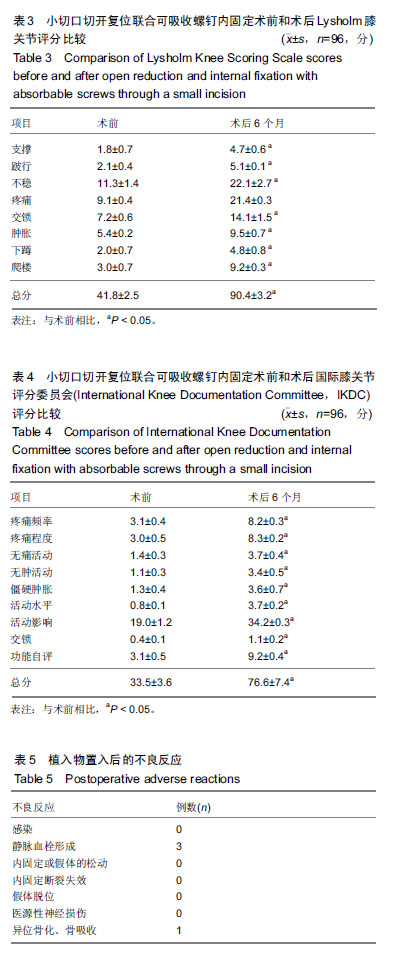
| [1]Albtoush OM, Horger M, Springer F, et al. Avulsion fracture of the medial collateral ligament association with Segond fracture. Clin Imaging. 2019;53:32-34. [2]Yao J, Wang H, Quan S, et al. Suture-bridge fixation under arthroscopy in treatment of tibial eminence avulsion fracture of anterior cruciate ligament in adolescents. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(11):1402-1405. [3]Lombardo-Torre M, Espejo-Reina A, García-Gutiérrez G, et al. Arthroscopic treatment of concurrent avulsion fracture of anterior and posterior cruciate ligament with suspension device. J Orthop Case Rep. 2018;8(2):81-85. [4]Strauss EJ, Kaplan DJ, Weinberg ME, et al. Arthroscopic management of tibial spine avulsion fractures: principles and techniques. J Am Acad Orthop Surg. 2018;26(10):360-367.[5]Liang X, Tian Y, Wang S, et al. Clinical efficacy of arthroscopic simultaneous treatment for anterior cruciate ligament injury combined with meniscus bucket-handle tear. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(5):547-552.[6]Hsieh YL, Yang CC. Early intervention of swimming exercises attenuate articular cartilage destruction in a rat model of anterior cruciate ligament and meniscus knee injuries. Life Sci. 2018;212: 267-274. [7]Park JY, Jeong BS, Roh YS, et al. Evaluation of an arthroscopic stifle lever for stifle joint distraction in toy breed dogs. J Vet Sci. 2018;19(5): 693-698.[8]Chmielnicki M, Siebert W, Prokop A. Foreign material-free anterior cruciate ligament repair with hamstring graft, felmet technique. Z Orthop Unfall. 2018;156(2):223-225.[9]Lombardo-Torre M, Espejo-Reina A, García-Gutiérrez G, et al. Arthroscopic treatment of concurrent avulsion fracture of anterior and posterior cruciate ligament with suspension device. J Orthop Case Rep. 2018;8(2):81-85. [10]Sekiya H, Takatoku K, Kimura A, et al. Arthroscopic fixation with EndoButton for tibial eminence fractures visualised through a proximal superomedial portal: a surgical technique. J Orthop Surg (Hong Kong). 2016;24(3):417-420.[11]Sueyoshi T, Emoto G, Yato T. Correlation between single assessment numerical evaluation score and lysholm score in primary total knee arthroplasty patients. Arthroplast Today. 2017; 4(1):99-102.[12]Kose O, Deniz G, Ozcan H, et al. A comparison of telephone interview versus on-site completion of Lysholm knee score in patients who underwent arthroscopic ACL reconstruction: are the results equivalent? Eur J Orthop Surg Traumatol. 2015;25(6): 1069-1072. [13]Ra HJ, Kim HS, Choi JY, et al. Comparison of the ceiling effect in the Lysholm score and the IKDC subjective score for assessing functional outcome after ACL reconstruction. Knee. 2014;21(5): 906-910. [14]Kümmel D, Preiss S, Harder LP, et al. Measurement properties of the German version of the IKDC subjective knee form (IKDC-SKF). J Patient Rep Outcomes. 2018;2:31. [15]Huang CC, Chen WS, Tsai MW, et al. Comparing the Chinese versions of two knee-specific questionnaires (IKDC and KOOS): reliability, validity, and responsiveness. Health Qual Life Outcomes. 2017;15(1):238. [16]Dietvorst M, Reijman M, van Groningen B, et al. PROMs in paediatric knee ligament injury: use the Pedi-IKDC and avoid using adult PROMs. Knee Surg Sports Traumatol Arthrosc. 2017. DOI: 10.1007/s00167-017-4687-3. [17]Tigerstrand Grevnerts H, Grävare Silbernagel K, Sonesson S, et al. Translation and testing of measurement properties of the Swedish version of the IKDC subjective knee form. Scand J Med Sci Sports. 2017;27(5):554-562. |