Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (21): 3378-3385.doi: 10.3969/j.issn.2095-4344.1750
Previous Articles Next Articles
Basic fibroblast growth factor-transfected bone marrow mesenchymal stem cell transplantation for chronic obstructive pulmonary disease
Wu Huala, Zhou Xiangxiang, Zhong Yulan, Gan Xin
- Department of Respiration, the First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China
-
Revised:2019-02-13Online:2019-07-28Published:2019-07-28 -
Contact:Gan Xin, MD, Chief physician, Department of Respiration, the First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China -
About author:Wu Huala, Master, Physician, Department of Respiration, the First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China -
Supported by:the National Natural Science Foundation of China, No. 81660009 (to GX) and 81260004 (to GX); the Natural Science Foundation of Jiangxi Province, No. 20161ACB20012 (to GX)
CLC Number:
Cite this article
Wu Huala, Zhou Xiangxiang, Zhong Yulan, Gan Xin. Basic fibroblast growth factor-transfected bone marrow mesenchymal stem cell transplantation for chronic obstructive pulmonary disease[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(21): 3378-3385.
share this article
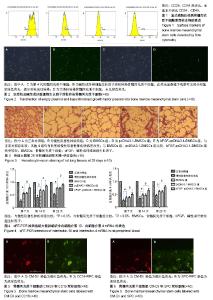
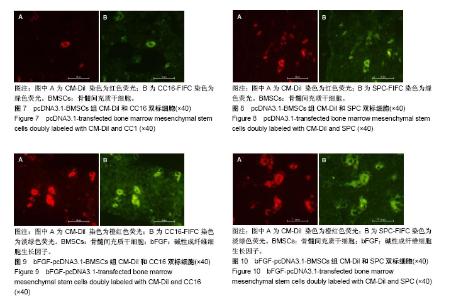
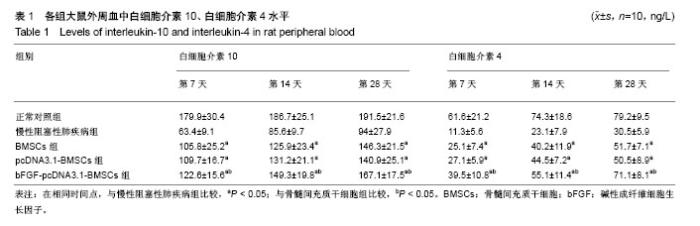
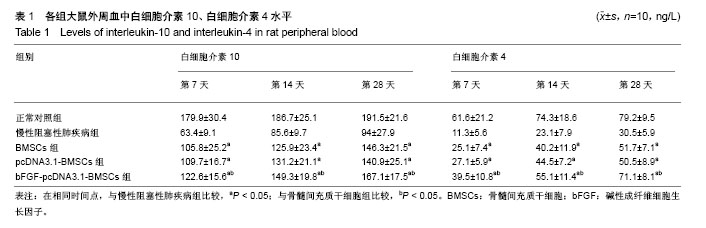
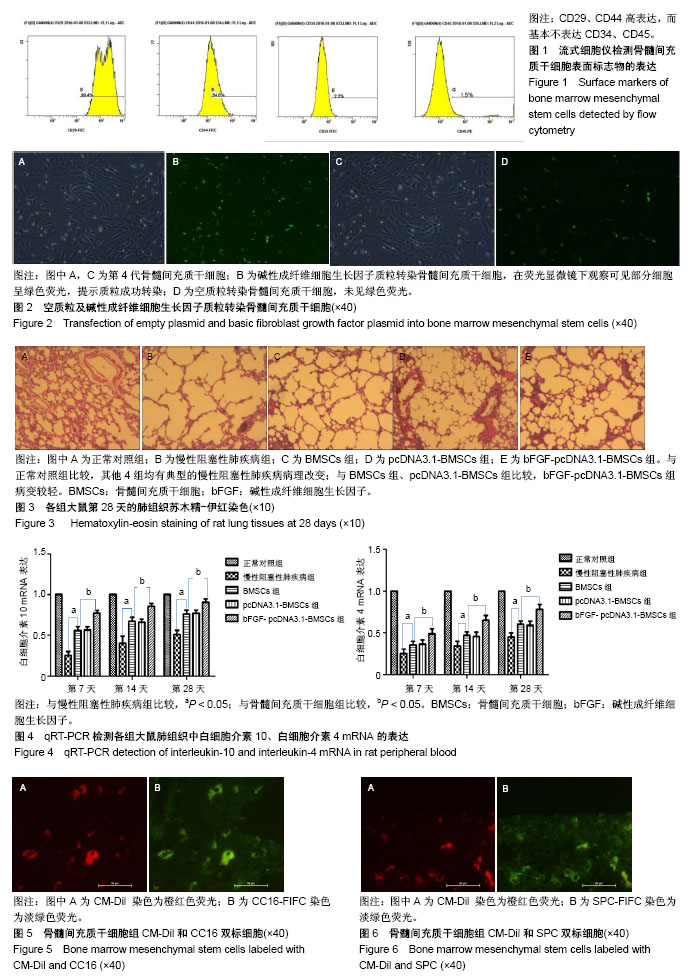
2.1 骨髓间充质干细胞的形态、鉴定和标记、转染结果 原代骨髓间充质干细胞为典型的纺锤形、梭形或三角形,局部可见小集落形成。提取、培养的第4代细胞经流式细胞术检测显示CD29、CD44高表达,而基本不表达造血干细胞的标记CD34、CD45,见图1。碱性成纤维细胞生长因子质粒转染骨髓间充质干细胞24-48 h,在荧光显微镜下观察可见部分细胞呈绿色荧光,提示质粒成功转染,见图2。对照组未见绿色荧光。 2.2 肺组织病理学改变 各实验组分别在干预第28天留取部分右肺中叶进行病理苏木精-伊红染色,在光学显微镜下观察病理改变,慢性阻塞性肺疾病组病理改变相对于正常对照组:肺泡腔不规则扩大,可见部分肺间隔断裂,数个肺泡融合成一个肺大疱;支气管、肺间质血管周围可见炎症细胞浸润,见图3B,符合典型慢性阻塞性肺疾病的病理改变。BMSCs组、pcDNA3.1-BMSCs组、bFGF-pcDNA3.1-BMSCs组:均有典型的慢性阻塞性肺疾病病理改变。在相同视野下,肺泡腔不规则扩大,可见部分肺泡间隔断裂,肺大疱形成,气管管壁及肺间质血管管壁有少数炎性细胞浸润。BMSCs组、pcDNA3.1-BMSCs组之间无明显差异;与BMSCs组、pcDNA3.1-BMSCs组比较,bFGF-pcDNA3.1-BMSCs组病变较轻,见图3C-E。 2.3 各组大鼠外周血白细胞介素10、白细胞介素4水平 各组在干预第7,14,28天留取外周血,通过双抗夹心ELISA法检测外周血中白细胞介素10、白细胞介素4水平,结果显示:在相同时间节点,3个治疗组外周血中白细胞介素10、白细胞介素4水平高于慢性阻塞性肺疾病组(均P < 0.01),BMSCs组、pcDNA3.1-BMSCs组外周血白细胞介素10、白细胞介素4水平差别不大,但bFGF-pcDNA3.1- BMSCs组较之二者相对较高,差异有显著性意义(均P < 0.05),见表1。 2.4 各组大鼠肺组织中白细胞介素10、白细胞介素4 mRNA的表达 各组分别在干预第7,14,28天留取部分右肺中叶,匀浆研磨后通过qRT-PCR检测肺组织中白细胞介素10、白细胞介素4 mRNA表达。在相同时间节点,3个治疗组肺组织中白细胞介素10、白细胞介素4表达高于慢性阻塞性肺疾病组(均P < 0.01),BMSCs组、pcDNA3.1-BMSCs组肺组织中的白细胞介素10、白细胞介素4表达差别不大,但bFGF-pcDNA3.1-BMSCs组较之二者相对较高,差异有显著性意义(均P < 0.05),见图4。 2.5 骨髓间充质干细胞在肺组织中的分化 各实验组分别在干预第28天留取部分右肺中叶进行新鲜冰冻切片,在荧光显微镜下观察CM-Dil染色阳性细胞分布情况,选择CM-Dil阳性细胞较多的切片进行免疫荧光染色,观察骨髓间充质干细胞在肺组织中分化情况。荧光显微镜下3个治疗组的肺组织切片中均有少数橙红色荧光的细胞,同时显淡绿色荧光(CM-Dil和SPC/CC16双染色标记),见图5-10。肺泡壁有少量显橙红色荧光的细胞(CM-Dil标记),同时显淡绿色荧光(SPC-FIFC标记),支气管壁有少量显橙红色荧光的细胞(CM-Dil标记),同时显淡绿色荧光(CC16-FIFC标记),相对于BMSCs组、pcDNA3.1-BMSCs组,bFGF-pcDNA3.1-BMSCs组可发现双染的阳性细胞较多。"
| [1] Weiss DJ. Stem cells, cell therapies, and bioengineering in lung biology and diseases. Comprehensive review of the recent literature 2010-2012. Ann Am Thorac Soc. 2013;10(5):S45-97.[2] Rodrigo SF, van Ramshorst J, Hoogslag GE, et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res. 2013;6(5):816-825.[3] Zhou Z, You Z. Mesenchymal Stem Cells Alleviate LPS-Induced Acute Lung Injury in Mice by MiR-142a-5p-Controlled Pulmonary Endothelial Cell Autophagy. Cell Physiol Biochem. 2016;38(1): 258-266.[4] Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179(3):1855-1863.[5] Li D, Pan X, Zhao J, et al. Bone Marrow Mesenchymal Stem Cells Suppress Acute Lung Injury Induced by Lipopolysaccharide Through Inhibiting the TLR2, 4/NF-κB Pathway in Rats with Multiple Trauma. Shock. 2016;45(6):641-646.[6] Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33(2):145-152.[7] Zhen G, Liu H, Gu N, et al. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008; 13:3415-3422.[8] Liu HM, Liu YT, Zhang J, et al. Bone marrow mesenchymal stem cells ameliorate lung injury through anti-inflammatory and antibacterial effect in COPD mice. J Huazhong Univ Sci Technolog Med Sci. 2017;37(4):496-504.[9] Ambalavanan N, Novak ZE. Peptide growth factors in tracheal aspirates of mechanically ventilated preterm neonates. Pediatr Res. 2003;53(2):240-244.[10] Morino S, Nakamura T, Toba T, et al. Fibroblast growth factor-2 induces recovery of pulmonary blood flow in canine emphysema models. Chest. 2005;128(2):920-926.[11] Morino S, Toba T, Tao H, et al. Fibroblast growth factor-2 promotes recovery of pulmonary function in a canine models of elastase-induced emphysema. Exp Lung Res. 2007;33(1):15-26.[12] Tanni SE, Pelegrino NR, Angeleli AY, et al. Smoking status and tumor necrosis factor-alpha mediated systemic inflammation in COPD patients. J Inflamm (Lond). 2010;7:29.[13] Gu W, Song L, Li XM, et al. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci Rep. 2015;5:8733.[14] Srivastava PK, Dastidar SG, Ray A. Chronic obstructive pulmonary disease: role of matrix metalloproteases and future challenges of drug therapy. Expert Opin Investig Drugs. 2007;16(7):1069-1078.[15] Tzouvelekis A, Laurent G, Bouros D. Stem cell therapy in chronic obstructive pulmonary disease. Seeking the Prometheus effect. Curr Drug Targets. 2013;14(2):246-252.[16] Badri L, Walker NM, Ohtsuka T, et al. Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am J Respir Cell Mol Biol. 2011;45(4):809-816.[17] Robinson AB, Stogsdill JA, Lewis JB, et al. RAGE and tobacco smoke: insights into modeling chronic obstructive pulmonary disease. Front Physiol. 2012;3:301.[18] Gupta V, Banyard A, Mullan A, et al. Characterization of the inflammatory response to inhaled lipopolysaccharide in mild to moderate chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2015;79(5):767-776.[19] Parasuramalu BG, Huliraj N, Prashanth Kumar SP, et al. Prevalence of chronic obstructive pulmonary disease and its association with tobacco smoking and environmental tobacco smoke exposure among rural population. Indian J Public Health. 2014;58(1):45-49.[20] Kobayashi S, Fujinawa R, Ota F, et al. A single dose of lipopolysaccharide into mice with emphysema mimics human chronic obstructive pulmonary disease exacerbation as assessed by micro-computed tomography. Am J Respir Cell Mol Biol. 2013; 49(6):971-977.[21] Matsui R, Brody JS, Yu Q. FGF-2 induces surfactant protein gene expression in foetal rat lung epithelial cells through a MAPK-independent pathway. Cell Signal. 1999;11(3):221-228.[22] Guzy RD, Stoilov I, Elton TJ, et al. Fibroblast growth factor 2 is required for epithelial recovery, but not for pulmonary fibrosis, in response to bleomycin. Am J Respir Cell Mol Biol. 2015;52(1): 116-128.[23] Zhao YF, Luo YM, Xiong W, et al. Mesenchymal stem cell-based FGF2 gene therapy for acute lung injury induced by lipopolysaccharide in mice. Eur Rev Med Pharmacol Sci. 2015;19(5):857-865.[24] 赵悦,马琳,彭珊珊,等.碱性成纤维细胞生长因子基因转染大鼠骨髓间充质干细胞的基因表达[J].医学研究生学报, 2015,28(12):1246-1251.[25] Ding X, Li M, Cao Y, et al. Effects of Plasmid Fibroblast Growth Factor-2 Magnetic Chitosan Gelatin Microspheres on Proliferation and Differentiation of Mesenchymal Stem Cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2015;32(5):1083-1089.[26] Tong L, Zhou J, Rong L, et al. Fibroblast Growth Factor-10 (FGF-10) Mobilizes Lung-resident Mesenchymal Stem Cells and Protects Against Acute Lung Injury. Sci Rep. 2016;6:21642.[27] 宋燕峰,曹洁.碱性成纤维细胞生长因子转染骨髓间充质干细胞移植治疗肺动脉高压[J].中国组织工程研究, 2015,19(6):918-922.[28] 崔利德,李铁民.碱性成纤维细胞生长因子基因修饰骨髓间充质干细胞移植修复急性肾损伤[J].中国组织工程研究, 2016,20(28):4169-4175.[29] 田雪品,刘海英.碱性成纤维细胞生长因子基因真核表达载体转染骨髓间充质干细胞移植治疗糖尿病[J].中国组织工程研究, 2016,20(36): 5385-5391.[30] 张琳,居士明,王敢,等.成纤维细胞生长因子修饰的骨髓间充质干细胞移植对颅脑损伤模型大鼠神经功能的改善机制分析[J].临床和实验医学杂志,2016,15(12):1137-1140.[31] Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114-1121.[32] Vitenberga Z, Pilmane M, Babjoniševa A. The evaluation of inflammatory, anti-inflammatory and regulatory factors contributing to the pathogenesis of COPD in airways. Pathol Res Pract. 2019; 215(1):97-105.[33] Silva BSA, Lira FS, Ramos D, et al. Severity of COPD and its relationship with IL-10. Cytokine. 2018;106:95-100.[34] Saxena A, Khosraviani S, Noel S, et al. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74(1):27-34.[35] Huang AX, Lu LW, Liu WJ, et al. Plasma Inflammatory Cytokine IL-4, IL-8, IL-10, and TNF-α Levels Correlate with Pulmonary Function in Patients with Asthma-Chronic Obstructive Pulmonary Disease (COPD) Overlap Syndrome. Med Sci Monit. 2016;22:2800-2808.[36] Zobel K, Martus P, Pletz MW, et al. Interleukin 6, lipopolysaccharide- binding protein and interleukin 10 in the prediction of risk and etiologic patterns in patients with community-acquired pneumonia: results from the German competence network CAPNETZ. BMC Pulm Med. 2012;12:6.[37] Sapan HB, Paturusi I, Jusuf I, et al. Pattern of cytokine (IL-6 and IL-10) level as inflammation and anti-inflammation mediator of multiple organ dysfunction syndrome (MODS) in polytrauma. Int J Burns Trauma. 2016;6(2):37-43.[38] Barnes PJ. Cytokine modulators as novel therapies for airway disease. Eur Respir J Suppl. 2001;34:67s-77s.[39] Barnes PJ. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2018;18(7): 454-466.[40] Zhu J, Qiu Y, Valobra M, et al. Plasma cells and IL-4 in chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(11):1125-1133.[41] Luzina IG, Lockatell V, Lavania S, et al. Natural production and functional effects of alternatively spliced interleukin-4 protein in asthma. Cytokine. 2012;58(1):20-26.[42] Liu Y, Zhuo A, Liu W, et al. The -33C/T polymorphism in the interleukin 4 gene is associated with asthma risk: a meta-analysis. J Investig Allergol Clin Immunol. 2014;24(2):114-121. |
| [1] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [2] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [3] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [4] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [5] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [6] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [7] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [8] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| [9] | Jiang Tao, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Ma Chuang, Wei Qin. Platelet-derived growth factor BB induces bone marrow mesenchymal stem cells to differentiate into vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3937-3942. |
| [10] | Sun Jianwei, Yang Xinming, Zhang Ying. Effect of montelukast combined with bone marrow mesenchymal stem cell transplantation on spinal cord injury in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3962-3969. |
| [11] | Zhang Lishu, Liu Anqi, He Xiaoning, Jin Yan, Li Bei, Jin Fang. Alpl gene affects the therapeutic effect of bone marrow mesenchymal stem cells on ulcerative colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3970-3975. |
| [12] | Hao Xiaona, Zhang Yingjie, Li Yuyun, Xu Tao. Bone marrow mesenchymal stem cells overexpressing prolyl oligopeptidase on the repair of liver fibrosis in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3988-3993. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Wei Qin, Zhang Xue, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Jia Qiyu, Ma Chuang. Platelet-derived growth factor-BB induces the differentiation of rat bone marrow mesenchymal stem cells into osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2953-2957. |
| [15] | Guo Zhibin, Wu Chunfang, Liu Zihong, Zhang Yuying, Chi Bojing, Wang Bao, Ma Chao, Zhang Guobin, Tian Faming. Simvastatin stimulates osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2963-2968. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||