,才会使其向所需求方向分化。国内学者穆月等[51]研究发现通过构建不同刚度聚二甲基硅氧烷基底体外培养大鼠骨髓间充质干细胞,弹性模量(354.1±40.9) kPa时成骨标记物达到峰值,并发现弹性模量过高、过低并不是成骨分化的最适环境。作者所在课题组也将大鼠骨髓间充质干细胞接种到1,4,10,40,80 kPa聚丙烯酰胺水凝胶中,检测不同时间段骨钙素、成骨相关基因的变化,发现在10-40 kPa的刚度区间时,成骨基因表达与刚度正相关,而过高过低的刚度导致其成骨分化趋势不明显[52]。最近有国外学者制作了具有宽广范围刚度(20-200 kPa)梯度的圆柱形聚乙烯醇/透明质酸水凝胶基底并培养人骨髓间充质干细胞,观察到人骨髓间充质干细胞在20 kPa基底利于向神经分化,在40 kPa基底利于向肌肉细胞分化,在80 kPa基底利于向软骨细胞分化,在190 kPa基底利于向成骨细胞分化[53]。另一项研究中通过增加基质硬度可以使骨髓间充质干细胞向成骨分化,然而过高的刚度会对骨髓间充质干细胞成骨产生负面的影响[54]。为了更加明确找出基底刚度和间充质干细胞间的关系,Lee等[55]建立一种光可切换交联剂的水凝胶,通过适当波长光的照射可以可逆改变它们的刚度,为研究细胞响应刚度动态变化的机械信号提供了一个平台。 2.3 细胞组分响应机械力学信号的方式及其影响 细胞组分参与和反馈机械力学信号转导也是研究细胞感受力学微环境的重要方面,细胞感受外力信号传导至细胞核,从而影响基因的表达和蛋白质的合成,反馈到外部细胞的基质,很多种细胞成分在信号传导中起到重要的作用,如自身的形状、细胞骨架、整合素、黏着斑、离子通道等方面参与机械力学信号转导。 McBeath等[56]发现细胞形状会对间充质干细胞分化产生影响,细胞在微粒化的培养板上培养,来明确细胞形状对间充质干细胞分化产生的影响,在圆形培养岛上细胞更偏向于脂肪分化,而在分散形培养岛上细胞偏向于成骨分化。黏着斑也会将细胞骨架连接于细胞外基质并为细胞内部的机械传导与形貌特征提供物理联系。许多研究表明,形貌特征会减少细胞的扩散而种植在基底的细胞则会生成更多的黏着斑[57]。Loye等[58]在200 nm大小的不同图案上培养间充质干细胞可促进其向成脂、成骨方向分化,并认为成骨性增加的差异与黏着斑的形成增加有关。Gao等[59]在聚二甲基硅氧烷上使用光刻法制作微观形貌并培养间充质干细胞,当细胞扩散时转化生长因子β3激活了rac1并增加了黏钙蛋白的表达,使成肌基因表达上调,当细胞扩散受到抑制时转化生长因子β3未能激活rac1和未能增加黏钙蛋白表达,从而成软骨基因表达增加,细胞形状是推动间充质干细胞分化的重要微环境条件。 细胞骨架成分可能同样参与细胞的机械信号传导,最新研究通过不同分层纳米孔/纳米线结构培养MC3T3-E1细胞,细胞的成骨性可能随细胞骨架张力的增加而增强[60]。作者所在课题组前期研究通过对骨髓间充质干细胞施加不同时长的应力刺激,应用原子力显微镜和荧光成像表征了细胞骨架,总结了细胞网状应力结构中的张力分布特点,为说明细胞骨架可以传导机械信号提供了物质基础[61],见图3。细胞骨架张力在细胞形状介导间充质干细胞分化中为重要的影响因素,有一种假说是当细胞扩散受限时,细胞骨架的张力降低,并且会促进成脂肪分化,细胞完全伸展时细胞骨架张力升高,促进成骨分化[62]。Pan等[63]通过分层级纳米结构指出了通过细胞骨架诱导和Yap通路介导可以调控骨髓间充质干细胞的成骨分化能力。但是关于细胞骨架对底物刚度的响应仍不是很明确,于是研究者通过3D培养骨髓间充质干细胞模型,发现其分化不依赖于ROCK、NMMII和肌动蛋白的聚合[43]。最近也有研究使用3D打印的机械生物反应器来进一步观察干细胞骨架所受微观力学的影响[64]。"
| [1]Petite H, Viateau V, Bensaïd W, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18(9):959-963.[2]Oryan A, Kamali A, Moshiri A, et al. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence. Cells Tissues Organs. 2017;204(2):59-83.[3]Liu YS, Lee OK. In search of the pivot point of mechanotransduction: mechanosensing of stem cells. Cell Transplant. 2014;23(1):1-11.[4]Yim EK, Sheetz MP. Force-dependent cell signaling in stem cell differentiation. Stem Cell Res Ther. 2012;3(5):41.[5]Steward AJ, Kelly DJ. Mechanical regulation of mesenchymal stem cell differentiation. J Anat. 2015;227(6):717-731.[6]Kelly DJ, Jacobs CR. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today. 2010;90(1):75-85.[7]Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606-615.[8]Tan J, Xu X, Tong Z, et al. Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis. Bone Res. 2015;3:15003.[9]Wang J, Wang CD, Zhang N, et al. Mechanical stimulation orchestrates the osteogenic differentiation of human bone marrow stromal cells by regulating HDAC1. Cell Death Dis. 2016;7:e2221.[10]Zeng Z, Yin X, Zhang X, et al. Cyclic stretch enhances bone morphogenetic protein-2-induced osteoblastic differentiation through the inhibition of Hey1. Int J Mol Med. 2015;36(5): 1273-1281.[11]Baker BM, Shah RP, Huang AH, et al. Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage. Tissue Eng Part A. 2011; 17(9-10):1445-1455.[12]Sumanasinghe RD, Bernacki SH, Loboa EG. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006;12(12):3459-3465.[13]Rui YF, Lui PP, Ni M, et al. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res. 2011;29(3):390-396.[14]Kang MN, Yoon HH, Seo YK, et al. Effect of mechanical stimulation on the differentiation of cord stem cells. Connect Tissue Res. 2012;53(2):149-159.[15]Khetan S, Guvendiren M, Legant WR, et al. Degradation- mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12(5): 458-465.[16]Hanson AD, Marvel SW, Bernacki SH, et al. Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann Biomed Eng. 2009;37(5):955-965.[17]Jang JY, Lee SW, Park SH, et al. Combined effects of surface morphology and mechanical straining magnitudes on the differentiation of mesenchymal stem cells without using biochemical reagents. J Biomed Biotechnol. 2011;2011:860652.[18]Chen X, Liu Y, Ding W, et al. Mechanical stretch-induced osteogenic differentiation of human jaw bone marrow mesenchymal stem cells (hJBMMSCs) via inhibition of the NF-κB pathway. Cell Death Dis. 2018;9(2):207.[19]Wu Y, Zhang X, Zhang P, et al. Intermittent traction stretch promotes the osteoblastic differentiation of bone mesenchymal stem cells by the ERK1/2-activated Cbfa1 pathway. Connect Tissue Res. 2012;53(6):451-459. [20]Wu J, Zhao J, Sun L, et al. Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. Bone. 2018;108:62-70.[21]Huang CY, Hagar KL, Frost LE, et al. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22(3): 313-323.[22]Kupcsik L, Stoddart MJ, Li Z, et al. Improving chondrogenesis: potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng Part A. 2010;16(6):1845-1855.[23]Li Z, Yao SJ, Alini M, et al. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A. 2010;16(2): 575-584.[24]Haugh MG, Meyer EG, Thorpe SD, et al. Temporal and spatial changes in cartilage-matrix-specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng Part A. 2011;17(23-24):3085-3093.[25]赵永亮,冯军宇,王刚,等. 周期性动态压力对海藻酸钠培养间充质干细胞成软骨分化的作用[J].中华关节外科杂志(电子版), 2016,10(4): 413-418.[26]Xiao E, Yang HQ, Gan YH, et al. Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells. 2015;33(2): 615-621.[27]Miyashita S, Ahmed NE, Murakami M, et al. Mechanical forces induce odontoblastic differentiation of mesenchymal stem cells on three-dimensional biomimetic scaffolds. J Tissue Eng Regen Med. 2017;11(2):434-446.[28]Arnsdorf EJ, Tummala P, Kwon RY, et al. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122(Pt 4):546-553.[29]Liu X, Zhang X, Lee I. A quantitative study on morphological responses of osteoblastic cells to fluid shear stress. Acta Biochim Biophys Sin (Shanghai). 2010;42(3):195-201.[30]Stavenschi E, Labour MN, Hoey DA. Oscillatory fluid flow induces the osteogenic lineage commitment of mesenchymal stem cells: The effect of shear stress magnitude, frequency, and duration. J Biomech. 2017;55:99-106.[31]Yang Y, Kulangara K, Sia J, et al. Engineering of a microfluidic cell culture platform embedded with nanoscale features. Lab Chip. 2011;11(9):1638-1646.[32]Sonam S, Sathe SR, Yim EK, et al. Cell contractility arising from topography and shear flow determines human mesenchymal stem cell fate. Sci Rep. 2016;6:20415.[33]Elder SH, Sanders SW, McCulley WR, et al. Chondrocyte response to cyclic hydrostatic pressure in alginate versus pellet culture. J Orthop Res. 2006;24(4):740-747.[34]Steward AJ, Kelly DJ, Wagner DR. Purinergic Signaling Regulates the Transforming Growth Factor-β3-Induced Chondrogenic Response of Mesenchymal Stem Cells to Hydrostatic Pressure. Tissue Eng Part A. 2016;22(11-12):831-839.[35]Steward AJ, Thorpe SD, Vinardell T, et al. Cell-matrix interactions regulate mesenchymal stem cell response to hydrostatic pressure. Acta Biomater. 2012;8(6):2153-2159.[36]Tang X, Teng S, Liu C, et al. Influence of hydrodynamic pressure on the proliferation and osteogenic differentiation of bone mesenchymal stromal cells seeded on polyurethane scaffolds. J Biomed Mater Res A. 2017;105(12):3445-3455.[37]易飞舟,赵萤,张旻.流体压力对骨髓间充质干细胞软骨向分化影响的体外实验研究[J].口腔医学,2016,36(6):481-484. [38]Stavenschi E, Corrigan MA, Johnson GP, et al. Physiological cyclic hydrostatic pressure induces osteogenic lineage commitment of human bone marrow stem cells: a systematic study. Stem Cell Res Ther. 2018;9(1):276.[39]Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677-689.[40]郭江龙,何静,吴方.不同硬度聚丙烯酰胺调控骨髓间充质干细胞的定向分化[J].中国组织工程研究,2018,22(25):3981-3986. [41]刘洋,韩东,华闻达,等.基底硬度与形貌协同对大鼠骨髓间充质干细胞成骨分化的影响[J].医用生物力学,2016,31(3):218-226.[42]Park JS, Chu JS, Tsou AD, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. 2011;32(16):3921-3930.[43]Parekh SH, Chatterjee K, Lin-Gibson S, et al. Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeletal tension. Biomaterials. 2011;32(9):2256-2264.[44]Burke DP, Khayyeri H, Kelly DJ. Substrate stiffness and oxygen availability as regulators of mesenchymal stem cell differentiation within a mechanically loaded bone chamber. Biomech Model Mechanobiol. 2015;14(1):93-105.[45]Srinivasan A, Chang SY, Zhang S, et al. Substrate stiffness modulates the multipotency of human neural crest derived ectomesenchymal stem cells via CD44 mediated PDGFR signaling. Biomaterials. 2018;167:153-167.[46]Kim TH, An DB, Oh SH, et al. Creating stiffness gradient polyvinyl alcohol hydrogel using a simple gradual freezing-thawing method to investigate stem cell differentiation behaviors. Biomaterials. 2015;40:51-60.[47]Wang H, Su K, Su L, et al. The effect of 3D-printed Ti6Al4V scaffolds with various macropore structures on osteointegration and osteogenesis: A biomechanical evaluation. J Mech Behav Biomed Mater. 2018;88:488-496.[48]Wu L, Magaz A, Wang T, et al. Data of a stiffness softening mechanism effect on proliferation and differentiation of a human bone marrow derived mesenchymal stem cell line towards the chondrogenic and osteogenic lineages. Data Brief. 2018;21: 133-142.[49]Zhao W, Li X, Liu X, et al. Effects of substrate stiffness on adipogenic and osteogenic differentiation of human mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2014;40:316-323.[50]Chen G, Xu R, Zhang C, et al. Responses of MSCs to 3D Scaffold Matrix Mechanical Properties under Oscillatory Perfusion Culture. ACS Appl Mater Interfaces. 2017;9(2):1207-1218.[51]穆月,赵继志,杨春,等.聚二甲基硅氧烷基底弹性对大鼠骨髓间充质干细胞骨向诱导分化的影响[J].中华口腔医学杂志, 2017,52(8): 492-498.[52]陈林,许明卿,刘洋,等.组织界面刚度变化对大鼠BMSCs成骨分化的影响[J].中国修复重建外科杂志,2016,30(12):1524-1531. [53]Oh SH, An DB, Kim TH, et al. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016;35:23-31.[54]He XT, Wu RX, Xu XY, et al. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 2018;71: 132-147.[55]Lee IN, Dobre O, Richards D, et al. Photoresponsive Hydrogels with Photoswitchable Mechanical Properties Allow Time-Resolved Analysis of Cellular Responses to Matrix Stiffening. ACS Appl Mater Interfaces. 2018;10(9):7765-7776.[56]McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483-495.[57]Biggs MJ, Richards RG, Gadegaard N, et al. The effects of nanoscale pits on primary human osteoblast adhesion formation and cellular spreading. J Mater Sci Mater Med. 2007;18(2): 399-404.[58]Loye AM, Kinser ER, Bensouda S, et al. Regulation of Mesenchymal Stem Cell Differentiation by Nanopatterning of Bulk Metallic Glass. Sci Rep. 2018;8(1):8758.[59]Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28(3):564-572.[60]Pan HH, Xie YT, Li K, et al. ROCK-regulated synergistic effect of macropore/nanowire topography on cytoskeletal distribution and cell differentiation. Rsc Advances.2015;123(5): 101834-101842.[61]华闻达,韩东,吴迪,等.骨髓间充质干细胞对不同时长应力刺激的力学响应[J].医用生物力学,2015,30(1):43-49.[62]McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483-495.[63]Pan H, Xie Y, Zhang Z, et al. YAP-mediated mechanotransduction regulates osteogenic and adipogenic differentiation of BMSCs on hierarchical structure. Colloids Surf B Biointerfaces. 2017;152: 344-353.[64]Raveling AR, Theodossiou SK, Schiele NR. A 3D printed mechanical bioreactor for investigating mechanobiology and soft tissue mechanics. MethodsX. 2018;5:924-932.[65]Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677-689. |
| [1] | Liu Zhichao, Zhang Fan, Sun Qi, Kang Xiaole, Yuan Qiaomei, Liu Genzhe, Chen Jiang. Morphology and activity of human nucleus pulposus cells under different hydrostatic pressures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1172-1176. |
| [2] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [3] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [4] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [5] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [6] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [7] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [8] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [9] | Ma Zetao, Zeng Hui, Wang Deli, Weng Jian, Feng Song. MicroRNA-138-5p regulates chondrocyte proliferation and autophagy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 674-678. |
| [10] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| [11] | Li Quanxi, Shen Yu, Wan Wei, Sun Shanzhi. Changes of abdominal wall mechanics and pain after tension-free inguinal hernia repair with polypropylene mesh [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 548-552. |
| [12] | Sun Jianwei, Yang Xinming, Zhang Ying. Effect of montelukast combined with bone marrow mesenchymal stem cell transplantation on spinal cord injury in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3962-3969. |
| [13] | Zhang Lishu, Liu Anqi, He Xiaoning, Jin Yan, Li Bei, Jin Fang. Alpl gene affects the therapeutic effect of bone marrow mesenchymal stem cells on ulcerative colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3970-3975. |
| [14] | Hao Xiaona, Zhang Yingjie, Li Yuyun, Xu Tao. Bone marrow mesenchymal stem cells overexpressing prolyl oligopeptidase on the repair of liver fibrosis in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3988-3993. |
| [15] | Jiang Tao, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Ma Chuang, Wei Qin. Platelet-derived growth factor BB induces bone marrow mesenchymal stem cells to differentiate into vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3937-3942. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||