Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (21): 3439-3444.doi: 10.3969/j.issn.2095-4344.1724
Application and potential of stem cells in the repair of facial nerve injury
Xiao Guangqing1, Lun Zhigang1, Chen Huizhen2, Li Aimin2
- 1Graduate School of Xuzhou Medical University, Xuzhou 221004, Jiangsu Province, China; 2Lianyungang Hospital Affiliated to Xuzhou Medical University, Lianyungang 222002, Jiangsu Province, China
-
Revised:2019-01-28Online:2019-07-28Published:2019-07-28 -
Contact:Li Aimin, MD, Chief physician, Professor, Lianyungang Hospital Affiliated to Xuzhou Medical University, Lianyungang 222002, Jiangsu Province, China -
About author:Xiao Guangqing, Master candidate, Graduate School of Xuzhou Medical University, Xuzhou 221004, Jiangsu Province, China -
Supported by:the Youth Talent Fund of Lianyungang First People’s Hospital, No. QN1702 (to CHZ)
CLC Number:
Cite this article
Xiao Guangqing, Lun Zhigang, Chen Huizhen, Li Aimin. Application and potential of stem cells in the repair of facial nerve injury[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(21): 3439-3444.
share this article
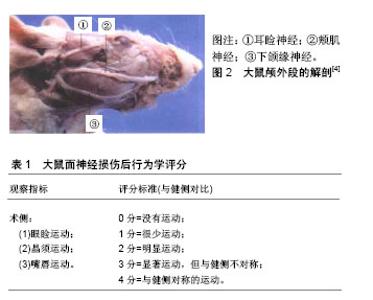
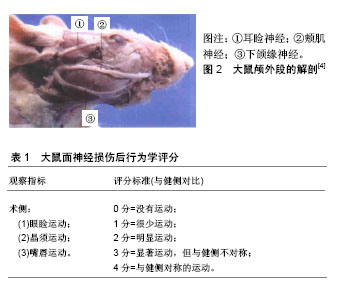
2.1 面神经损伤研究实验动物模型的应用 目前学者们使用灵长类、犬、家兔、猪、大鼠等建立了面神经损伤动物模型。灵长类、犬、猪等体型较大的动物相对来说面神经粗长,在实验中容易进行神经手术方面的操作,但需要较大的经费支持,术后动物管理难度也相对较大,因此运用并不广泛。家兔、大鼠等小型动物为目前基础实验应用较多的对象,但综合比较来看,大鼠是最为理想的实验动物,目前在面神经损伤修复基础实验中运用最为广泛,其优点是体型小、易于操作、便于管理,除了具有损伤修复和抗感染能力强之外,大鼠面神经的走行、分布和功能均和人类高度相似,术后的行为学改变也相对明显,便于术后评估,特别适合用于面神经损伤的研究。以大鼠为例,面神经损伤模型的建立主要集中在出颅段的主干及面部终末分支上[4],见图2,因主干较短,适合做夹闭、烧灼、切断损伤,离断缺损模型操作难度相对较大。大鼠面部终末分支主要包括耳睑神经、颊肌神经和下颌缘神经,耳睑神经相对较短,不利于操作,而颊肌神经和下颌缘神经较长,适合做离断缺损模型。主干损伤模型术后行为学表现相对明显,可用改良Most’s法进行术后行为学检测[5],见表1。分支损伤模型术后行为学变化较主干损伤差,可进行术后单一指标的行为学检测,有研究对面神经颊支进行离断缺损修复实验[6],术后仅通过晶须运动来进行行为学评估。"
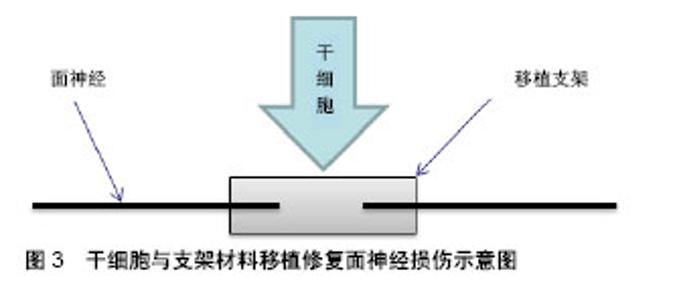
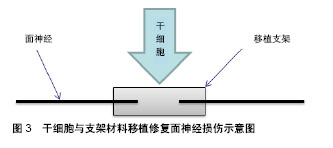
2.2 组织工程的应用 组织工程的核心是建立由细胞和生物材料构成的三维空间复合体,对病损组织进行形态、结构和功能的重建并达到永久性替代,其在面神经损伤实验研究应用中主要体现在移植支架及生物技术两个方面。目前,干细胞修复面神经损伤的主流研究方法是利用支架辅助干细胞移植,来替代自体神经移植作为轴突靶向生长的结构,见图3。支架的应用主要包括源自脉管系统的动静脉导管和一些生物可吸收性材料,特点是移植后可以在体内被降解吸收,从而降低移植排斥反应,同时为移植干细胞营造一个良好的微环境,为干细胞增殖、分化、代谢、营养交换等提供空间[7],还可以减少干细胞的流失以及外部因素的干扰等。目前,应用到面神经损伤修复实验中的支架材料主要有脱细胞动静脉导管[8]、壳聚糖、聚乙醇酸、胶原蛋白、纤维蛋白等。除了支架应用之外,一些生物技术也参与到干细胞修复面神经损伤研究中,如3D贴片移植系统可以将干细胞制成球形薄片用于移植[9];3D打印技术可以使干细胞累叠成生物导管用于神经修复等[10]。生物技术主要是将干细胞进行处理,使干细胞演变成生物材料的同时还保留着干细胞的特性,然后直接用于面神经损伤修复,从而提高干细胞的移植效果。"
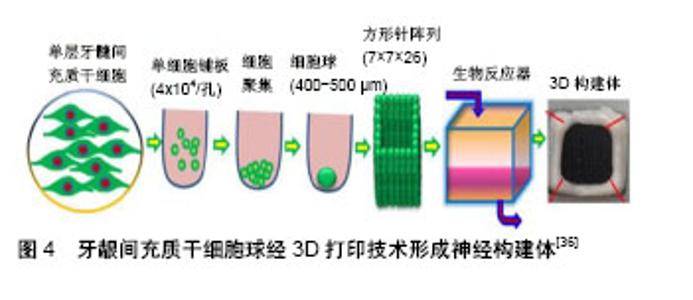
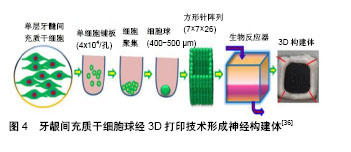
2.3 各类干细胞的特点及其在面神经损伤修复中的相关研究 2.3.1 影响干细胞移植修复面神经损伤的因素 干细胞修复面神经损伤是一个既复杂又漫长的过程,在移植过程中,有许多因素可以影响面神经的再生[11],包括损伤的部位和程度、干细胞移植的时间窗、干细胞的分化状态等都会影响其效果。另外,各种神经营养因子如脑源性神经营养因子、神经营养因子3、胶质细胞源性神经营养因子和睫状神经营养因子等也可以改善干细胞修复面神经损伤的效果,还有研究表明富血小板血浆也可以促进干细胞对面神经损伤的修复效果[12]。总之,组织工程与干细胞联合应用修复面神经损伤具有较好的发展前景[13]。 2.3.2 神经干细胞 胚胎期哺乳动物神经干细胞主要分布于海马、脑室下区、纹状体和皮质,成年哺乳动物神经干细胞主要分布于侧脑室的下室区和海马齿状回的颗粒下层[14-15]。神经干细胞在面神经修复中的应用是许多研究的重点,其可以分化成为神经元、星形胶质细胞和少突胶质细胞[16],在特定的条件下也可以诱导分化为许旺细胞[17]。神经干细胞移植具有可塑性强、高迁移率和低免疫原性等优点,但其需要在哺乳动物的大脑内取材,来源相对局限,因此在临床试验中开展相对较难,临床研究应用价值有限。Guo等[18]用含有胶原蛋白、神经生长因子和神经干细胞的壳聚糖导管移植治疗家兔面神经10 mm缺损,移植12周后进行电生理、组织学、免疫组织化学检测,其修复面神经再生效果明显优于不含神经干细胞的对照组,并且与自体神经移植组效果相似,证实了神经干细胞可以作为面神经损伤修复的种子细胞。Shi等[19]利用表达胶质细胞源性神经营养因子基因的慢病毒转染神经干细胞,转染后的神经干细胞与聚乙醇酸导管复合移植治疗大鼠面神经横断损伤,12周后进行电生理、免疫组织化学染色和神经形态学分析,结果发现胶质细胞源性神经营养因子转染神经干细胞可以明显提高再生神经轴突的数量,对面神经损伤修复的效果优于未转染神经干细胞,该研究表明,神经干细胞可以促进面神经损伤的修复,胶质细胞源性神经营养因子可以提高神经干细胞对神经损伤的修复效果。 2.3.3 骨髓间充质干细胞 骨髓间充质干细胞是一类存在于骨髓中且具有高度自我更新和多向分化潜能的干细胞,在体内或体外特定的诱导条件下,可以向脂肪、骨、肌肉及神经等多种组织细胞分化,也可以诱导分化为许旺细胞表型细胞,支持神经再生,其特性与许旺细胞很相似[20]。与神经干细胞相比,骨髓间充质干细胞的增殖分化能力相对较低,但其来源于骨髓,更易获得,取材相对方便,移植后不良反应小,在传代多次后仍然具有干细胞的特性[21],其缺点是采集过程具有侵入性和痛苦性,同时细胞增殖能力受供者年龄的影响,但骨髓间充质干细胞仍然是目前用于面神经损伤研究的重要种子细胞。Wang等[22]以自体静脉导管为支架将骨髓间充质干细胞和转分化后具有许旺细胞表型的骨髓间充质干细胞移植到家兔1 cm面神经缺损模型中,4周后进行行为学、免疫组织化学以及形态学检查分析,结果发现移植具有许旺细胞表型的骨髓间充质干细胞组家兔面神经损伤部位更早地出现神经元以及更多数量的髓鞘轴突,8周后利用RT-PCR技术检测发现具有许旺细胞表型的骨髓间充质干细胞组家兔面神经损伤部位的髓鞘蛋白mRNA水平高于骨髓间充质干细胞组,该研究表明,与正常的骨髓间充质干细胞相比,具有许旺表型的骨髓间充质干细胞可以加速横断神经轴突再生,达到更好的修复效果。Costa等[23]将骨髓间充质干细胞或分化后具有许旺细胞表型的骨髓间充质干细胞联合自体神经填充的聚乙醇酸导管移植到大鼠面神经下颌支损伤模型中,6周后进行轴突形态测量和复合肌肉动作电位分析,发现骨髓间充质干细胞组和具有许旺表型的骨髓间充质干细胞组面神经轴突数量以及动作电位振幅均优于未移植细胞组,且具有许旺表型的骨髓间充质干细胞对损伤神经的修复效果优于骨髓间充质干细胞。 2.3.4 脂肪干细胞 脂肪干细胞来源于脂肪组织,是相对于骨髓间充质干细胞来说具有更高增殖和分化潜力的干细胞[24],在一定条件下可以分化成许多有特定功能的细胞。因哺乳动物体内脂肪含量丰富,可以通过微创手术获得,便于提取,因此脂肪干细胞具有侵入性小、来源相对广泛等特点,适合用于自体移植,与骨髓间充质干细胞一样,脂肪干细胞也可以分化为具有许旺细胞表型细胞,同样具有移植后不良反应小的特点,同时脂肪干细胞可以释放许多生长因子,促进内源性许旺细胞的聚集[25],有利于神经的修复和再生;缺点是脂肪干细胞的活性受来源部位、脂肪层以及供者年龄的影响[26]。在面神经乃至整个神经系统领域中,脂肪干细胞都具有极大的研究价值,也是目前常用于面神经损伤修复研究的种子细胞之一。Ghoreishian等[27]将来源于混种狗自体脂肪干细胞填充于聚四氟乙烯管并用藻酸盐水凝胶固定,移植修复狗7 mm面神经缺损,12周后发现实验组轴突数量、神经传导速度和神经动作电位的最大振幅明显高于对照组,证实脂肪干细胞可以从功能的角度促进损伤面神经的恢复。Watanabe等[28]将未分化的脂肪干细胞、分化的脂肪干细胞以及许旺细胞植入胶原凝胶中,修复7 mm大鼠面神经缺损,13周后经形态学定量分析发现3组大鼠面神经功能恢复接近自体神经移植,该研究表明3种细胞具有类似的神经再生潜力。Kamei等[29]利用脂肪干细胞联合聚乙醇酸导管通过介入跳跃移植技术将大鼠夹闭损伤的面神经主干与同侧舌下神经连接起来,术后13周进行生理学和组织病理学评估,结果发现面神经主干与同侧舌下神经之间的导管内出现神经再生且脂肪干细胞对神经的再生具有促进作用,所有治疗组都显示出再生的神经纤维,由舌下神经核和面神经核双重支配,该研究成果表明,脂肪干细胞联合聚乙醇酸导管可以促进神经再生,介入跳跃移植技术也是一种可以用于修复神经损伤的方法。 2.3.5 牙源性干细胞 牙源性干细胞具有类似间充质干细胞的特性,包括自我更新和多向分化潜能。牙源性干细胞包括牙髓干细胞[30]、牙龈间充质干细胞、来自人体脱落乳牙的干细胞、来自根尖乳头的干细胞和牙周韧带干细胞等[31]。牙源性干细胞来源于牙周组织,资源丰富,临床应用潜力大,除了具有高度自我更新和多向分化特性外,还具有易获得性和侵入性小等优点。在特定刺激的存在下,牙源性干细胞可以分化为成骨细胞[32]、脂肪细胞和肌原细胞等各种细胞类型。Sasaki等[33]将嵌入胶原凝胶中的牙髓干细胞联合聚乳酸管移植到大鼠面神经颊支7 mm损伤模型中,移植5 d后免疫荧光染色发现聚乳酸管中出现轴突再生,移植9周后免疫荧光染色检测发现再生神经中出现大量轴突,证实了牙髓干细胞联合聚乳酸管的应用可以促进神经再生。另外,牙源性干细胞还可以通过诱导分化成神经系干细胞用于面神经损伤的修复,有研究指出这种诱导分化后的神经系干细胞在周围神经修复中拥有更好的效果[34]。Zhang等[35]将牙龈间充质干细胞通过一种非遗传方法诱导成神经嵴类干细胞,用诱导后的神经嵴类干细胞与神经导管组合移植修复大鼠面神经损伤,证实了牙龈间充质干细胞衍生的神经嵴类干细胞可以促进面神经再生,有可能成为组织工程的种子细胞,替代自体神经移植改善周围神经的再生。在Zhang等[36]的另一项研究中,运用3D生物打印技术将牙龈间充质干细胞球制成导管状的神经构建体[10],见图4,利用这种神经构建体移植修复大鼠面神经颊支的节段性缺损,移植后第7周神经构建体移植组大鼠的行为学评分高于硅胶管对照组,移植后12周神经构建体移植组肌肉动作电位与自体神经移植组类似,该研究证实运用3D生物打印技术制成的牙龈间充质干细胞神经构建体具有促进大鼠面神经损伤再生的效果。Pereira等[37]用人体脱落乳牙干细胞联合聚乙醇酸管并结合自体神经移植修复大鼠面神经下颌支损伤模型,通过电生理和组织学评估移植效果,发现人体脱落乳牙干细胞可以促进面神经的再生,还能在体内分化为许旺细胞。"
| [1] Hadlock TA, Kowaleski J, Lo D, et al. Rodent facial nerve recovery after selected lesions and repair techniques. Plast Reconstr Surg. 2010;125(1):99-109.[2] Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin. 2013;29(3):331-348.[3] Watt FM, Driskell RR. The therapeutic potential of stem cells. Philos Trans R Soc Lond B Biol Sci. 2010;365(1537):155-163.[4] 单增强,王小标,赵莉,等.大鼠面神经颅外段的解剖及其应用[J].解剖学杂志,2005, 28(1):82-83.[5] Most SP. Facial nerve recovery in bcl2 overexpression mice after crush injury. Arch Facial Plast Surg. 2004;6(2):82-87.[6] Sun F, Zhou K, Mi WJ, et al. Repair of facial nerve defects with decellularized artery allografts containing autologous adipose-derived stem cells in a rat model. Neurosci Lett. 2011; 499(2):104-108.[7] 葛振,邹刚,刘毅,等.组织工程支架材料:特征及在组织工程中的应用[J].中国组织工程研究,2018,22(26):4222-4228.[8] Sun F, Zhou K, Mi WJ, et al. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials. 2011; 32(32):8118-8128.[9] Tamaki T, Soeda S, Hashimoto H, et al. 3D reconstitution of nerve-blood vessel networks using skeletal muscle-derived multipotent stem cell sheet pellets. Regen Med. 2013;8(4): 437-451.[10] Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773-785.[11] Nelke KH, ?uczak K, Pawlak W, et al. Stem cells and related factors involved in facial nerve function regeneration. Postepy Hig Med Dosw (Online). 2015;69:996-1002.[12] 孙妍娜,张荣明,毛旭,等.复合脂肪来源干细胞的脱细胞异种神经联合富血小板血浆修复兔面神经损伤的实验研究[J].中国修复重建外科杂志, 2018, 32(6):736-744.[13] Euler de Souza Lucena E, Guzen FP, Lopes de Paiva Cavalcanti JR, et al. Experimental considerations concerning the use of stem cells and tissue engineering for facial nerve regeneration: a systematic review. J Oral Maxillofac Surg. 2014;72(5):1001-1012.[14] Jin K, Galvan V. Endogenous neural stem cells in the adult brain. J Neuroimmune Pharmacol. 2007;2(3):236-242.[15] Choi BY, Kim JH, Kim HJ, et al. Zinc chelation reduces traumatic brain injury-induced neurogenesis in the subgranular zone of the hippocampal dentate gyrus. J Trace Elem Med Biol. 2014;28(4): 474-481.[16] Parker MA, Anderson JK, Corliss DA, et al. Expression profile of an operationally-defined neural stem cell clone. Exp Neurol. 2005; 194(2):320-332.[17] He Y, Zhang PZ, Sun D, et al. Wnt1 from cochlear schwann cells enhances neuronal differentiation of transplanted neural stem cells in a rat spiral ganglion neuron degeneration model. Cell Transplant. 2014; 23(6):747-760.[18] Guo BF, Dong MM. Application of neural stem cells in tissue- engineered artificial nerve. Otolaryngol Head Neck Surg. 2009; 140(2):159-164.[19] Shi Y, Zhou L, Tian J, et al. Transplantation of neural stem cells overexpressing glia-derived neurotrophic factor promotes facial nerve regeneration. Acta Otolaryngol. 2009;129(8):906-914.[20] Ladak A, Olson J, Tredget EE, et al. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol. 2011;228(2):242-252.[21] Lotfy A, Salama M, Zahran F, et al. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: a comparative study. Int J Stem Cells. 2014;7(2):135-142.[22] Wang X, Luo E, Li Y, et al. Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res. 2011;1383:71-80.[23] Costa HJ, Bento RF, Salomone R, et al. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res. 2013;1510:10-21.[24] Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3): 132-141.[25] Sowa Y, Kishida T, Imura T, et al. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo without Differentiation into Schwann-Like Lineage. Plast Reconstr Surg. 2016;137(2):318e-330e.[26] Jiang L, Jones S, Jia X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int J Mol Sci. 2017;18(1): E94.[27] Ghoreishian M, Rezaei M, Beni BH, et al. Facial nerve repair with Gore-Tex tube and adipose-derived stem cells: an animal study in dogs. J Oral Maxillofac Surg. 2013;71(3):577-587.[28] Watanabe Y, Sasaki R, Matsumine H, et al. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J Tissue Eng Regen Med. 2017;11(2):362-374.[29] Kamei W, Matsumine H, Osaki H, et al. Axonal supercharged interpositional jump-graft with a hybrid artificial nerve conduit containing adipose-derived stem cells in facial nerve paresis rat model. Microsurgery. 2018;38(8):889-898.[30] 邵苗苗,刘钟西,许诺,等.牙髓干细胞在组织工程研究中的进展和应用前景[J].中国组织工程研究, 2018, 22(13):2126-2132.[31] Chalisserry EP, Nam SY, Park SH, et al. Therapeutic potential of dental stem cells. J Tissue Eng. 2017;8:2041731417702531.[32] Laino G, Carinci F, Graziano A, et al. In vitro bone production using stem cells derived from human dental pulp. J Craniofac Surg. 2006; 17(3):511-515.[33] Sasaki R, Aoki S, Yamato M, et al. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med. 2011;5(10):823-830.[34] Zhang Q, Nguyen P, Xu Q, et al. Neural Progenitor-Like Cells Induced from Human Gingiva-Derived Mesenchymal Stem Cells Regulate Myelination of Schwann Cells in Rat Sciatic Nerve Regeneration. Stem Cells Transl Med. 2017;6(2): 458-470.[35] Zhang Q, Nguyen PD, Shi S, et al. Neural Crest Stem-Like Cells Non-genetically Induced from Human Gingiva-Derived Mesenchymal Stem Cells Promote Facial Nerve Regeneration in Rats. Mol Neurobiol. 2018;55(8):6965-6983.[36] Zhang Q, Nguyen PD, Shi S, et al. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci Rep. 2018;8(1): 6634.[37] Pereira LV, Bento RF, Cruz DB, et al. Stem Cells from Human Exfoliated Deciduous Teeth (SHED) Differentiate in vivo and Promote Facial Nerve Regeneration. Cell Transplant. 2019; 28(1): 55-64.[38] Tamaki T, Uchiyama Y, Okada Y, et al. Functional recovery of damaged skeletal muscle through synchronized vasculogenesis, myogenesis, and neurogenesis by muscle-derived stem cells. Circulation. 2005; 112(18):2857-2866.[39] Beane OS, Fonseca VC, Cooper LL, et al. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose- derived mesenchymal stem/stromal cells. PLoS One. 2014; 9(12): e115963.[40] Zhou J, Cui H, Lu H, et al. Muscle-derived stem cells in peripheral nerve regeneration: reality or illusion. Regen Med. 2017;12(4): 459-472.[41] Saito K, Tamaki T, Hirata M, et al. Reconstruction of Multiple Facial Nerve Branches Using Skeletal Muscle-Derived Multipotent Stem Cell Sheet-Pellet Transplantation. PLoS One. 2015;10(9): e0138371.[42] Murrell W, Féron F, Wetzig A, et al. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005;233(2):496-515.[43] Batioglu-Karaaltin A, Karaaltin MV, Oztel ON, et al. Human olfactory stem cells for injured facial nerve reconstruction in a rat model. Head Neck. 2016;38 Suppl 1:E2011-2020.[44] Ziegler L, Grigoryan S, Yang IH, et al. Efficient generation of schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Rev. 2011;7(2):394-403.[45] Rippon HJ, Bishop AE. Embryonic stem cells. Cell Prolif. 2004; 37(1): 23-34.[46] Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007; 448(7151):318-324.[47] Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318 (5858): 1917-1920.[48] Ikeda M, Uemura T, Takamatsu K, et al. Acceleration of peripheral nerve regeneration using nerve conduits in combination with induced pluripotent stem cell technology and a basic fibroblast growth factor drug delivery system. J Biomed Mater Res A. 2014;102(5):1370-1378.[49] Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3(6): 595-605.[50] 鲁斌,王岩松.脐带血干细胞的研究进展[J].实用医学杂志,2016, 32(7): 1189-1191.[51] Ko KR, Lee J, Lee D, et al. Hepatocyte Growth Factor (HGF) Promotes Peripheral Nerve Regeneration by Activating Repair Schwann Cells. Sci Rep. 2018;8(1):8316.[52] Bosse F. Extrinsic cellular and molecular mediators of peripheral axonal regeneration. Cell Tissue Res. 2012;349(1):5-14.[53] Sowa Y, Imura T, Numajiri T, et al. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev. 2012;21(11):1852-1862.[54] Walsh S, Midha R. Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurg Focus. 2009; 26(2):E2. |
| [1] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [2] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [3] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [4] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [5] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [6] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [7] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [8] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [9] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| [10] | Jiang Tao, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Ma Chuang, Wei Qin. Platelet-derived growth factor BB induces bone marrow mesenchymal stem cells to differentiate into vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3937-3942. |
| [11] | Sun Jianwei, Yang Xinming, Zhang Ying. Effect of montelukast combined with bone marrow mesenchymal stem cell transplantation on spinal cord injury in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3962-3969. |
| [12] | Zhang Lishu, Liu Anqi, He Xiaoning, Jin Yan, Li Bei, Jin Fang. Alpl gene affects the therapeutic effect of bone marrow mesenchymal stem cells on ulcerative colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3970-3975. |
| [13] | Hao Xiaona, Zhang Yingjie, Li Yuyun, Xu Tao. Bone marrow mesenchymal stem cells overexpressing prolyl oligopeptidase on the repair of liver fibrosis in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3988-3993. |
| [14] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [15] | Wei Qin, Zhang Xue, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Jia Qiyu, Ma Chuang. Platelet-derived growth factor-BB induces the differentiation of rat bone marrow mesenchymal stem cells into osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2953-2957. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||