Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (9): 1432-1438.doi: 10.3969/j.issn.2095-4344.2017.09.022
Previous Articles Next Articles
In vitro EdU labeling of peripheral blood mononuclear cells in rabbits
Zhao Ming-lei, Zhen Dong-qin, Huang Jian-fa, Li Wei-hua, Wang Wen-cong, Li Zhi-quan, Zhang He-ning,Xian Bi-kun, Peng Yu-ting, Zhou Min-yi, Huang Bing
- State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China
-
Online:2017-03-28Published:2017-03-31 -
Contact:Huang Bing, Researcher, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China -
About author:Zhao Ming-lei, Studying for master’s degree, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China -
Supported by:the Scientific Plan Project of Guangdong Province, No. 2013B040200020, 2013B020400003; the Scientific Plan Project of Guangzhou City, No. 15570001
CLC Number:
Cite this article
Zhao Ming-lei, Zhen Dong-qin, Huang Jian-fa, Li Wei-hua, Wang Wen-cong, Li Zhi-quan, Zhang He-ning,Xian Bi-kun, Peng Yu-ting, Zhou Min-yi, Huang Bing. In vitro EdU labeling of peripheral blood mononuclear cells in rabbits[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(9): 1432-1438.
share this article
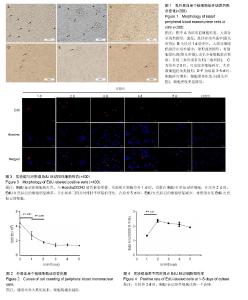
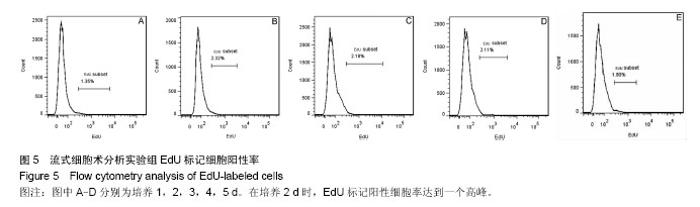
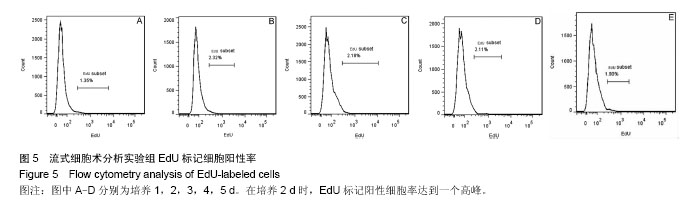
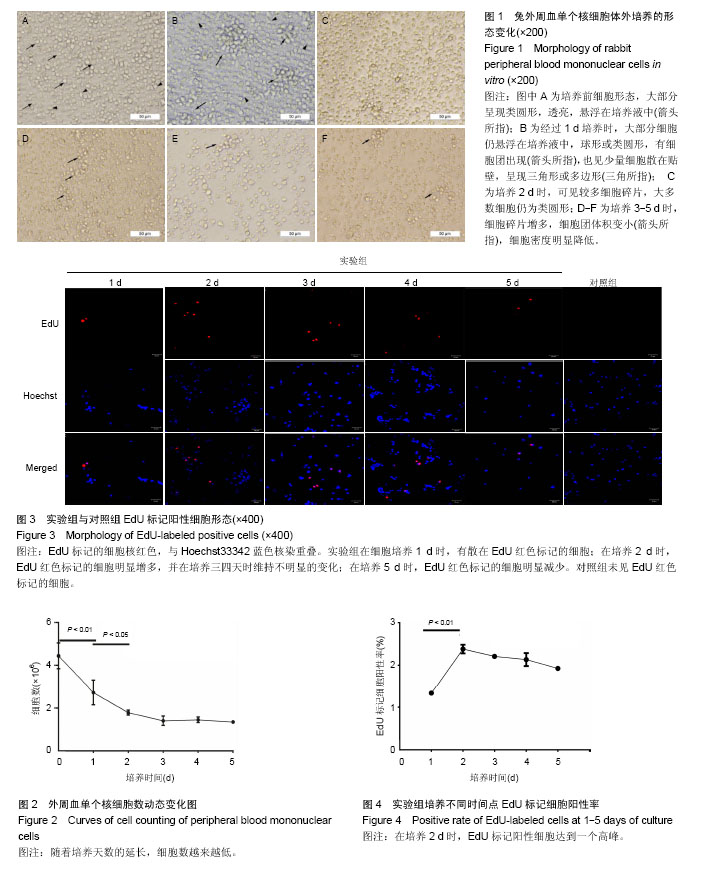
2.1 外周血单个核细胞体外培养时的形态变化 刚分离的兔外周血单个核细胞在普通光学显微镜下大部分呈现类圆形,透亮,悬浮在培养液中(图1A,箭头所指),少数细胞有尖的突起(图1A,三角所指);经过1 d培养时(图1B),大部分细胞仍悬浮在培养液中,球形或类圆形;有细胞团出现(图1B,箭头所指),也见少量细胞散在贴壁,呈现三角形或多边形(图1B,三角所指);培养2 d时,镜下可见较多细胞碎片;大多数细胞仍为类圆形(图1C);培养3-5 d时,细胞碎片增多,细胞团体积变小(图中箭头所指),细胞密度明显降低(图1D、E、F)。 2.2 外周血单个核细胞体外培养时细胞数的变化 兔外周血单个核细胞刚分离和经1-5 d体外培养时细胞数分别为(4.44±0.61)×106、(2.73±0.57)×106、(1.79±0.12)×106、(1.41±0.22)×106、(1.45±0.13)×106、(1.35±0.05)×106。随着培养时间的延长,细胞数越来越低,在刚分离时和培养1 d时、培养1 d和培养2 d时细胞数比较差异有显著性意义(P < 0.05),2 d与3 d、3 d与4 d、4 d与5 d间细胞数无差异(P > 0.05),培养到3-5 d时,维持在一个最低期,见图2。 2.3 EdU标记阳性细胞观察 EdU标记的兔外周血单个核细胞呈现细胞核红色,与Hoechst33342蓝色核染重叠。 实验组在细胞培养1 d时,有散在EdU红色标记的细胞;在培养2 d时,EdU红色标记的细胞明显增多,并在培养三四天时维持不明显的变化;在培养5 d时,EdU红色标记的细胞才有明显减少(图3)。对照组(不加EdU培养)未见EdU红色标记的细胞。 2.4 流式细胞术检测EdU标记阳性细胞率的变化 流式细胞仪检测显示,实验组培养1-5 d的EdU标记细胞阳性率分别为(1.33±0.02)%、(2.38±0.10)%、(2.21±0.03)%、(2.13±0.15)%、(1.93±0.03)%,见图4,5,在培养2 d时,EdU标记阳性细胞达到一个高峰,与培养1 d时比较差异有显著性意义(P < 0.05);在培养3-5 d是维持在一个平台期,培养2 d与3 d、3 d与4 d、4 d与5 d间细胞阳性率比较差异无显著性意义(P > 0.05)。"
| [1] 王佃亮.种子细胞——组织工程连载之三[J].中国生物工程杂志, 2014,34(7):108-113.[2] 裴雪涛,刘大庆.干细胞:组织器官重建的理想种子细胞[J].中国修复重建外科杂志, 2006,20(4):344-348.[3] Nakamura T,Okamoto I,Sasaki K,et al.A developmental coordinate of pluripotency among mice, monkeys and humans. Nature.2016;537(7618):57-62.[4] Saurat NG,Livesey FJ,Moore S.Cortical Differentiation of Human Pluripotent Cells for In Vitro Modeling of Alzheimer's Disease//Castrillo JI,Oliver SG.Methods in Molecular Biology. 2016:267-278. [5] McConnell G,Tragardh J,Amor R,et al.A novel optical microscope for imaging large embryos and tissue volumes with sub-cellular resolution throughout.Elife.2016;5. pii: e18659. doi: 10.7554/eLife.18659.[6] Beaudin AE,Boyer SW,Perez-Cunningham J,et al.A Transient Developmental Hematopoietic Stem Cell Gives Rise to Innate-like B and T Cells.Cell Stem Cell.2016;19(6): 768-783.[7] 习佳飞,王韫芳,裴雪涛.成体干细胞及其在再生医学中的应用[J].生命科学, 2006,18(4):328-332.[8] Tomasetti C,Vogelstein B.Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions.Science.2015;347(6217):78-81.[9] Fuentealba LC,Rompani SB,Parraguez JI,et al.Embryonic Origin of Postnatal Neural Stem Cells].Cell. 2015; 161(7): 1644-1655.[10] Guo G,von Meyenn F,Santos F,et al.Naive Pluripotent Stem Cells Derived Directly from Isolated Cells of the Human Inner Cell Mass.Stem Cell Reports.2016;6(4):437-446.[11] Zhang Y,Huang B.Peripheral Blood Stem Cells: Phenotypic Diversity and Potential Clinical Applications.Stem Cell Rev..2012;8(3):917-925.[12] Pelttari K,Pippenger B,Mumme M,et al.Adult human neural crest-derived cells for articular cartilage repair.Sci Transl Med. 2014;6(251):119r-251r.[13] Palumbo A,Cavallo F,Gay F,et al.Autologous transplantation and maintenance therapy in multiple myeloma.N Engl J Med. 2014;371(10):895-905.[14] Moskowitz CH,Nademanee A,Masszi T,et al.Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double- blind, placebo-controlled, phase 3 trial.Lancet. 2015; 385(9980):1853-1862.[15] Martins VC,Busch K,Juraeva D,et al.Cell competition is a tumour suppressor mechanism in the thymus.Nature.2014; 509(7501):465-470.[16] Sebastiano V,Zhen HH,Haddad B,et al.Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa.Sci Transl Med.2014;6(264):163r-264r.[17] Bertolo A,Gemperli A,Gruber M,et al.In vitro cell motility as a potential mesenchymal stem cell marker for multipotency. Stem Cells Transl Med.2015;4(1):84-90.[18] Konomi K,Tobita M,Kimura K,et al.New Japanese initiatives on stem cell therapies. Cell Stem Cell.2015;16(4):350-352.[19] Castiglione F, Dewulf K, Hakim L,et al.Adipose-derived Stem Cells Counteract Urethral Stricture Formation in Rats.Eur Urol. 2016;70(6):1032-1041.[20] Huang P,Li S,Han M.Autologous Transplantation of Granulocyte Colony–Stimulating Factor–Mobilized Peripheral Blood Mononuclear Cells Improves Critical Limb Ischemia in Diabetes.Diabetes Care.2006;29(2):478-479.[21] Zhao Y,Glesne D,Huberman E.A human peripheral blood monocyte-derived subset acts as pluripotent stem cells.Proc Natl Acad Sci U S A.2003;100(5):2426-2431.[22] Seta N,Okazaki Y,Izumi K,et al.Fibronectin Binding Is Required for Acquisition of Mesenchymal/Endothelial Differentiation Potential in Human Circulating Monocytes. Clin Dev Immunol.2012;2012:1-9.[23] Kuwana M,Okazaki Y,Kodama H,et al.Endothelial Differentiation Potential of Human Monocyte-Derived Multipotential Cells.Stem Cells.2006;24(12):2733-2743.[24] Seta N,Kuwana M.Human circulating monocytes as multipotential progenitors.Keio J Med.2007;56(2):41-47.[25] Seta N,Kuwana M.Derivation of multipotent progenitors from human circulating CD14+ monocytes.Exp Hematol.2010; 38(7):557-563.[26] 王德峰,张喜善,武京国,等.自体外周血单核细胞移植联合多孔髓芯减压修复股骨头坏死:11个月随访评价[J].中国组织工程研究, 2015,19(1):114-118.[27] Zhang M,Huang B.The multi-differentiation potential of peripheral blood mononuclear cells.Stem Cell Res Ther. 2012; 3(6):48.[28] 谷涌泉,张建,苏力,等.自体外周血单个核细胞移植治疗下肢缺血53例的临床研究[J].中华普通外科杂志,2006,21(12):848- 851.[29] Xian B, Zhang Y, Peng Y, et al. Adult Human Peripheral Blood Mononuclear Cells Are Capable of Producing Neurocyte or Photoreceptor-Like Cells That Survive in Mouse Eyes After Preinduction With Neonatal Retina.Stem Cells Transl Med.2016.pii: sctm.2015-0395. [Epub ahead of print][30] Peng Y,Zhang Y,Huang B,et al.Survival and migration of pre-induced adult human peripheral blood mononuclear cells in retinal degeneration slow (rds) mice three months after subretinal transplantation.Curr Stem Cell Res Ther. 2014; 9(2):124-133.[31] Zhang Y,Luo Y,Li K,et al. Pre-induced adult human peripheral blood mononuclear cells migrate widely into the degenerative retinas of rd1 mice.Cytotherapy. 2013;15(11):1416-1425.[32] Liu Q,Guan L,Huang B,et al.Adult peripheral blood mononuclear cells transdifferentiate in vitro and integrate into the retina in vivo.Cell Biol Int. 2011;35(6):631-638.[33] Qu D,Wang G,Wang Z,et al.5-Ethynyl-2′-deoxycytidine as a new agent for DNA labeling: Detection of proliferating cells. Anal Biochem.2011;417(1):112-121.[34] Chehrehasa F,Meedeniya ACB,Dwyer P,et al.EdU, a new thymidine analogue for labelling proliferating cells in the nervous system.J Neurosci Methods. 2009 ;177(1):122-130. [35] Painter RB,Schaefer AW.Variation in the rate of DNA chain growth through the S phase in HeLa cells.J Mol Biol.1971; 58(1):289-295.[36] Foley J,Ton T,Maronpot R,et al.Comparison of proliferating cell nuclear antigen to tritiated thymidine as a marker of proliferating hepatocytes in rats.Environ Health Perspect. 1993;101 Suppl 5:199-205.[37] Fulcher DA,Wong SWJ.Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T cell function in the diagnostic laboratory.Immunol Cell Biol.1999; 77(6):559-564.[38] Quah BJC,Wijesundara DK,Ranasinghe C,et al.The Use of Fluorescent Target Arrays for Assessment of T Cell Responses In vivo.J Vis Exp.2014(e5162788).[39] Stockinger B,Barthlott T,Kassiotis G.The concept of space and competition in immune regulation.Immunology.2004;111(3): 241-247.[40] 刘雷,杨立业.EdU在检测细胞增殖中的应用[J].医学综述,2010, 16(19):2901-2904.[41] 韩建群,修瑞娟.两种细胞增殖分析方法的比较性研究[J].山东医药,2012,52(12):70-72.[42] Rehman J.Peripheral Blood "Endothelial Progenitor Cells" Are Derived From Monocyte/Macrophages and Secrete Angiogenic Growth Factors.Circulation. 2003;107(8): 1164-1169.[43] Bianco JN,Poli J,Saksouk J,et al.Analysis of DNA replication profiles in budding yeast and mammalian cells using DNA combing.Methods.2012;57(2):149-157.[44] Anda S, Boye E, Grallert B. Cell-cycle analyses using thymidine analogues in fission yeast.PLoS One.2014;9(2): e88629.[45] Wei Z,Hurtt R,Ciccarelli M,et al.Growth inhibition of human hepatocellular carcinoma cells by overexpression of G-protein-coupled receptor kinase 2.J Cell Physiol. 2012; 227(6):2371-2377.[46] Lake JI,Avetisyan M,Zimmermann AG,et al.Neural crest requires Impdh2 for development of the enteric nervous system, great vessels, and craniofacial skeleton. Dev Biol.2016;409(1):152-165.[47] Mohr MA,Sisk CL.Pubertally born neurons and glia are functionally integrated into limbic and hypothalamic circuits of the male Syrian hamster.Proc Natl Acad Sci U S A. 2013; 110(12):4792-4797.[48] Zainal AS,Mohamed RN,Megat AWR,et al.Analyses of basal media and serum for in vitro expansion of suspension peripheral blood mononucleated stem cell.Cytotechnology. 2016;68(4):675-686.[49] Dixit P,Donnelly H,Edamatsu M,et al.Progenitor cells from atria, ventricle and peripheral blood of the same patients exhibit functional differences associated with cardiac repair.Int J Cardiol.2016;228:412-421.[50] 娄向新,袁卉华,包敏,等.模拟干细胞生长微环境以促进间充质干细胞的扩增[J].中国细胞生物学学报,2013,35(11):1681-1688.[51] 徐海龙,丁越,谢洪,等.碱性成纤维细胞生长因子及胰岛素样生长因子影响骨髓间充质干细胞增殖及胶原合成[J].中国组织工程研究,2016,20(6):891-897.[52] Linh NT,Abueva CD,Lee BT.Enzymatic in situ formed hydrogel from gelatin-tyramine and chitosan-4-hydroxylphenyl acetamide for the co-delivery of human adipose-derived stem cells and platelet-derived growth factor towards vascularization.Biomed Mater. 2017; 12(1):015026.[53] Xu B,Zhao Y,Xiao Z,et al.A Dual Functional Scaffold Tethered with EGFR Antibody Promotes Neural Stem Cell Retention and Neuronal Differentiation for Spinal Cord Injury Repair.Adv Healthc Mater.2017.doi:10.1002/adhm.201601279.[Epub ahead of print][54] Naudin C,Hattabi A,Michelet F,et al.PUMILIO/FOXP1 signaling drives expansion of hematopoietic stem/progenitor and leukemia cells.Blood.2017.pii: blood-2016-10-747436.[55] Muñoz M,Martin D,Carrocera S,et al.Localisation of stem cell factor, stanniocalcin-1, connective tissue growth factor and heparin-binding epidermal growth factor in the bovine uterus at the time of blastocyst formation.Reprod Fertil Dev.2017.doi: 10.1071/RD16383.[Epub ahead of print][56] Ratajczak MZ,Bartke A,Darzynkiewicz Z.Prolonged Growth Hormone/Insulin/Insulin-like Growth Factor Nutrient Response Signaling Pathway as a Silent Killer of Stem Cells and a Culprit in Aging.Stem Cell Rev.2017.doi: 10.1007/s12015-017-9728-2.[Epub ahead of print] Review.[57] Cassell P. Review of article: Coadministration of adipose-derived stem cells and control-released basic fibroblast growth factor facilitates angiogenesis in a murine ischemic hind limb model by Horikoshi-Ishihara, Tobita, Tajima, et al. Journal of Vascular Surgery 12/2016; pp1825-1834. J Vasc Nurs.2017;35(1):36-37.[58] Sarveazad A,Newstead GL,Mirzaei R,et al.A new method for treating fecal incontinence by implanting stem cells derived from human adipose tissue: preliminary findings of a randomized double-blind clinical trial.Stem Cell Res Ther. 2017;8(1):40.[59] Zhuo HL,Bai LP,Liu D,et al.Effects of retinol on expressions of epidermal growth factor, stem cell factor, colony-stimulating factor 1 and leukemia inhibitory factor in human umbilical cord-derived mesenchymal stem cells.Nan Fang Yi Ke Da Xue Xue Bao. 2016;37(2):221-225.[60] Wang C,Deng Y,Chen F,et al.Basic fibroblast growth factor is critical to reprogramming buffalo (Bubalus bubalis) primordial germ cells into embryonic germ stem cell-like cells. Theriogenology. 2017;91:112-120. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Cui Xing, Sun Xiaoqi, Zheng Wei, Ma Dexin. Huangqin Decoction regulates autophagy to intervene with intestinal acute graft-versus-host disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1057-1062. |
| [4] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [5] | Yang Sidi, Wang Qian, Xu Nuo, Wang Ronghan, Jin Chuanqi, Lu Ying, Dong Ming. Biodentine enhances the proliferation and differentiation of osteoblasts through upregulating bone morphogenetic protein-2 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 516-520. |
| [6] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [7] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [8] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [9] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [10] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [11] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [12] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [13] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [14] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [15] | Feng Dongfei, He Hongxu, Xie Qi, Zhang Lili, Zhou Hui, Li Wei. Selection of key genes related to biological functions and regulation pathway in periodontal reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 253-259. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||