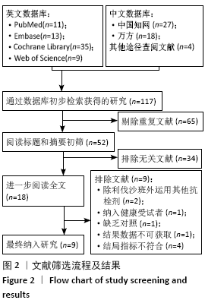
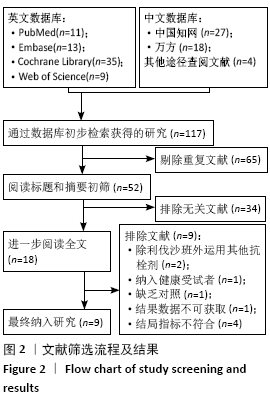
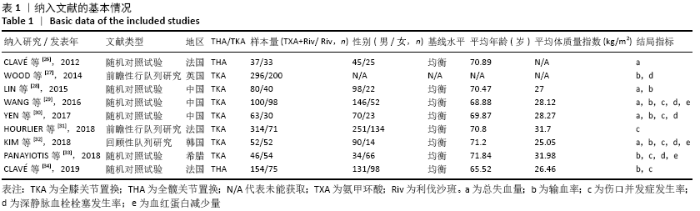
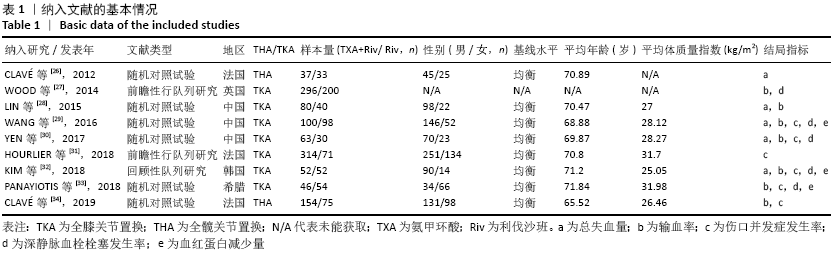
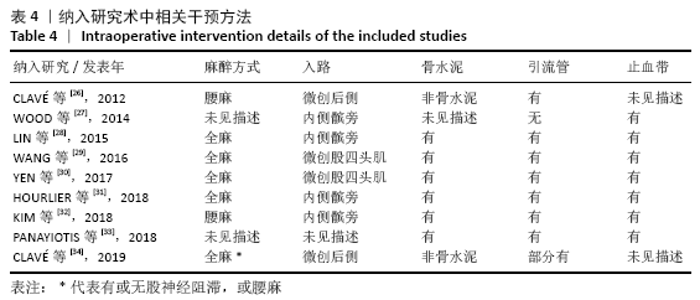
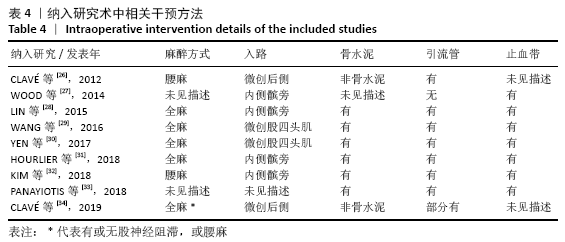
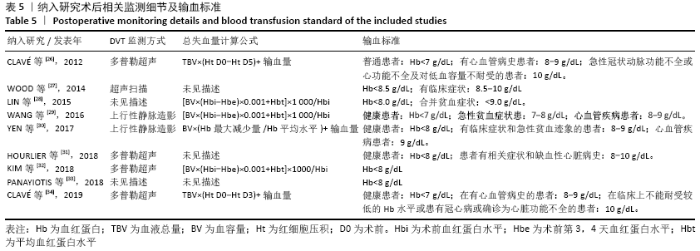
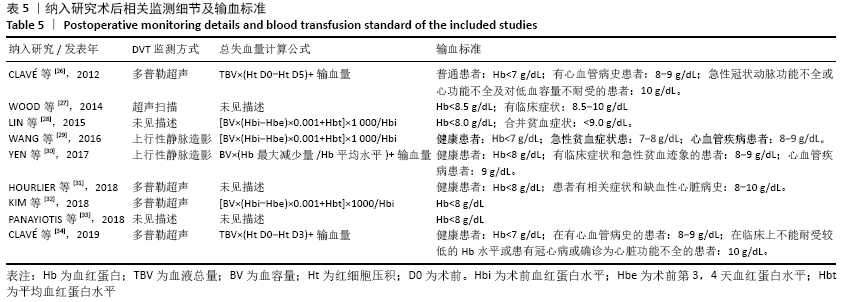
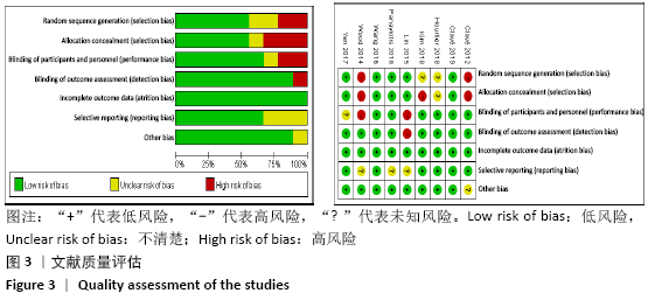
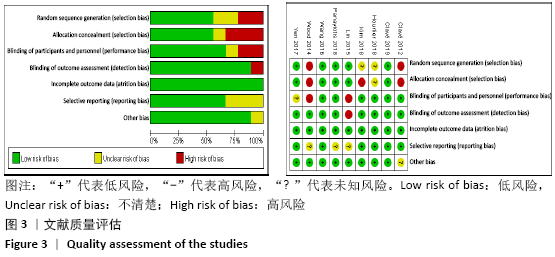
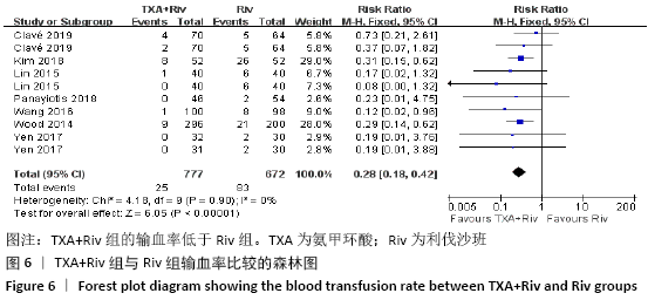
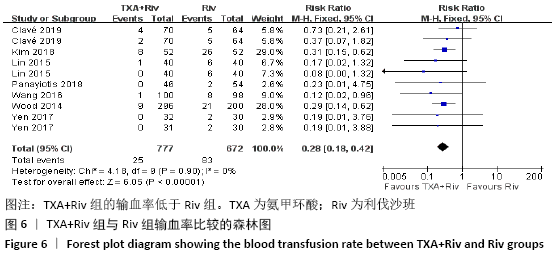
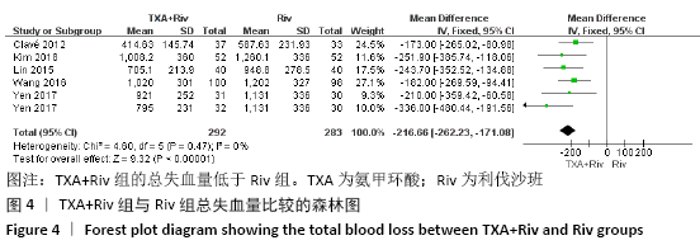
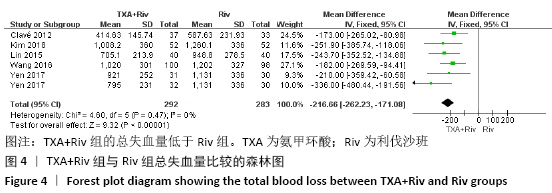
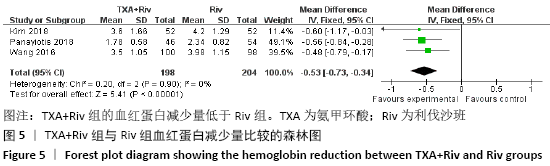
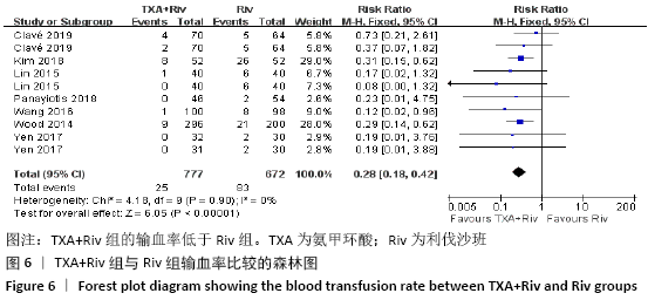
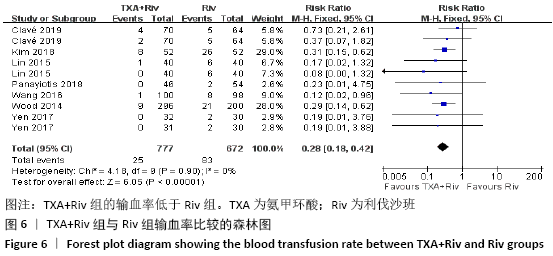
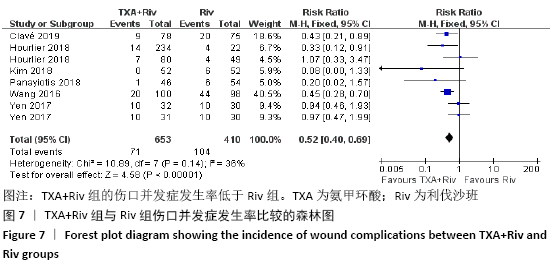
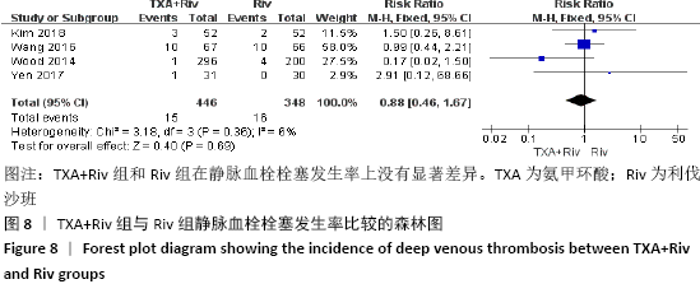
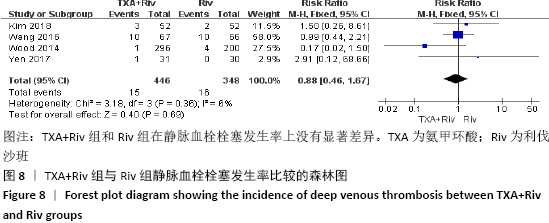
Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (15): 2453-2460.doi: 10.3969/j.issn.2095-4344.3824
Efficacy and safety of tranexamic acid combined with rivaroxaban in primary total knee and hip arthroplasties: a meta-analysis
Gao Fenghe1, Chen Tongying1, Lin Jiebin1, Liang Zujian1, 2
- 1Third School of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 2The Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510240, Guangdong Province, China
-
Received:2020-06-17Revised:2020-06-24Accepted:2020-07-23Online:2021-05-28Published:2021-01-05 -
Contact:Liang Zujian, MD, Chief TCM physician, Master’s supervisor, Third School of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; The Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510240, Guangdong Province, China -
About author:Gao Fenghe, Master candidate, Third School of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
Supported by:the Natural Science Foundation of Guangdong Province, No. 2018A0303130103 (to LZJ)
CLC Number:
Cite this article
Gao Fenghe, Chen Tongying, Lin Jiebin, Liang Zujian. Efficacy and safety of tranexamic acid combined with rivaroxaban in primary total knee and hip arthroplasties: a meta-analysis[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(15): 2453-2460.
share this article
| [1] DOMINIQUE S, BRICE RD, JULIA G, et al. Total knee arthroplasties from the origin to navigation: history, rationale, indications. Int Orthop. 2019; 43(3):597-604. [2] SHARKEY PETER F, HOZACK WILLIAM J, ROTHMAN RICHARD H, et al. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13. [3] SHARKEY PF, LICHSTEIN PM, SHEN C, et al. Why are total knee arthroplasties failing today-has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778. [4] JONES CA, VOAKLANDER DC, JOHNSTON DW, et al. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752. [5] TRIANTAFYLLOPOULOS GEORGIOS K, SORANOGLOU VASILEIOS G, MEMTSOUDIS STAVROS G, et al. Rate and Risk Factors for Periprosthetic Joint Infection Among 36,494 Primary Total Hip Arthroplasties. J Arthroplasty. 2018;33(4):1166-1170. [6] KIM KI, EGOL KA, HOZACK WJ, et al. Periprosthetic fractures after total knee arthroplasties. Clin. Orthop. Relat. Res. 2006;446:167-75. [7] CARLING MS, JEPPSSON A, ERIKSSON BI, et al. Transfusions and blood loss in total hip and knee arthroplasty: a prospective observational study. J Orthop Surg Res. 2015;10(1):48. [8] SEHAT KR, EVANS R, NEWMAN JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151-155. [9] LIU X, ZHANG X, CHEN Y, et al. Hidden Blood Loss After Total Hip Arthroplasty. J Arthroplasty. 2011;26(7):1100-11050. [10] SIHLER KRISTEN C, NAPOLITANO LENA M. Complications of massive transfusion. Chest. 2010;137(1):209-220. [11] SHARMA S, SHARMA P, TYLER LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician. 2011;83(6):719-724. [12] DUNN CJ, GOA KL. Tranexamic Acid: a review of its use in surgery and other indications. Drugs. 1999;57(6):1005-1032. [13] NAPOLITANO LM, COHEN MJ, COTTON BA, et al. Tranexamic acid in trauma: how should we use it? J Trauma Acute Care Surg. 2013;74(6):1575-1586. [14] FILLINGHAM YA, RAMKUMAR DB, JEVSEVAR DS, et al. The efficacy of tranexamic acid in total knee arthroplasty: a network meta-analysis. J Arthroplasty. 2018;33(10):3090-3098. [15] JANUEL JM, CHEN G, RUFFIEUX C, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012;307(3):294-303. [16] AST MICHAEL P, GORAB ALEXANDRA H, BANKA TREVOR R, et al. Clinical outcomes of patients with non-fatal VTE after total knee arthroplasty. J Arthroplasty. 2014;29(1):37-39. [17] CHAPELLE C, ROSENCHER N, JACQUES ZUFFEREY P, et al. Prevention of venous thromboembolic events with low-molecular-weight heparin in the non-major orthopaedic setting: meta-analysis of randomized controlled trials. Arthroscopy. 2014;30(8):987-996. [18] 中华医学会骨科学分会(COA).中国骨科大手术静脉血栓栓塞症预防指南[J].中华骨科杂志, 2009,29(6):602-604. [19] FALCK-YTTER Y, FRANCIS CW, JOHANSON NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S. [20] EINSTEIN INVESTIGATORS, BAUERSACHS R, BERKOWITZ SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010; 363(26):2499-2510. [21] CHAN NC, WEITZ JI. Rivaroxaban for prevention and treatment of venous thromboembolism. Future Cardiol. 2019;15(2):63-77. [22] GALANIS T, MERLI GJ. New oral anticoagulants: prevention of VTE in phase III studies in total joint replacement surgery and the hospitalized medically-ill patients. J Thromb Thrombolysis. 2013;36(2):141-148. [23] YUAN X, LI B, WANG Q, et al. Comparison of 3 routes of administration of tranexamic acid on primary unilateral total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplasty. 2017;32(9):2738-2743. [24] HU KZ, SUN HY, SUI C. Effects of five treatment regimens on blood loss and blood transfusion in total knee arthroplasty: a preliminary study in China. Int J Clin Pharmacol Ther. 2017;55(5):433-441. [25] YEN SH, LIN PC, KUO FC, et al. Thromboprophylaxis after minimally invasive total knee arthroplasty: a comparison of rivaroxaban and enoxaparin. Biomed J. 2014;37(4):199-204. [26] CLAVÉ A, FAZILLEAU F, DUMSER D, et al. Efficacy of tranexamic acid on blood loss after primary cementless total hip replacement with rivaroxaban thromboprophylaxis: a case-control study in 70 patients. Orthop Traumatol Surg Res. 2012;98(5):484-490. [27] WOOD AM, SMITH R, KEENAN A, et al. Using a combination of tranexamic acid and rivaroxaban in total knee replacements reduces transfusion requirements: a prospective cohort study. J Arthrosc Joint Surg. 2014;1(2):76-81. [28] LIN SY, CHEN CH, FU YC, et al. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015;30(5):776-780. [29] WANG JW, CHEN B, LIN PC, et al. The efficacy of combined use of rivaroxaban and tranexamic acid on blood conservation in minimally invasive total knee arthroplasty a double-blind randomized, controlled trial. J Arthroplasty. 2017;32(3):801-806. [30] YEN SH, LIN PC, CHEN B, et al. Topical tranexamic acid reduces blood loss in minimally invasive total knee arthroplasty receiving rivaroxaban. Biomed Res Int. 2017;2017:1-8. [31] HERVÉ H, FENNEMA P. Tranexamic acid use and risk of thrombosis in regular users of antithrombotics undergoing primary total knee arthroplasty: a prospective cohort study. Trasfusione Del Sangue. 2016;16(1):1-9. [32] KIM YT, KANG MW, LEE JK, et al. Combined use of topical intraarticular tranexamic acid and rivaroxaban in total knee arthroplasty safely reduces blood loss, transfusion rates, and wound complications without increasing the risk of thrombosis. BMC Musculoskelet Disord. 2018;19(1):227. [33] KARAMPINAS PK, MEGALOIKONOMOS PD, LAMPROPOULOU-ADAMIDOU K, et al. Similar thromboprophylaxis with rivaroxaban and low molecular weight heparin but fewer hemorrhagic complications with combined intra-articular and intravenous tranexamic acid in total knee arthroplasty. Eur J Orthop Surg Traumatol. 2018,29(2):455-460. [34] CLAVÉ A, GÉRARD R, LACROIX J, et al. A randomized, double-blind, placebo-controlled trial on the efficacy of tranexamic acid combined with rivaroxaban thromboprophylaxis in reducing blood loss after primary cementless total hip arthroplasty. Bone Joint J. 2019;101-B(2):207-212. [35] TENGBORN L, BLOMBÄCK M, BERNTORP E. Tranexamic acid-an old drug still going strong and making a revival. Thromb Res. 2015;135(2): 231-242. [36] GIANAKOS AL, HURLEY ET, Haring RS, et al. Reduction of blood loss by tranexamic acid following total hip and knee arthroplasty: a meta-analysis. JBJS Rev. 2018;6(5):e1. [37] ADIGWEME OO, LEE GC. Tranexamic acid: the new gold standard? Tech Orthop. 2017;32(1):17-22. [38] GUZEL Y, GURCAN OT, GOLGE UH, et al. Topical tranexamic acid versus autotransfusion after total knee arthroplasty. J Orthop Surg. 2016;24(2): 179-182. [39] ELISABETH P, SUSANNE R, ALEXANDER S, et al. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov. 2011;10(1):61-75. [40] David G, Helen R, Andrea D, et al. A Phase IV Study of Thromboembolic and Bleeding Events Following Hip and Knee Arthroplasty Using Oral Factor Xa Inhibitor. J Arthroplasty. 2017;32(3): 958-964. [41] HUISMAN MENNO V, QUINLAN DANIEL J, DAHL OLA E, et al. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: Results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes. 2010;3(6):652-660. [42] MONREAL M, FOLKERTS K, DIAMANTOPOULOS A, et al. Cost-effectiveness impact of rivaroxaban versus new and existing prophylaxis for the prevention of venous thromboembolism after total hip or knee replacement surgery in France, Italy and Spain. Thromb Haemost. 2013;110(5):987-994. [43] MENG BY, MA J, LIU Z, et al. Efficacy and safety of tranexamic acid combined with rivaroxaban in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. J Invest Surg. 2019;(1):1-10. [44] GIBSON CAITLIN M, YUET WEI C, Racial and ethnic differences in response to anticoagulation: a review of the literature. J Pharm Pract. 2019. Doi:10.1177/0897190019894142. [45] LIEW NGOH C, ALEMANY GINA V, PANTEP A, et al. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol. 2017; 36(1):1-20. [46] LACHIEWICZ PAUL F, Comparison of ACCP and AAOS guidelines for VTE prophylaxis after total hip and total knee arthroplasty. Orthopedics. 2009;32(12):74-78. [47] DARZI ANDREA J, KARAM SAMER G, RANA C, et al. Prognostic factors for VTE and bleeding in hospitalized medical patients: a systematic review and meta-analysis. Blood. 2020;135(20): 1788-1810. [48] KRAUSS ES, CRONIN M, DENGLER N, et al. The effect of BMI and gender on bleeding events when rivaroxaban is administered for thromboprophylaxis following total hip and total knee arthroplasty. Semin Thromb Hemost. 2019;45(2):180-186. [49] YUAN FZ, ZHANG JY, JIANG D, et al. Quadriceps-sparing versus traditional medial parapatellar approaches for total knee arthroplasty: a meta-analysis. BMC Musculoskelet Disord. 2019;20(1): 117. [50] CAI DF, FAN QH, ZHONG HH, et al. The effects of tourniquet use on blood loss in primary total knee arthroplasty for patients with osteoarthritis: a meta-analysis. J Orthop Surg Res. 2019;14(1):348. [51] WANG C, ZHOU CH, QU H, et al. Comparison of tourniquet application only during cementation and long-duration tourniquet application in total knee arthroplasty: a meta-analysis. J Orthop Surg Res. 2018;13:216. [52] PU X, SUN JM. General anesthesia vs spinal anesthesia for patients undergoing total-hip arthroplasty: a meta-analysis. Medicine (Baltimore). 2019;98(6):e14925. |
| [1] | Li Dadi, Zhu Liang, Zheng Li, Zhao Fengchao. Correlation of total knee arthroplasty efficacy with satisfaction and personality characteristics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1346-1350. |
| [2] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [3] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [4] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [5] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [6] | Zhang Chong, Liu Zhiang, Yao Shuaihui, Gao Junsheng, Jiang Yan, Zhang Lu. Safety and effectiveness of topical application of tranexamic acid to reduce drainage of elderly femoral neck fractures after total hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1381-1386. |
| [7] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [8] | Chen Junming, Yue Chen, He Peilin, Zhang Juntao, Sun Moyuan, Liu Youwen. Hip arthroplasty versus proximal femoral nail antirotation for intertrochanteric fractures in older adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1452-1457. |
| [9] | Chen Jinping, Li Kui, Chen Qian, Guo Haoran, Zhang Yingbo, Wei Peng. Meta-analysis of the efficacy and safety of tranexamic acid in open spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1458-1464. |
| [10] | Hu Kai, Qiao Xiaohong, Zhang Yonghong, Wang Dong, Qin Sihe. Treatment of displaced intra-articular calcaneal fractures with cannulated screws and plates: a meta-analysis of 15 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1465-1470. |
| [11] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [12] | Wang Yongsheng, Wu Yang, Li Yanchun. Effect of acute high-intensity exercise on appetite hormones in adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1305-1312. |
| [13] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148. |
| [14] | Zhong Hehe, Sun Pengpeng, Sang Peng, Wu Shuhong, Liu Yi. Evaluation of knee stability after simulated reconstruction of the core ligament of the posterolateral complex [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 821-825. |
| [15] | Zhao Zhongyi, Li Yongzhen, Chen Feng, Ji Aiyu. Comparison of total knee arthroplasty and unicompartmental knee arthroplasty in treatment of traumatic osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 854-859. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||