Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (28): 4492-4497.doi: 10.3969/j.issn.2095-4344.2310
Previous Articles Next Articles
Effects of sustained-release atorvastatin calcium nanofiber scaffold on cell adhesion and proliferation
Wang Le, Hui Min, Dong Xiling, Zhou Han, Dong Hongliang, Zhang Xiaoming, Liu Tongbin
Department of Prosthodontics, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China
-
Received:2019-12-02Revised:2019-12-05Accepted:2020-01-02Online:2020-10-08Published:2020-08-31 -
Contact:Zhang Xiaoming, Master, Chief physician, Master’s supervisor, Department of Prosthodontics, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China Liu Tongbin, Master, Department of Prosthodontics, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China -
About author:Wang Le, Master candidate, Department of Prosthodontics, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China -
Supported by:the Medical and Health Science and Technology Development Plan Project of Shandong Province, No. 2016WS0121; the Science and Technology Program of Binzhou Medical University, No. BY2017KJ05
CLC Number:
Cite this article
Wang Le, Hui Min, Dong Xiling, Zhou Han, Dong Hongliang, Zhang Xiaoming, Liu Tongbin. Effects of sustained-release atorvastatin calcium nanofiber scaffold on cell adhesion and proliferation[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4492-4497.
share this article
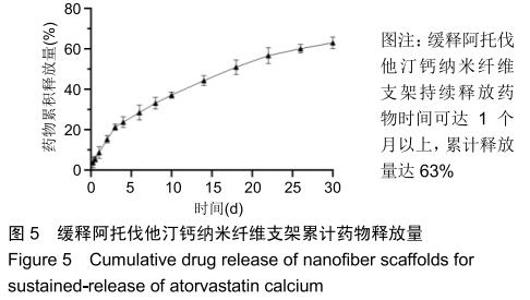
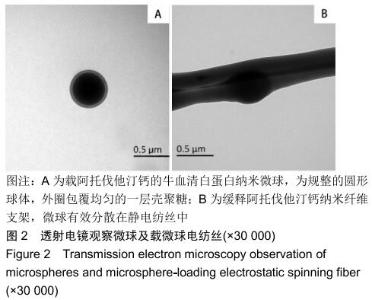
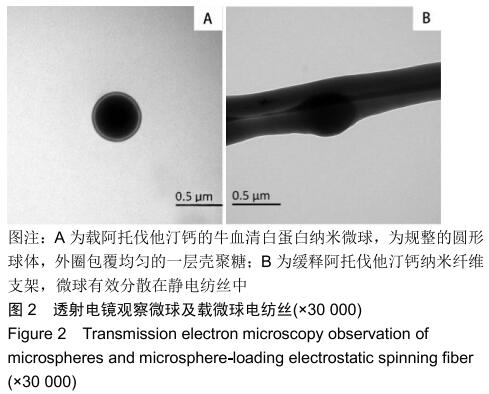
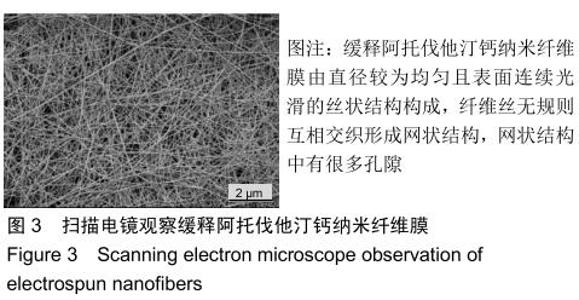
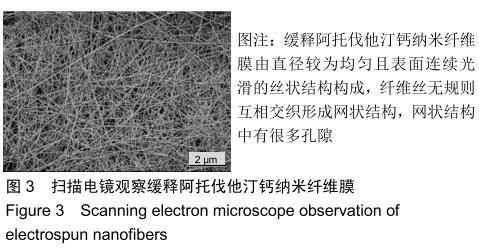
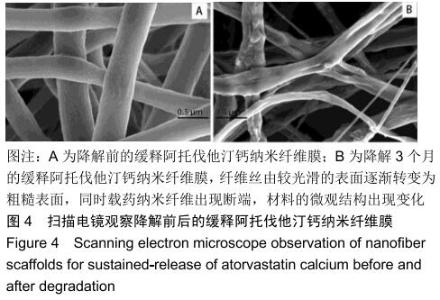
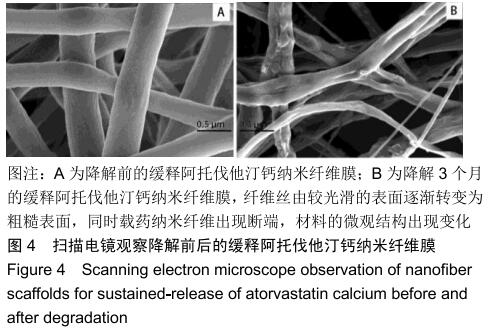
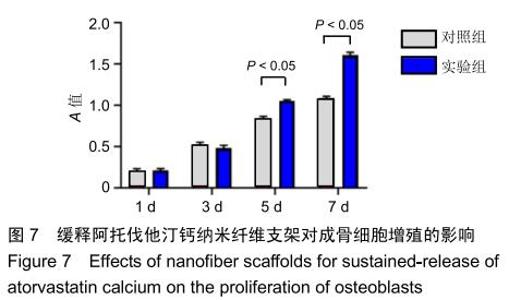
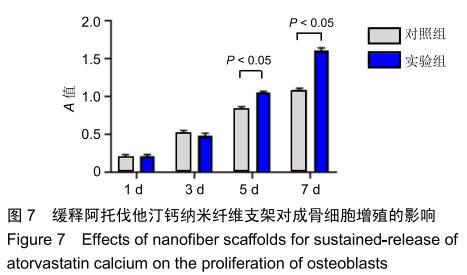
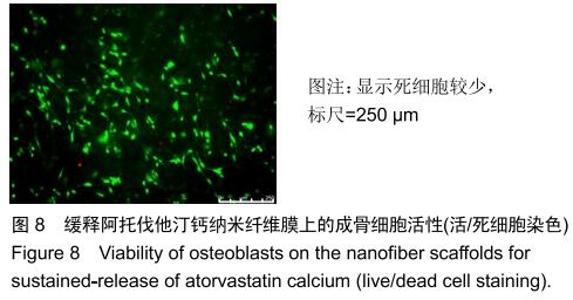
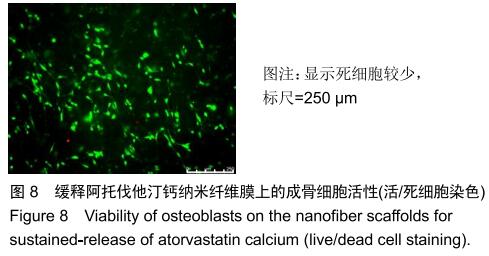
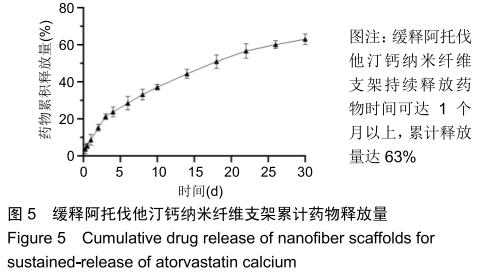
2.3 缓释阿托伐他汀钙纤维膜的失重率 聚己内酯作为高分子聚合物其分子链中具有众多酯键,酯键发生断裂会导致高分子材料整体结构破坏,导致分子质量下降。缓释阿托伐他汀钙纤维膜1,2,3个月时的失重率分别为18.33%,29.54%,35.70%。第1个月时材料降解速度是最快的,后期降解速度减缓,这是因为高分子聚合物包含的酯键水解断裂是随意的,降解初期链包含酯键分子位点多。降解1个月时,明显感觉材料变得松软,机械性能下降,但是尚可成膜,降解3个月时材料已经不完整。 2.4 缓释阿托伐他汀钙纳米纤维支架的药物释放 药物包封率70%左右,大部分药物可以包裹在牛血清白蛋白微球中。药物缓释支架前期释放较快,前3 d已达21%,体现了短期的爆发式的释放规律,后期释放逐渐变慢,3-30 d的释放模式类似药物零级释放动力学。药物缓释支架持续释放药物时间可达1个月以上,累计释放量达63%,1个月以后药物仍然缓慢释放,见图5。多重屏障半衰期明显延长,整体分为2个阶段,前期短暂的突释放可迅速使药物达到有效作用浓度,后期的缓慢释放可维持药物浓度并明显延长药物作用时间。 "
|
[1] 李莺,尤亚鹏,李宝娥,等.纯钛表面TiO_2纳米管结合Ⅰ型胶原促进成骨细胞黏附和骨结合[J].中国组织工程研究,2019,23(14): 2169-2176.
[2] 杨玉鹏,赵海静,谷建琦,等.钛芯与骨形成蛋白复合材料修复即刻种植牙槽骨缺陷的评价[J].中国组织工程研究,2017,21(22): 3536-3540.
[3] SEVIMAY M, TURHAN F, KILIÇARSLAN MA, et al. Three-dimensional finite element analysis of the effect of different bone quality on stress distribution in an implant-supported crown.J Prosthet Dent. 2005;93(3): 227-234.
[4] LEE S, GANTES B, RIGGS M, et al. Bone density assessments of dental implant sites: 3. Bone quality evaluation during osteotomy and implant placement.Int J Oral Maxillofac Implants.2007;22(2):208-212.
[5] OFFERMANNS V, ZOFFMANN ANDERSEN O, RIEDE G, et al. Bone regenerating effect of surface-functionalized titanium implants with sustained-release characteristics of strontium in ovariectomized rats.Int J Nanomed.2016;11:2431-2442.
[6] CORCUERA-FLORES JR, ALONSO-DOMÍNGUEZ AM, SERRERA-FIGALLO MÁ, et al. Relationship Between Osteoporosis and Marginal Bone Loss in Osseointegrated Implants: A 2-Year Retrospective Study. J Periodontol. 2016; 87(1):14-20.
[7] WANG M, LAN L, LI T, et al. The effect of oxytocin on osseointegration of titanium implant in ovariectomized rats. Connect Tissue Res.2016;57(3):220-225.
[8] SANTOS PL,GULINELLI JL, TELLES CS, et al. Bone substitutes for peri-implant defects of postextraction implants. Int J Biomater.2013;2013:307136.
[9] WANG Z, LI Y, ZHOU F, et al. Effects of Statins on Bone Mineral Density and Fracture Risk. Medicine. 2016;95(22):e3042.
[10] TSARTSALIS AN, DOKOS C, KAIAFA GD, et al. Statins, bone formation and osteoporosis: hope or hype?Hormones(Athens, Greece).2012;11(2):126.
[11] ORYAN A, ALIDADI S, MOSHIRI A, et al. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res.2014;9(1):18.
[12] GUTIERREZ GE, LALKA D, GARRETT IR, et al. Transdermal application of lovastatin to rats causes profound increases in bone formation and plasma concentrations.Osteoporosis Int. 2006;17(7):1033-1042.
[13] ZHENG IC, LI CC. Multiblock copolymers composed of poly (butylenes succinate)and poly(1,2-propylenesuccinate): Effect of molar ratio of diisocyanate to polyester diols oncrosslink densities, thermal properties, mechanical properties and biodegradability.Polym Degrad Stabil. 2010; 9(95):1-8.
[14] ZENG JB, LI YD. A novel biodegradable multiblock poly(ester urethane)containing poly(L-lactic acid)and poly(butylene sue-cinate)blocks.Polymer.2009;(50):1178-1186.
[15] DASH TK, KONKIMALLA VB. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J Control Release.2012;158(1):15-33.
[16] YOSHIMOTO H, SHIN YM, TERAI H, et al. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering.Biomaterials.2003;24(12):2077-2082.
[17] EICHHORN SJ, SAMPSON WW. Statistical geometry of pores and statistics of porous nanofibrous assemblies.J R Soc Interface.2005;2(4):309-318.
[18] FU R, LI C, YU C, et al. A novel electrospun membrane based on moxifloxacin hydrochloride/poly(vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Delivery.2014;23(3):818-829.
[19] WENG L, XIE J. Smart electrospun nanofibers for controlled drug release: recent advances and new perspectives.Curr Pharm Design.2015;21(15):1944.
[20] HAN D, STECKL AJ. Triaxial Electrospun Nanofiber Membranes for Controlled Dual Release of Functional Molecules.ACS Appl Mater Inter.2013;5(16):8241-8245.
[21] WADE RJ, BASSIN EJ, GRAMLICH WM, et al. Nanofibrous Hydrogels with Spatially Patterned Biochemical Signals to Control Cell Behavior.Adv Mater.2015;27(8):1356-1362.
[22] SANTANA-MELO GF, RODRIGUES BVM, DA SILVA E, et al. Electrospun ultrathin PBAT/nHAp fibers influenced the in vitro and in vivo osteogenesis and improved the mechanical properties of neoformed bone.Colloid Surface B. 2017;155: 544-552.
[23] LI L, ZHOU G, WANG Y, et al. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect.Biomaterials.2015;37:218-229.
[24] LI D, SUN H, JIANG L, et al. Enhanced Biocompatibility of PLGA Nanofibers with Gelatin/Nano-Hydroxyapatite Bone Biomimetics Incorporation.ACS Appl Mater Inter. 2014;6(12): 9402-9410.
[25] WANG J, SUN B, TIAN L, et al. Evaluation of the potential of rhTGF- β3 encapsulated P(LLA-CL)/collagen nanofibers for tracheal cartilage regeneration using mesenchymal stems cells derived from Wharton's jelly of human umbilical cord[J]. Mat Sci Eng C.2017;70:637-645.
[26] ZHANG X, REAGAN MR, KAPLAN DL. Electrospun silk biomaterial scaffolds for regenerative medicine.Adv Drug Deliver Rev.2009;61(12):988-1006.
[27] KIM K, LUU YK, CHANG C, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds.J Control Release.2004;98(1):47-56.
[28] KHALF A, SINGARAPU K, MADIHALLY SV. Influence of solvent characteristics in triaxial electrospun fiber formation. React Funct Polym.2015;90:36-46.
[29] GAUTAM S, DINDA AK, MISHRA NC. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method.Mat Sci Eng C.2013;33(3):1228-1235. |
| [1] | Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074. |
| [2] | Zou Gang, Xu Zhi, Liu Ziming, Li Yuwan, Yang Jibin, Jin Ying, Zhang Jun, Ge Zhen, Liu Yi. Human acellular amniotic membrane scaffold promotes ligament differentiation of human amniotic mesenchymal stem cells modified by Scleraxis in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1037-1044. |
| [3] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [4] | Ma Zetao, Zeng Hui, Wang Deli, Weng Jian, Feng Song. MicroRNA-138-5p regulates chondrocyte proliferation and autophagy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 674-678. |
| [5] | Li Wenjing, Li Haobo, Liu Congna, Cheng Dongmei, Chen Huizhen, Zhang Zhiyong. Comparison of different bioactive scaffolds in the treatment of regenerative pulp of young permanent teeth [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 499-503. |
| [6] | Wang Yujiao, Liu Dan, Sun Song, Sun Yong. Biphasic calcium phosphate loaded with advanced platelet rich fibrin can promote the activity of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 504-509. |
| [7] | Zhou Jihui, Yao Meng, Wang Yansong, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Influence of novel nanoscaffolds on biological behaviors of neural stem cells and the related gene expression [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 532-536. |
| [8] | Ma Zhijie, Li Jingyu, Cao Fang, Liu Rong, Zhao Dewei. Influencing factors and biological property of novel biomedical materials: porous silicon carbide coated with bioactive tantalum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 558-563. |
| [9] | Liu Liu, Zhou Qingzhu, Gong Zhuo, Liu Boyan, Yang Bin, Zhao Xian. Characteristics and manufacturing techniques of collagen/inorganic materials for constructing tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 607-613. |
| [10] | Ye Haimin, Ding Linghua, Kong Weihao, Huang Zutai, Xiong Long. Role and mechanism of hierarchical microchanneled bone scaffolds in promoting osteogenesis and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 621-625. |
| [11] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [12] | Chen Yang, Huang Denggao, Gao Yuanhui, Wang Shunlan, Cao Hui, Zheng Linlin, He Haowei, Luo Siqin, Xiao Jingchuan, Zhang Yingai, Zhang Shufang. Low-intensity pulsed ultrasound promotes the proliferation and adhesion of human adipose-derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3949-3955. |
| [13] | Zhou Wu, Wang Binping, Wang Yawen, Cheng Yanan, Huang Xieshan. Transforming growth factor beta combined with bone morphogenetic protein-2 induces the proliferation and differentiation of mouse MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3630-3635. |
| [14] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [15] | Liu Jun, Yang Long, Wang Weiyu, Zhou Yuhu, Wu Ying, Lu Tao, Shu Liping, Ma Minxian, Ye Chuan. Preparation and properties of poly3-hydroxybutyrate 4-hydroxybutyrate/polyethylene glycol/graphene oxide tissue-engineered scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3466-3472. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||